 |
Q4B Evaluation and Recommendation of Pharmacopoeial Texts for Use in the ICH Regions
Annex 5: Disintegration Test General Chapter
(PDF version of this document)
| This draft guidance, when finalized, will represent the Food and Drug Administration's (FDA's) current thinking on this topic. It does not create or confer any rights for or on any person and does not operate to bind FDA or the public. You can use an alternative approach if the approach satisfies the requirements of the applicable statutes and regulations. If you want to discuss an alternative approach, contact the FDA staff responsible for implementing this guidance. If you cannot identify the appropriate FDA staff, call the appropriate number listed on the title page of this guidance. |
For questions regarding this draft document contact (CDER) Robert King 301-796-1242, or (CBER) Christopher Joneckis 301-827-0373.
Final signoff on Step 2 Annex 5 Disintegration Test June 5, 2008
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL
REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE
Draft Consensus Guideline
Q4B - Annex 5
Evaluation and Recommendation of Pharmacopoeial Texts
for Use in the ICH Regions
ON
Disintegration Test General Chapter
Current Step 2 Version
Dated June 5, 2008
At Step 2 of the ICH Process, a consensus draft text or guideline, agreed by the appropriate ICH Expert Working Group, is transmitted by the ICH Steering Committee to the regulatory authorities of the three ICH regions (the European Union, Japan and the USA) for internal and external consultation, according to national or regional procedures.
Q4B -- Annex 5
Evaluation and Recommendation of Pharmacopoeial Texts
for Use in the ICH Regions
ON
Disintegration Test General Chapter
ICH Consensus Guideline
Released for Consultation on June 5, 2008, at Step 2 of the ICH Process
1. Introduction
This annex is the result of the Q4B process for Disintegration Test General Chapter.
The proposed texts were submitted by the Pharmacopoeial Discussion Group (PDG).
2. Q4B Outcome
2.1 Analytical Procedures
The ICH Steering Committee, based on the evaluation by the Q4B Expert Working Group (EWG), recommends that for tablets and capsules, the official pharmacopoeial texts, Ph.Eur. 2.9.1. Disintegration of Tablets and Capsules, JP 6.09 Disintegration Test, and USP <701> Disintegration can be used as interchangeable in the ICH regions subject to the conditions detailed below. Testing conditions for specific dosage forms are outside the scope of the harmonization of this chapter.
2.1.1 The Disintegration Test is not considered to be interchangeable in the three regions for tablets and capsules larger than 18 millimeters (mm) long.
2.1.2 The Disintegration Test is not considered to be interchangeable in the three regions for enteric coated preparations.
2.1.3 Product-specific parameters such as media and the use of discs should be specified in the application dossier.
2.2 Acceptance Criteria
Acceptance criteria are outside the scope of the harmonization of this chapter and should be specified in the application dossier.
3. Timing of Annex Implementation
When this annex is implemented (incorporated into the regulatory process at ICH Step 5) in a region, it can be used in that region. Timing might differ for each region.
4. Considerations for Implementation
4.1 General consideration: When sponsors or manufacturers change their existing methods to the implemented Q4B-evaluated pharmacopoeial texts that are referenced in Section 2.1 of this annex, any change notification, variation, and/or prior approval procedures should be handled in accordance with established regional regulatory mechanisms pertaining to compendial changes.
4.2 FDA consideration: Based on the recommendation above, and with reference to the conditions set forth in this annex, the pharmacopoeial texts referenced in Section 2.1 of this annex can be considered interchangeable. However, FDA might request that a company demonstrate that the chosen method is acceptable and suitable for a specific material or product, irrespective of the origin of the method.
4.3 EU consideration: For the European Union, the monographs of the Ph. Eur. have mandatory applicability. Regulatory authorities can accept the reference in a marketing authorisation application, renewal or variation application citing the use of the corresponding text from another pharmacopoeia as referenced in Section 2.1, in accordance with the conditions set out in this annex, as fulfilling the requirements for compliance with the Ph. Eur. Chapter 2.9.1., on the basis of the declaration of interchangeability made above.
4.4 MHLW consideration: The pharmacopoeial texts referenced in Section 2 of this annex can be used as interchangeable in accordance with the conditions set out in this annex. Details of implementation requirements will be provided in the notification by MHLW when this annex is implemented.
