CBER Presentation
How fast can a new vaccine for an emerging respiratory virus be developed and available for use?
Jesse L. Goodman, MD, MPH
Director, Center for Biologics Evaluation and Research (CBER), FDA
CDC ICEID, March 22, 2006
Overview
- Big picture - how much risk vs. how much benefit?
- Progress --- but flies in ointment
- Special regulatory tools
- So how quickly can we respond now?
- Two scenarios providing examples of current range
- Evolving technologies and approaches
- A "roll out" concept for phased development, regulatory status and use?
- Not considered but important needs (e.g. immunization process itself and non-medical interventions)
Big picture - how much risk vs. how much benefit?
- All medicine, public health and regulation is (or should be) risk/benefit based
- Challenge with developing and testing new vaccines for EIDs frequently is uncertainty of benefit (e.g. what is risk of disease) -evolves continuously and then changes dramatically when/if outbreak "arrives"
- All products themselves also have uncertain risks
- While vaccines are generally very safe, unexpected events occur, even following appropriate development, clinical studies and review (e.g. GBS with Swine flu, IS with RotaShield)
- Even uncommon AEs can have large impacts in setting of broad immunization of healthy population (1/100,000 = 3500 deaths) of course far less than a potential pandemic
- Uncertainty about risk can be reduced by:
- Appropriate initial studies and continuing data acquisition and analysis during use
- Use of technologic approaches with historic experience
- Quality and experience in manufacturing and product testing
Progress- yes- but flies in ointment
- Major determinants of innovation,production speed and capacity are:
- Economics of industry
- Vaccine industry and capacity stabilized-improving
- First blockbuster vaccines, investments in CT and influenza are helping but maintenance will be critical
- Limitations: EID market inherently uncertain - government dependent if actions to be taken ahead of time, when needed
- Limits of technology and manufacturing
- Science has provided many new tools
- Rapid detection and cloning of new antigens (e.g. PCR for SARS, RG for flu), new adjuvants, production methods, delivery systems, platform technologies
- Limitations: knowledge gaps and inexperience - many approaches not predictable for given application. Lack of redundancy and resilience in manufacturing base.
How quickly can we respond now?
- Not as fast as we like or may need to!
- Components of response:
- Isolation of agent
- Preparation of seed strain/antigen
- Pilot manufacturing (bulk, purification, formulation)
- Proof of concept
- Clinical immunogenicity/efficacy and safety data as needed
- Scaled manufacturing, bulk, purification
- Fill and finish, product testing
- Delivery and administration
- Production, testing and quality does take time

Tools to Speed Product Availability and Facilitate Evaluation/Approval
- Early and frequent consultation between sponsor, end user (if different) and FDA.
- Fast track
- Priority review
- Accelerated approval - surrogate
- Approval under "Animal Rule"
- Availability for emergency use under IND or Emergency Use Authorization (EUA)
"Animal Rule"
- Products to reduce or prevent serious conditions caused by exposure to lethal or permanently disabling toxic chemical, biological, radiological, or nuclear substances
- Human efficacy studies not feasible or ethical
- Use of animal data scientifically appropriate
- Not applicable if approval can be based on standards described elsewhere in FDA regulations
Animal Rule (cont.)
