Research Highlights
June 2006
Isolation and Validation of a Metal-reducing Protein Complex from Shewanella published in the Journal of Bacteriology
A manuscript describing work by Pacific Northwest National Laboratory (PNNL) scientists in which a protein complex implicated in the reduction of manganese and iron was purified and its subunits identified appears in a July 2006 issue of the Journal of Bacteriology. Identifying these proteins and their metal reduction capabilities is important to understand bacterial physiology and to facilitate bioremediation of contaminated sites, because the metal-reducing capability may also apply to toxic metals such as uranium, technetium, and chromium.
To clarify the roles of cytochromes OmcA and MtrC in the bacterium Shewanella oneidensis MR-1, the PNNL scientists cloned, expressed, and purified the cytochromes, permitting, for the first time, direct measurements of their metal-reducing activity and functional association. Cytochromes are enzymes that function in respiration and photosynthesis processes by transferring electrons across a gradient or membrane. OmcA and MtrC have been implicated in reduction of iron or manganese oxide.
The results of this study will help provide an important understanding of how metal reductase activities can function in the reductive immobilization of toxic metals in contaminated sites. Future experiments should measure the possible association and function of a reconstituted OmcA-MtrC complex against solid metals to determine whether binding and metal reduction involve a direct association with individual proteins.
Authors of the paper are Liang Shi, Baowei Chen, Zheming Wang, Dwayne Elias, Uljana Mayer, Yuri Gorby, Shuisong Ni, Brian Lower, David Kennedy, David Wunschel, Heather Mottaz, Matthew Marshall, Eric Hill, Alex Beliaev, John Zachara, James Fredrickson, and Thomas Squier.
This research was supported by the U.S. Department of Energy (DOE) Office of Biological and Environmental Research (BER) under the Genomics: GTL program and an EMSL Scientific Grand Challenge project at the W.R. Wiley Environmental Molecular Sciences Laboratory, a national scientific user facility sponsored by DOE-BER.
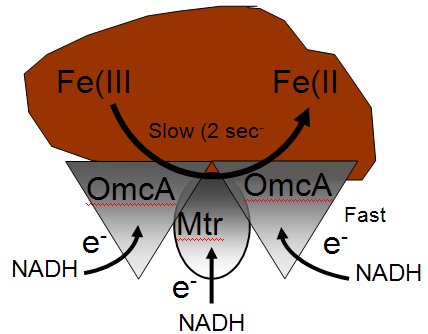
Model of Protein Complex Between OmcA and MtrC. Oligomeric complex involving outer membrane (OM) decaheme cytochromes OmcA and MtrC permits coupling between NADH and reduction of Fe3+. Rates of metal reduction are rate limiting (2 sec-1), as evidenced by the virtually identical rate constants obtained from direct measurements of OmcA or MtrC oxidation relative to that associated with the NADH-dependent reduction of Fe(III)-NTA.
Reference
Shi L, B Chen, Z Wang, DA Elias, MU Mayer, YA Gorby, S Ni, BH Lower, DW Kennedy, DS Wunschel, HM Mottaz, MJ Marshall, EA Hill, AS Beliaev, JM Zachara, JK Fredrickson, and TC Squier. 2006. "Isolation of high-affinity functional protein complex between OmcA and MtrC: two outer membrane decaheme c-type cytochromes of Shewanella oneidensis MR-1." Journal of Bacteriology 188(13):4705-4714.