5. References Used for the Q4B Evaluation
5.1 The PDG Stage 5B sign-off document (Rev. 1): Japanese Pharmacopoeial Forum, Volume 16, number 4 (December 2007)
5.2 The pharmacopoeial references for Disintegration Test General Chapter for this annex are:
5.2.1 European Pharmacopoeia (Ph. Eur.): 6.3 Edition (official January 2009) Disintegration of Tablets and Capsules (reference 01/2009: 20901) [Advance copy]
5.2.2 Japanese Pharmacopoeia (JP): 6.09 Disintegration Test as it will appear in Supplement II to the JP Fifteenth Edition. The draft English version of the JP text provided by MHLW is appended.
5.2.3 United States Pharmacopeia (USP): Revision Bulletin <701> Disintegration issued June 6, 2008, and official August 1, 2008 [Advance copy]
Draft Supplement II to the JP15
6.09 Disintegration Test
This test is harmonized with the European Pharmacopoeia and the U. S. Pharmacopeia. The parts of the text that are not harmonized are marked with symbols (♦ ♦).
Disintegration Test is provided to determine whether tablets, capsules, ♦granules or pills♦ disintegrate within the prescribed time when placed in a liquid medium at the experimental conditions presented below.
For the purposes of this test, disintegration does not imply complete solution of the unit or even of its active constituent.
Apparatus
The apparatus consists of a basket-rack assembly, a 1000-mL, low-form beaker, 138 to 160 mm in height and having an inside diameter of 97 to 115 mm for the immersion fluid, a thermostatic arrangement for heating the fluid between 35 º and 39 º, and a device for raising and lowering the basket in the immersion fluid at a constant frequency rate between 29 and 32 cycles per minute through a distance of not less than 53 mm and not more than 57 mm. The volume of the fluid in the vessel is such that at the highest point of the upward stroke the wire mesh remains at least 15 mm below the surface of the fluid and descends to not less than 25 mm from the bottom of the vessel on the downward stroke. At no time should the top of the basket-rack assembly become submerged. The time required for the upward stroke is equal to the time required for the downward stroke, and the change in stroke direction is a smooth transition, rather than an abrupt reversal of motion. The basket-rack assembly moves vertically along its axis. There is no appreciable horizontal motion or movement of the axis from the vertical. º and 39 º, and a device for raising and lowering the basket in the immersion fluid at a constant frequency rate between 29 and 32 cycles per minute through a distance of not less than 53 mm and not more than 57 mm. The volume of the fluid in the vessel is such that at the highest point of the upward stroke the wire mesh remains at least 15 mm below the surface of the fluid and descends to not less than 25 mm from the bottom of the vessel on the downward stroke. At no time should the top of the basket-rack assembly become submerged. The time required for the upward stroke is equal to the time required for the downward stroke, and the change in stroke direction is a smooth transition, rather than an abrupt reversal of motion. The basket-rack assembly moves vertically along its axis. There is no appreciable horizontal motion or movement of the axis from the vertical.
Basket-rack assembly - The basket-rack assembly consists of six open-ended transparent tubes, each 77.5 ± 2.5 mm long and having an inside diameter of 20.7 to 23 mm and a wall 1.0 to 2.8 mm thick; the tubes are held in a vertical
 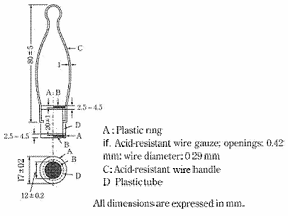
Fig. 6.09-1 Disintegration apparatus ♦Fig. 6.09-2 Auxilary tube♦
position by two plates, each 88 to 92 mm in diameter and 5 to 8.5 mm in thickness, with six holes, each 22 to 26 mm in diameter, equidistant from the center of the plate and equally spaced from one another. Attached to the under surface of the lower plate is a woven stainless steel wire cloth, which has a plain square weave with 1.8- to 2.2-mm apertures and with a wire diameter of 0.57 to 0.66 mm. The parts of the apparatus are assembled and rigidly held by means of three bolts passing through the two plates. A suitable means is provided to suspend the basket-rack assembly from the raising and lowering device using a point on its axis. The basket-rack assembly conforms to the dimensions found in Fig. 6.09-1. The design of the basket-rack assembly may be varied somewhat pro-vided the specifications for the glass tubes and the screen mesh size are maintained: ♦for example, in order to secure the glass tubes and the upper and the lower plastic plates in position at the top or the bottom, an acid-resistant metal plate, 88 - 92 mm in diameter and 0.5 - 1 mm in thickness, having 6 perforations, each about 22 to 26 mm in diameter, may be used which coincide with those of the upper plastic plate and upper open ends of the glass tubes♦.