- Still need human clinical data
- PK/immunogenicity
- Safety
- Approval subject to post-marketing studies and any needed restrictions on use
- Potential limitations
- No valid or comparable animal model of disease
- How to predictably bridge animal data to humans
- Confidence an issue, even with valid models
Product Availability under IND
- Facilitated implementation to use products under IND in an emergency (e.g., smallpox or anthrax release)
- "Streamlined" IND - flexible requirements
- Informed consent required per regulations
- Frequently significant uncertainty re: risks/benefits
- Potentially cumbersome for widespread use
Emergency Use Authorization (EUA):
- Sec. of HHS can declare emergency after Sec. of Defense, Homeland Security, or HHS determines an emergency (or potential for one) exists, affecting national security
- Sec. of HHS (FDA) can authorize use of product:
- For serious or life-threatening condition
- No adequate, approved, available alternative
- Known & potential benefits outweigh known & potential risks
- EUA granted for up to 1 yr: can be renewed
EUA: Conditions of Authorization
- Inform health care workers or recipients, if feasible
- Product authorized for emergency use
- Significant known & potential risks and benefits
- Alternatives
- Option to accept or refuse (vs. written consent)
- Appropriate conditions for monitoring and reporting AEs, record keeping
- Authority for additional conditions, e.g., who may distribute or administer, data collection & analysis
Groundwork is Needed for Broad Emergency Use Under IND or EUA
- Product may be used very widely in multiple populations
- Therefore, should have reasonable evidence of safety and support for efficacy or likely surrogate such as immunogenicity
- Primary time challenge in development is proof of principle and making product consistently - not clinical studies or review
- If product can be made, core data can be generated rapidly - example GSK Fluarix: 1 month/900 patients
- This should be done before emergency (or pre-pandemic/epidemic) wherever possible
- Managed, prioritized, funded processes needed to identify and develop candidates, assure data will be available to support use in an emergency
Risk/Benefit for Emergency Use Products
- FDA assesses risk/benefit for each product/use and the situation on the ground at that time
- Treatment:for otherwise untreatable, serious illness, reasonable to tolerate significant risk
- Prevention:if given to well individuals, balance shifts, especially if pre-exposure (or pre-outbreak)
- Lack of efficacy can be a safety issue
- Something is not always better than nothing
- Ineffective therapy can inhibit development of effective therapies
- All such products need objective and effective risk communication
How Quickly: continued
- Time and data needs in each stage are dependent on disease and vaccine specific factors
- Experience with similar/related pathogens re: biology and protective response correlates
- Experience/capacity with needed technology, related vaccine(s)
- Resultant clinical data needs: immunogenicity/effectiveness and safety
- Disease/host specific challenges, unknowns/concerns
- Capacity also depends on # of doses, amount of antigen needed
