

The Center for Devices and Radiological Health (CDRH) is accepting and encouraging sponsors of premarket medical device submissions to include a copy of their submission in the prescribed electronic form along with the required paper copies.
The answers to the following questions explain how to create a complete electronic copy. Please read all of the information carefully before submitting an electronic copy. To quickly navigate to a particular question and answer, click on the specific link below.
A: An electronic copy is an exact duplicate of a paper submission, created and submitted on a CD or DVD, accompanied by a copy of the signed cover letter and the complete original paper submission. An electronic copy is not an electronic submission. Electronic copies of premarket submissions accompanying the paper submission should be submitted to the CDRH Document Mail Center.
For more information on electronic submissions, refer to:
A: Any submission type (510(k), IDE, PMA, 513(g), Pre-IDE, HDE, PDP, Emergency Use Authorization, and subsequent amendments or supplements ) can be submitted as an electronic copy. Just indicate in the cover letter that an electronic copy of the submission is being provided and that it is an exact duplicate of the paper copy.
A: The electronic copy should be sent in an Acrobat Portable Document Format (PDF) because Center staff will use Adobe’s Acrobat 7.0 to view the submission. This will assure that what a reviewer sees on the screen is the same as what has been submitted on paper. It is also important to follow the correct naming conventions for files and folders when submitting an electronic copy. For documents that are scanned, Optical Character Recognition (OCR) should be performed prior to submission.
![]() Note: At this time, we cannot support the new features in Adobe Acrobat 8.0.
Note: At this time, we cannot support the new features in Adobe Acrobat 8.0.
Additional Files (non-PDF)
In addition to the PDF files, the submission may contain other data files typically provided electronically (e.g., clinical data) in support of the review. The following files types will be accepted: SAS, XPORT, XML, SGML, and Molfile. Microsoft Word (or comparable products) is not an accepted format for electronic copies.
 Note: If you want to submit a file type that is not listed above, please send your request to: cdrhesub@cdrh.fda.gov and we will contact you to discuss the alternatives.
Note: If you want to submit a file type that is not listed above, please send your request to: cdrhesub@cdrh.fda.gov and we will contact you to discuss the alternatives.
A: Electronic copies should be sent on CD-ROM or DVD. We encourage applicants to select media that allows the submission to be contained on one CD-ROM or DVD, whenever possible.
A: All CDRH submissions for ODE and OIVD, whether or not they contain an electronic copy, are sent to the same location:
FDA Center for Devices and Radiological Health
Document Mail Center, HFZ-401
9200 Corporate Boulevard
Rockville, MD 20850
A: At present, documents that require signatures cannot be submitted with an electronic signature. This includes, but is not limited to: IDE; PMA; 510(k); Master Access File (MAF) cross-reference letters; financial disclosure documents; 510(k) truth and accuracy statements; 510(k) statements; 510(k) class III certifications; declarations of conformity to consensus standards; and third party documents.
To ensure the electronic copy is complete, it is essential that you:
A checklist has been provided at the end of this document after the questions and answers. Before submitting your electronic copy, please ensure you are submitting a formatted electronic copy.
![]() Important Note: A scanned PDF is not a substitute for the original paper copy. The submission will not be complete without the paper copy of the cover letter with an original handwritten signature. The electronic copy (CD/DVD) and the accompanying cover letter should be identified as the electronic copy. (See Figure 1 in the question below.)
Important Note: A scanned PDF is not a substitute for the original paper copy. The submission will not be complete without the paper copy of the cover letter with an original handwritten signature. The electronic copy (CD/DVD) and the accompanying cover letter should be identified as the electronic copy. (See Figure 1 in the question below.)
A: Currently, an electronic copy that follows this document will serve as one of the required number of copies for the various premarket submission types. For the submission to be complete, the electronic copy should be considered one unit of the regular paper submission requirements. Figure 1 depicts each unit that is required for the submission.
Figure 1: Submission contents and its discrete units

As per this document, copies of a submission are required as indicated below. In the case of an electronic copy (which includes the paper original), the electronic media counts as:
A: The structure of an electronic copy is highly dependent on the overall file size of the submission and can be organized using files and folders in one of the following ways:
![]() Note: File size should not exceed 50MB.
Note: File size should not exceed 50MB.
Figures are presented below to illustrate how to name and structure a submission. Figure 2 provides a sample PMA submission that contains volumes. Figure 3 provides an example of a PMA submission that contains multiple volumes as PDF files. Figure 4 provides an example of a submission that contains multiple PDF files, as in the case of a 510(k) submission. Figure 5 provides an example of an IDE with volumes. Figure 6 provides an example of a 510(k) structured in one PDF document with bookmarks.
