Research Highlights
July 2007
A New Fluorescent Probe for Protein Interactions
Research published in JACS, highlighted in Nature
Results: Scientists at Pacific Northwest National Laboratory have created a new red fluorescent probe to label proteins and quantitatively monitor their interactions in living cells. This probe adds to a toolkit of biarsenical dyes developed into multiuse affinity probes (MAPs) at PNNL to study macromolecular machines and protein networks.
The research, published in the Journal of the American Chemistry Society, appears as a research highlight in the July 5 issue of Nature.
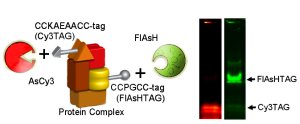 Stylized figure of the molecular machine (protein complex) containing a protein (yellow) with the FlAsHTAG and a protein (orange) with the Cy3TAG. FlAsH (green) and AsCy3(red) bind specifically to the protein containing its cognate tag, as shown in the SDSPAGE gel, which is imaged using light at the two different wavelengths. Enlarged View
Stylized figure of the molecular machine (protein complex) containing a protein (yellow) with the FlAsHTAG and a protein (orange) with the Cy3TAG. FlAsH (green) and AsCy3(red) bind specifically to the protein containing its cognate tag, as shown in the SDSPAGE gel, which is imaged using light at the two different wavelengths. Enlarged View
Methods: The PNNL team, led by biochemist M. Uljana Mayer, made a new fluorescent affinity probe, AsCy3, targeted to a small tag that can be engineered onto a protein of interest. This new probe can then be used in conjunction with the first-generation probe, called FlAsH, to study macromolecular machines and protein networks. The small tags are especially useful because, for example, proteins that need to be exported across the inner membrane of bacteria cannot be engineered to contain large tags such as fluorescent proteins.
This new probe is in a class of small-molecule compounds called biarsenical MAPs. The researchers derivatized Cy3, a fluorescent dye that has good photostability and quantum yield, low pH sensitivity and high absorbance. Quantum yield measures the efficiency with which absorbed light produces effects. "Biarsenical" refers to the two arsenics used in the derivatization because of their ability to bind to cysteines-sulfur—containing amino acids.
AsCy3 is directed specifically to a peptide containing four cysteines, Cy3TAG, in the presence of other tetracysteine tags. The new probe is a partner to the previously developed green MAP. This discovery is an important step toward a whole toolkit of colored probes directed to different small peptides—strings of amino acids, which form specific structural elements.
Why it matters: Small-molecule biarsenical MAPs, in conjunction with complementary protein tags, are important new tools for analyzing cellular function through live-cell imaging, targeted protein inactivations, and the measurement of protein dynamics and binding. In addition, MAPs serve as affinity reagents for isolating intact protein complexes for complementary structural measurements.
The work is part of a long-term goal to develop the necessary reagents and technology to rapidly identify a substantial fraction of the multi-protein complexes in an organism. This will lead to a systems-level understanding of microbial pathways. Knowledge of real-time changes in enzyme interactions will permit the reengineering of microbial pathways for improved biofuel production, mitigation of climate change, and for bioremediation. Critical to this goal is the development of new affinity reagents and protein tags that permit the rapid identification and validation of protein complexes.
What's next: There is increased demand for cell-permeable fluorescent dyes that can bind to relatively small tags and do not hinder protein export. The PNNL team has expanded the biarsenical toolkit and is working toward developing a whole color palette of targetable probes for studying protein-protein interaction networks. Such studies can be applied to environmental remediation, national security, and energy problems.
Acknowledgments: Team members are Haishi Cao, Yijia Xiong, Ting Wang, Baowei Chen, Thomas Squier, and Uljana Mayer, all at PNNL. This research was supported by the Genomics: GTL program of the U.S. Department of Energy's Office of Biological and Environmental Research. Some of the work was conducted in the Environmental Molecular Sciences Laboratory, a DOE national scientific user facility located at PNNL.
References: Cao H, Y Xiong, T Wang, B Chen, TC Squier, and MU Mayer. 2007. "A red Cy3-based biarsenical fluorescent probe targeted to a complementary binding peptide." Journal of the American Chemical Society 129(28):8672 -8673.
Cao H, B Chen, TC Squier, and MU Mayer. 2006. "CrAsH: A Biarsenical Multi-use Affinity Probe with low non-specific fluorescence." Chemical Communications (24):2601-2603.
Mayer MU, L Shi, and TC Squier. 2005. "One-step, non-denaturing isolation of an RNA polymerase enzyme complex using an improved multi-use affinity probe resin." Molecular Biosystems 1(1):53-56.


