You are here:
Research
- Lab Overview
- Laboratory Chief Office
- Muscle Assembly Group
- Muscle Physiology Section
- Muscle Proteomics and Nanotechnology Section

Muscle Proteomics and Nanotechnology Section
Kuan Wang, Ph.D.
Section Head, Muscle Proteomics and Nanotechnology Section
Phone: (301) 496-4097
Fax: (301) 402-8566
E-mail: wangk@mail.nih.gov
Research Overview
Under Dr. Wang's direction, scientists in the Muscle Proteomics and Nanotechnology Section of the Laboratory of Muscle Biology (LMB), engage in multidisciplinary research in five major areas of muscle biology. These are 1) giant proteins, slender filaments, and elastic cytoskeleton; 2) proteomics in muscle and motile systems; 3) nanotechnology in muscle and motile systems; and 4) stress-bearing structures in muscle and motile systems; 5) MRI imaging of muscle tissues. Project 1 represents long-standing interests at the laboratory in the pursuit of the cellular and physiological functions of titin, nebulin, nebulette and other giant proteins in muscle and non-muscle cells. Project 2 deals with the development of a systematic chemical cross-linking approach to map protein contacts between interfacing proteins in complex cellular structures. This represents an effort to leapfrog in the structural genomics field and make extensive use of our expertise in protein chemistry and the new instrumentation resources at the LMB. Project 3 seeks to apply nanotechnology to life sciences. This new bioengineering initiative enables our researchers to study the actions and interactions of single molecules, single filaments, and single organelles in real time. Project 4 aims to discover the design principles of the stress bearing and transmitting structure, with a view towards tissue engineering and the pathogenesis of muscle diseases. Project 5 aims to determine muscle structure at the fiber level in intact tissue and the effects of disease states on the organization of the fibers. Animal models of nemaline myopathy and muscle knots are under investigation.
Project 1: Giant proteins, slender filaments, and elastic cytoskeleton
The resting muscle, in the absence of any stimulation, is remarkably elastic when stretched and released. When muscle is activated, it develops contractile force, shortens and then lengthens again to its original dimension when stimulation stops. It is well known that muscle develops active force by the cycling of a molecular motor, myosin, to actin filaments in the contractile machinery (sarcomere). Muscle shortens when actin filaments are pulled to slide past myosin-thick filaments. Little is known, however, of how contracted muscle restores its length and how resting muscle responds to stretch and compression. It is also unclear how muscle cells manage to control the uniform and precise length of thick and thin filaments when sarcomeres are assembled in developing muscle tissues.
Recent studies of muscle cytoskeletal lattices begin to shed light on both questions. The cytoplasm of striated muscle cells contains, besides actin and myosin filaments, at least two interconnected lattices. An intermediate filament lattice envelops and links all sarcomeres to the membrane skeleton (costamere), mitochondria, nuclei, and sarcoplasmic reticulum. Inside the sarcomere, a cytoskeletal matrix consisting of a set of elastic titin filaments and a set of inextensible nebulin filaments provides structural continuity. Both lattices generate restoring force.
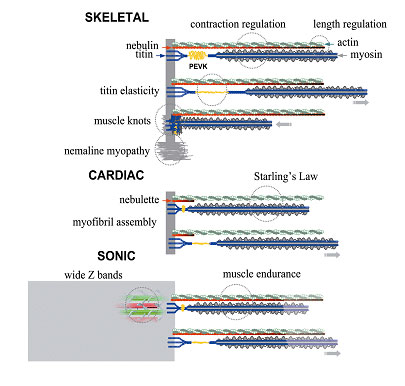
Both titin and nebulin are unprecedented giant proteins, discovered and named in this Laboratory. Recent research in the Muscle Proteomics and Nanotechnology Section has focused on the roles of titin and nebulin in the structure, regulation, mechanics, and assembly of sarcomeres in striated muscles. A multidisciplinary approach was adopted that draws on methodologies and concepts of biochemistry, biophysics, cell and molecular biology, and physiology.