Disks -- The use of disks is permitted only where specified or allowed. Each tube is provided with a cylindrical disk 9.5 ± 0.15 mm thick and 20.7 ± 0.15 mm in diameter. The disk is made of a suitable, transparent plastic material having a specific gravity of between 1.18 and 1.20. Five parallel 2 ± 0.1 mm holes extend between the ends of the cylinder. One of the holes is centered on the cylindrical axis. The other holes are centered 6 ± 0.2 mm from the axis on imaginary lines perpendicular to the axis and parallel to each other. Four identical trapezoidal-shaped planes are cut into the wall of the cylinder, nearly perpendicular to the ends of the cylinder. The trapezoidal shape is symmetrical; its parallel sides coincide with the ends of the cylinder and are parallel to an imaginary line connecting the centers of two adjacent holes 6 mm from the cylindrical axis. The parallel side of the trapezoid on the bottom of the cylinder has a length of 1.6 ± 0.1 mm, and its bottom edges lie at a depth of 1.5 ~ 1.8 mm from the cylinder's circumference. The parallel side of the trapezoid on the top of the cylinder has a length of 9.4 ± 0.2 mm, and its center lies at a depth of 2.6 ± 0.1 mm from the cylinder's circumference. All surfaces of the disk are smooth. If the use of disks is specified, add a disk to each tube, and operate the apparatus as directed under Procedure. The disks conform to dimensions found in Fig. 6.09-1. The use of automatic detection employing modified disks is permitted where the use of disks is specified or allowed. Such disks must comply with the requirements for density and dimension given in this chapter.
♦Auxiliary tube -- The auxiliary tube, as illustrated in Fig. 6.09-2, consists of a plastic tube D, 12 ± 0.2 mm in inside diameter, 17 ± 0.2 mm in outside diameter, 20 ± 1 mm in length, having both outside ends screw-cut, and two plastic rings A, each 12 ± 0.2 mm in inside diameter, 17 ± 0.2 mm in outside diameter, 2.5 - 4.5 mm in length, having one inside end screw-cut. Acid-resistant woven wire gauze having 0.42-mm openings and 0.29-mm wire diameter is placed in each plastic ring and the rings are attached by screws to each end of the plastic tube. The distance between two wire gauzes is 20 ± 1 mm. A handle of an acid-resistant wire, 1 mm in diameter and 80 ± 5 mm in length, is attached to the mid portion of the plastic tube. The auxiliary tube is used for the test of granules and capsules containing enteric coated granules.♦
Procedure
1) Immediate-release preparation
In case of tablets, capsules ♦and pills (except for pills containing crude drugs)♦  place 1 dosage unit in each of the six tubes of the basket, and if prescribed add a disk. ♦Unless otherwise specified, operate the apparatus, using water as the immersion fluid,♦ maintained at 37 ± 2º C as the immersion fluid. ♦Unless otherwise specified, carry out the test for 20 minutes for capsules, 30 minutes for plain tablets, and 60 minutes for coated tablets and pills.♦ Lift the basket from the fluid, and observe the dosage units. ♦Complete disintegration is defined as that state in which any residue of the unit, except fragments of insoluble coating or capsule shell, remaining on the screen of the test apparatus or adhering to the lower surface of the disks, if used, is a soft mass having no palpably firm core. ♦ The test is met if all of the dosage units have disintegrated completely. If 1 or 2 dosage units fail to disintegrate, repeat the test on 12 additional dosage units. The test is met if not less than 16 of the total of 18 dosage units tested are disintegrated. place 1 dosage unit in each of the six tubes of the basket, and if prescribed add a disk. ♦Unless otherwise specified, operate the apparatus, using water as the immersion fluid,♦ maintained at 37 ± 2º C as the immersion fluid. ♦Unless otherwise specified, carry out the test for 20 minutes for capsules, 30 minutes for plain tablets, and 60 minutes for coated tablets and pills.♦ Lift the basket from the fluid, and observe the dosage units. ♦Complete disintegration is defined as that state in which any residue of the unit, except fragments of insoluble coating or capsule shell, remaining on the screen of the test apparatus or adhering to the lower surface of the disks, if used, is a soft mass having no palpably firm core. ♦ The test is met if all of the dosage units have disintegrated completely. If 1 or 2 dosage units fail to disintegrate, repeat the test on 12 additional dosage units. The test is met if not less than 16 of the total of 18 dosage units tested are disintegrated.