- Two illustrative possible scenarios
- Fast: High experience, likely correlate, similar vaccines made, substantial capacity, no special concerns
- Moderate - uncertain: Biology and/or correlate not understood and/or special concerns
- And then there are "black holes" e.g. retrovirus, prion disease
"Fast": New Influenza Strain
- Positives:
- High familiarity, annual experience
- Many licensed processes/facilities
- Ability to rapidly obtain antigen and develop seed strain
- Good safety record, limited need for clinical data for existing processes/vaccines
- Likely immune surrogate and bridge to effective licensed vaccines
- Negatives:
- Rapid antigenic changes and egg based technologies difficult to scale up
- Limited industrial capacity
- Manufacturing risks
- Testing for contamination important
Rapid Flu Vaccine Production
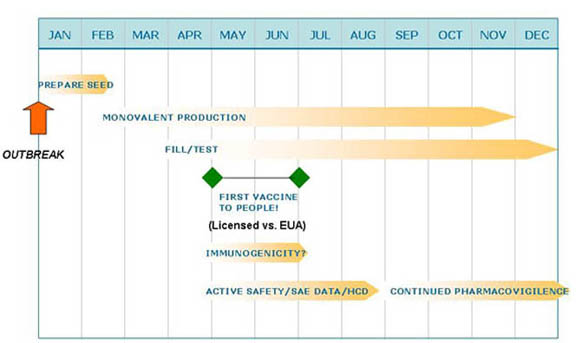
Rapid Flu Vaccine Production Moderate - ?: SARS CoV
- Positives:
- Animals and people make protective neutralizing antibodies
- Small animal and primate models developed
- Negatives:
- No familiarity, experience, licensed processes/facilities or products
- Possible safety concern- Ab dependent enhancement
- Killed vaccines not maximally effective
- Major proteins have complex glycosylation
- Need for immunogenicity and safety data - lack of correlate of protection
- No clinical link to effectiveness, animal disease models imperfect
Hypothetical Crash Program for Inactivated or rSARS/CoV Vaccine
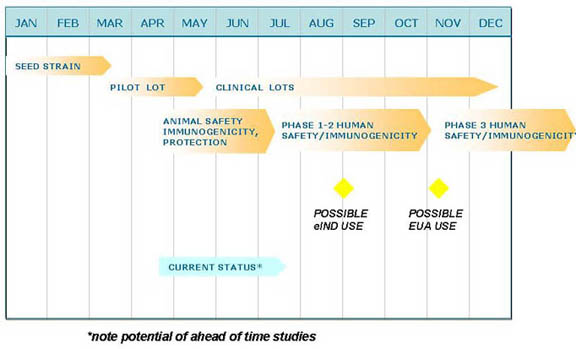
Vaccine technologies to accelerate production or improve immunogenicity
- Reverse vaccinology (sequence based) - prior to culture
- Immunogen identification technology- xreactive epitopes
- Reassortants, reverse genetics*
- Cell culture* - scalability, potential use of contract facility
- Live atten* - rapid, broad antigenicity, Ab+CTL but safety
- Viral/bacterial vectored: "
- DNA (poor human responses) & "prime/boost": Ab + CTL
- Recombinant protein(s)*: mono-multi-antigen
- insect/animal cells for glycosylation
- plant, edible: dosing, environmental issues
- Synthetic or natural peptide*: "
- Virosome/pseudovirus/liposome - Ab + CTL
Platform Technologies
- Use of standard platform as cassette/carrier for immunogen
- Examples: viral vector, virosome
- Potential benefits: with adequate experience, likely to gain predictability in safety, immunogenicity
- Problem: not there yet
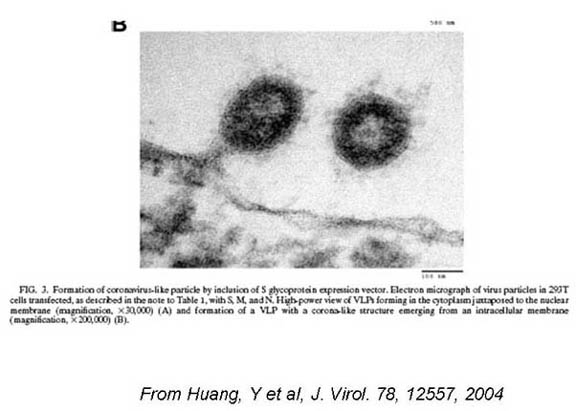
SARs: Virus Like Particles
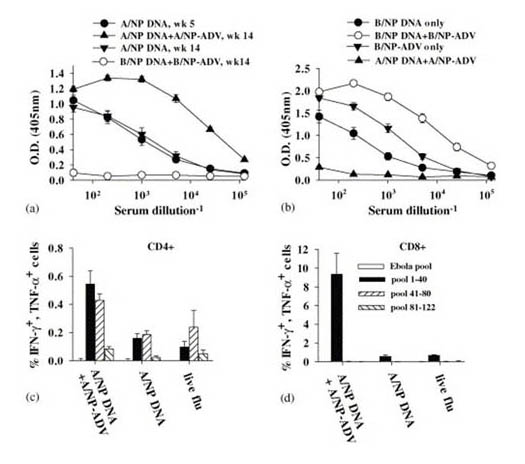
PRIME BOOST: From Epstein, SM et al, Protection against multiple influenza A subtypes by vaccination with highly conserved nucleoprotein, Vaccine 23, 5404, 2005
Adjuvants
- Highly variable in actions and effectiveness
- Increased potency often correlates with reactogenicity
- Mineral salts (e.g. alum) - most widely used, predominantly stimulate Ab response
- Emulsions/oils (e.g. MF59) may be stronger adjuvants and stimulate more cross-reactive Abs and Th-1 CTL
- MF59 licensed flu vax in Europe - reactogenicity in children
- Microbial "derivatives" (e.g. lipid A, CpG, toxins) - most stimulate innate immunity through different TLRs
- Microparticles and virus like particles - traffic antigen to APC's, can also serve as platform vectors/adjuvants