Titin
A primary theme of our research is the understanding of the structural and physiological roles of titin, a family of giant structural proteins that constitute an elastic matrix in the striated muscle sarcomeres and some nonmuscle cells. Titin and the elastic matrix may play major physiological roles, including the genesis of long-range elasticity, the maintenance of sarcomere stability, the assembly of nascent sarcomeres in developing muscle cells, and the assembly of mysoin in the microfilaments in nonmuscle cells.
Nebulin and Nebulette
Nebulin is thought to act as a protein ruler that regulates the length of actin filaments in skeletal sarcomeres. Our recent studies indicate that nebulin tethers myosin heads to actin in a calcium/calmodium-sensitive manner; raising the intriguing possibility that nebulin may act as a calcium-linked regulatory protein that is distinct from the classic troponin/tropomyosin system. Nebulette is a small homolog of nebulin in heart cells, and plays an important role in the assembly of myofibrils.
Project 2: Proteomics in muscle and motile systems
A second area of study incorporates recent advances in protein separation, mass spectrometry and bioinformatics that enabled the simultaneous analysis of a large number of proteins in cells and tissues. The interaction of these proteins, especially those that respond to specific challenges, are rapidly identified by ingenious screening techniques such as phage expression or yeast-two hybrid systems. These studies, collectively termed "proteomics," have greatly enriched our knowledge of cellular physiology and pathology in normal and diseased tissues.
A systematic identification of molecular interfaces between interacting proteins in a natural cellular environment represents the next major challenge toward a molecular understanding of the complex and dynamic cellular events. Such a profile of molecular interfaces would also be invaluable as a tool in drug development by targeting crucial molecular interfaces.
This Section initiated such a proteomics project focusing on the application of a chemical cross-linking approach toward a systematic and global identification of protein-protein interfaces in complex systems such as the contractile machinery of muscle cells, the cytoskeletal filaments and organelles of non-muscle cells.
Project 3: Nanotechnology in muscle and motile systems
A third area of research is nanotechnology in muscle and motility systems. Spectacular advances in the ability to manipulate, fabricate and alter tiny subjects at the nanometer scale (nanotechnology) have revolutionized material science in the past decade. The recent application of nanotechnology to life sciences has shown exciting promises in a wide range of disciplines. The development of laser-based technology such as the confocal microscope, laser tweezers, laser scissors, the multiphoton excitation confocal microscope, and the near field microscope opens many new possibilities. These allow functional imaging of living cells in thick tissues; manipulation of single molecules, single organelles and single cells; determination of the binding force and rate of interaction of DNA and other single molecules; surgery of chromosomes and organelles in a living cell, and the fabrication of miniature medical devices. The development of scanning probe microscopy (atomic force, scanning, tunneling, electrochemical probe, near field microscopy) has enabled the manipulation of single molecules, the preparation of novel biochips and biosensors, and the measurement of physical and spectroscopic properties of single molecules in living cells.
Scientists in the Section endeavor to establish state-of-the-art nanotechnology facilities (atomic force microscope, cellular force microscope, single fiber force station, real time confocal microscope, single molecule fluorescence microscope, and digital image analysis) to apply, adapt and develop bio-nanotechnology. These techniques are applied to study single motors such as myosin and kinesin as well elastic proteins such a titin and nebulin, muscle filaments, cytoskeletal filaments, and receptors in cellular membranes and cellular organelles such as myofibril, ribosome, and chromatin. Specifically, their goals are to measure the mechanical property and spectroscopy of single molecules, organelles and single cells; to measure the strength, speed, and movement of interacting single molecules/organelles/cells; to manipulate and alter intracellular organelles in living cells; and to fabricate and design new instrumentation in nanotechnology.
These direct measurements and manipulations of single molecules, filaments, and organelles will provide unique and important insights of the events in the assembly and function of contractile machinery in muscle and nonmuscle cells. These studies will also reveal important engineering principles for designing tissues with prescribed mechanical properties. Successful application of atomic force microscopy was completed that images and measures the elastic properties of single monomeric protein, oligomeric protein, and genetically engineered titin and nebulin molecules. The single molecule fluorescence microscope also was applied to study the morphology and dynamic interactions of titin, nebulin and myosin motors at the single molecule level.