♦For pills containing crude drugs, carry out the test for 60 minutes in the same manner, using 1st fluid for disintegration test as the immersion fluid. When any residue of the unit is observed, carry out the test successively for 60 minutes, using 2nd fluid for disintegration test.♦
♦In case of granules, shake granules on a No. 30 (500 pm) sieve as directed in (1) Granules under Particle Size Distribution Test for Preparations <6.03>, transfer 0.10g of the residue on the sieve to each of the 6 auxiliary tubes, secure the 6 tubes to the bottom of the basket tightly, and operate the apparatus, using water as the immersion fluid, maintained at 37 ±2º C as the immersion fluid, unless otherwise specified. Observe the samples after 30 minutes of operation for plain granules and after 60 minutes for coated granules, unless otherwise specified. Complete disintegration is defined as that state in which any residue of the granules, except fragments of insoluble coating in the auxiliary tube, is a soft mass having no palpably firm core. The test is met if all of 6 samples in the auxiliary tubes have disintegrated completely. If 1 or 2 samples fail to disintegrate, repeat the test on 12 additional samples. The test is met if not less than 16 of the total of 18 samples tested are disintegrated.♦ C as the immersion fluid, unless otherwise specified. Observe the samples after 30 minutes of operation for plain granules and after 60 minutes for coated granules, unless otherwise specified. Complete disintegration is defined as that state in which any residue of the granules, except fragments of insoluble coating in the auxiliary tube, is a soft mass having no palpably firm core. The test is met if all of 6 samples in the auxiliary tubes have disintegrated completely. If 1 or 2 samples fail to disintegrate, repeat the test on 12 additional samples. The test is met if not less than 16 of the total of 18 samples tested are disintegrated.♦
♦2) Enteric coated preparations
Unless otherwise specified, perform the following two tests, (a) the test with 1st fluid for disintegration test and (b) the test with the 2nd fluid for disintegration test, separately.
(1) Enteric coated tablet and capsule
(a) The test with 1st fluid for disintegration test
Carry out the test for 120 minutes, using 1st fluid for disintegration test according to the procedure described in immediate release preparations. Disintegration is defined as that state in which the tablet or capsule is broken or the enteric coating film is ruptured or broken. The test is met if none of six dosage units is disintegrated. If 1 or 2 dosage units are disintegrated, repeat the test on additional 12 dosage units. The test is met if not less than 16 of the total of 18 dosage units tested are not disintegrated.
(b) The test with 2nd fluid for disintegration test
According to the procedure described in immediate-release preparations, carry out the test with new dosage units for 60 minutes, using 2nd fluid for disintegration test and determine if the test is met or not.
(2) Enteric coated granules and capsules containing the enteric coated granules
Shake granules or contents taken out from capsules on a No. 30 (500 pm) sieve as directed in (1) Granules under Particle Size Distribution Test for Preparations <6.03>, transfer 0.10 g of the residue on the sieve to each of the 6 auxiliary tubes, secure the 6 tubes to the bottom of the basket tightly, and operate the apparatus, using the 1st and 2nd fluids for disintegration test.
(a) The test with 1st fluid for disintegration test
According to the procedure described in immediate-release preparations, carry out the test for 60 minutes, using 1st fluid for disintegration test. The test is met if particles fallen from the openings of the wire gauze number not more than 15.
(b) The test with 2nd fluid for disintegration test
According to the procedure described in immediate-release preparations, carry out the test with new samples for 30 minutes, using 2nd fluid for disintegration test and determine if test is met or not.♦
 Back
to Top Back
to Top  Back to Guidance Page
Back to Guidance Page
 PDF requires the free
Adobe Acrobat Reader PDF requires the free
Adobe Acrobat Reader
Date created: August 12, 2008
|
 |