- Cytokines
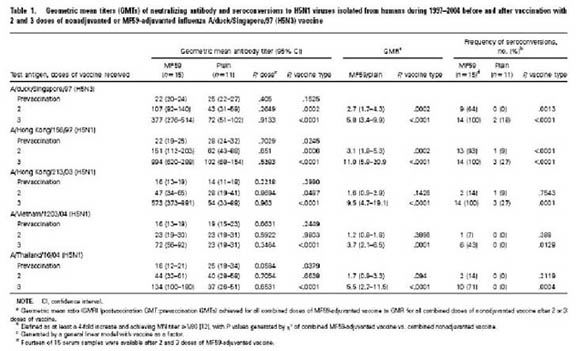
From I. Stephenson et al, Cross-Reactivity to Highly Pathogenic Avian Influenza H5N1 Viruses after Vaccination with Nonadjuvanted and MF59-Adjuvanted Influenza A/Duck/Singapore/97 (H5N3) Vaccine JID 191, 1210, 2005
Delivery Routes/Systems
- May enhance local humoral and cellular immunity, invoke APC and elicit CTL responses
- May allow more rapid practical delivery, delivery outside of health care settings, or self-immunization
- May conserve antigen
- Transcutaneous
- Mucosal
- Oral
Urgent Use? - Relevant Lessons of Swine Flu
- Communication re: benefit/risks critical
- Includes uncertainty of pandemic/epidemic - as vaccine benefit depends on it
- Likely better in non-crisis or routine situation - priming
- Ability and process to reevaluate changing situations
- Public's safety concerns and expectations are important and significant (and even more so today) and can affect, and even derail, vaccination plans
- Importance of safety monitoring in use
- Confidence in vaccines, governments and public health systems will be on the line
Relevant Lessons of CT Efforts
- Vaccine production complex, time consuming, not always predictable- vaccines are not widgets.
- Short-cuts seldom are.
- Most major delays have been in making a workable product, not clinical studies
- Less expensive seldom is.
- FDA and other global regulatory counterparts can play important and facilitating roles
- Help facilitate production, maximize the efficiency of investments
- Rapidly and objectively evaluate scientific findings re: safety, manufacturing and efficacy in face of urgency
A New Conceptual Framework: "Roll Out"?
- In true or evolving emergencies, even accelerated vaccine development and evaluation approaches likely to fall short
- Can we integrate and speed the process through a "roll out" approach integrating manufacturing and broadening clinical studies coordinated simultaneously with initial use?
- In any case, effective deployment, roll out, and data acquisition of safety and efficacy studies will be needed for new products early during emergency availability
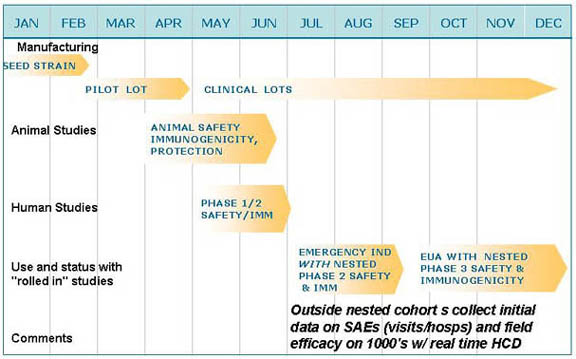
Hypothetical Emergency Roll Out Program for Novel Vaccine
Conclusions
- Much accomplished and ongoing to improve vaccine technology and nimble evaluation & regulatory pathways
- Many promising innovations are not yet "solutions"
- Even best case scenarios will require months from pathogen discovery to a vaccine
- Thus, while success is possible and closer than in past, we must also focus on:
- Enhanced surveillance and predictive sciences and ahead of time vaccine development against possible threats
- Better and predictive understanding of rapid platform vaccine technologies and manufacturing approaches
- Technologies to overcome antigenic variation, enhance stability
- Anti-infectives and nonspecific immune enhancement products
- Development and evaluation of early non-medical interventions (e.g. personal protection/masks, social measures etc.)
- Much can be evaluated during annual flu seasons, for example
Thanks! Your ideas welcome.....
CBER: INNOVATIVE TECHNOLOGY ADVANCING PUBLIC HEALTH