Project 4: Stress-bearing structures in muscle and motile systems
A fourth area of concentration is in stress-bearing structures in muscle and motile systems. The cytoplasm of striated muscle cells contains, besides actin and myosin filaments, at least two interconnected lattices. An intermediate filament lattice envelops and links all sarcomeres to the membrane skeleton (costamere), mitochondria, nuclei, and sarcoplasmic reticulum. Inside the sarcomere, a cytoskeletal matrix consists of a set of elastic titin filaments and a set of inextensible nebulin filaments that provide structural continuity. Both lattices generate restoring force. Active force and elastic force are transmitted through specialized anchor structures of the sarcomere.
One important stress-bearing structure is the Z-line, a dense and narrow structure that anchors and organizes four major filaments: actin, titin, nebulin, and desmin filaments. The Z-lines therefore play a fundamental role in both the structural organization of sarcomere and the transmission of mechanical forces of the sarcomere, as well as the intermediate filament lattice. Its dense structure however poses technical challenges and the variability of protein composition makes it difficult to generalize findings from one muscle to the next.
Projects in this area of research address the Z-line structure and function from several perspectives. 1. What are the roles of titin, nebulin (skeletal muscles), nebulette (a nebulin-like protein in the heart) in the assembly and integrity of the Z-line in vertebrate muscle? 2. What are the composition and structure of the unusually broad Z-line of sonic muscle of Midshipman fish? What is its relationship to the anomalous nemaline rod Z-bodies found in aging heart muscle and in diseased skeletal muscle known as nemaline myopathy? These studies are important in understanding how contractile machinery assembles during development, how it disassembles during remodeling of muscle tissues, how tension is transmitted during muscle activities, and how muscles malfunction in disease.
Project 5: Magnetic Resonance Imaging of muscle tissues
Our magnetic resonance imaging (MRI) projects are an integral part of the laboratory's aim of applying imaging techniques to the study of live or intact skeletal muscle tissues in health and disease. MRI is especially attractive because it is non-invasive and can provide a wealth of information beyond simple images: fiber orientation can be tracked in tissues, neuronal paths can be highlighted and the viscoelastic properties of tissue may be determined. These additional types of information make MR especially useful in studying the properties of muscle. The NIH has world-class MR imaging instrumentation and expertise in the MRI research Facility (NMRF) and Mouse Imaging Facility (MIF).
Our current projects are focused on understanding the role of fiber orientation in an unusually high endurance muscle and the structure and properties of muscle at and near the vicinity of muscle knots. MR is the only technique that can provide three-dimensional structural information in intact tissue at a resolution of 100 μm or less. These studies will lead to a better understanding of how muscle fibers behave in functioning muscle and how their derangement lead to muscle knots.
Selected Publications
Forbes JG, Jin AJ, Wang K. Forced Unfolding and Extension of Calmodulin. Probe Microscopy. In Press.
Ma K, Kan L, Wang K. Polyproline II helix is a key structural motif of the elastic PEVK segment of titin. Biochemistry. 2001; 40(12): 3427-38
![]()
Gutierrez-Cruz G, Van Heerden AH, Wang K. Modular motif, structural folds and affinity profiles of the PEVK segment of human fetal skeletal muscle titin. J Biol Chem. 2001; 276(10):7442-9.
![]()
Forbes JG, Jin AJ, Wang K. Atomic Force Microscope Study of the Effect of the Immobilization Substrate on the Structure of a Multimeric Protein. Langmuir. 2001; 17:3067-3075.
Adhikari BB, Wang K. S100A1 modulates skeletal muscle contraction by desensitizing calcium activation of isometric tension, stiffness and ATPase. FEBS Lett. 2001; 497(2-3):95-8.
![]()
See complete list of publications
Updated October 10, 2007