Figure 2: Example of a PMA submission with Volumes
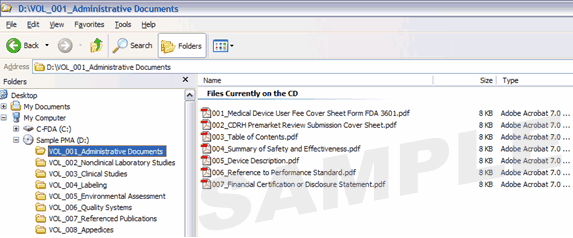
Figure 3: Example of a PMA with PDF Volumes
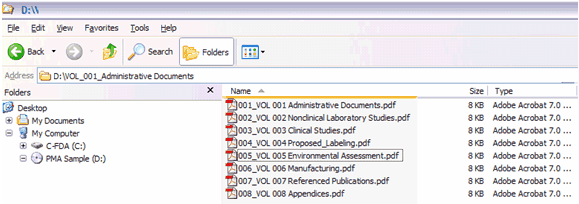
Figure 4: 510(k) submission without Volumes
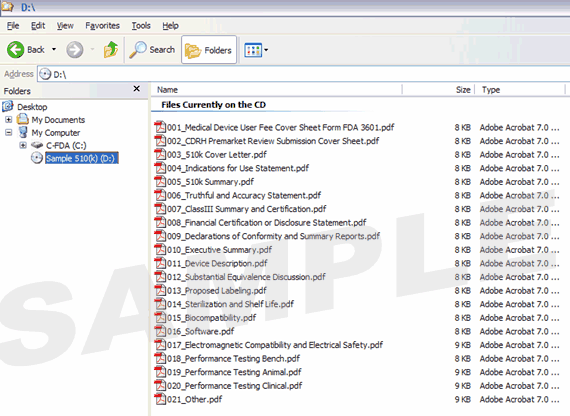
Figure 5: Investigational Device Exemption (IDE) with Volumes
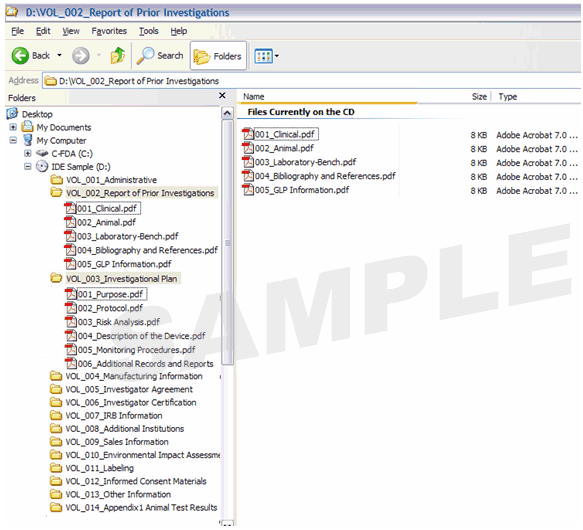
Figure 6: Sample of a 510(k) structured in one PDF file with Bookmarks
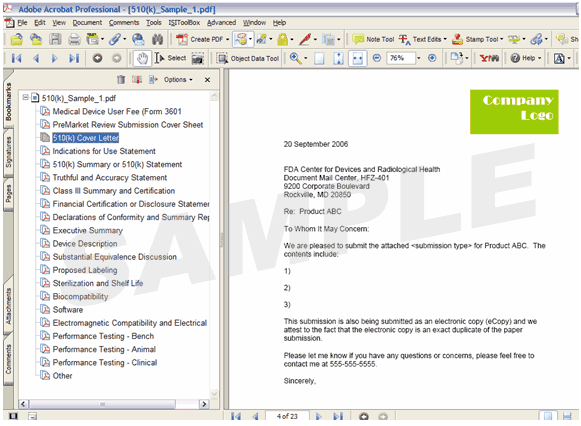
![]() Note: In Figure 6 above, the example illustrates how to consolidate the multiple sections of a submission into one file with the use of bookmarks. This is in contrast to the multi-file submission displayed in Figure 4.
Note: In Figure 6 above, the example illustrates how to consolidate the multiple sections of a submission into one file with the use of bookmarks. This is in contrast to the multi-file submission displayed in Figure 4.
 If you have questions about any one of the structures above, please contact CDRH at cdrhesub@cdrh.fda.gov to ensure that the format is acceptable prior to developing your submission.
If you have questions about any one of the structures above, please contact CDRH at cdrhesub@cdrh.fda.gov to ensure that the format is acceptable prior to developing your submission.
A: There are several ways to format an electronic copy. However, at a minimum, it is required that the following instructions are used to create a compliant electronic copy of the submission, to include: naming of folders and files; bookmarks and hypertext links; and Optical Character Recognition (OCR). The following instructions are provided with regard to (1) naming of folders and files; (2) bookmarks and hypertext links; (3) optical character recognition; and (4) file size.
1. Naming of folders and files
Submissions may be sent in any of the following structures:
The following section outlines the required naming convention for all folders and files:
![]() Note: Folders can only be named: Vol_001 thru Vol_999 or Vol_001 Description thru Vol_999 Description. It is important to follow the folder naming convention because it is used to upload the submission into CDRH’s document repository. If this naming convention is not used, the submission will fail the loading process. Volumes should be used when the submission has a large quantity of files.
Note: Folders can only be named: Vol_001 thru Vol_999 or Vol_001 Description thru Vol_999 Description. It is important to follow the folder naming convention because it is used to upload the submission into CDRH’s document repository. If this naming convention is not used, the submission will fail the loading process. Volumes should be used when the submission has a large quantity of files.
An example of a file name is as follows:
For files that are organized as volumes, the file name is as follows:
![]() Note: Files size should determine when to create more than one file in a submission. If the file size is greater than 50MB, consider splitting the contents into multiple files and use the next number in the sequence. It is important to follow the file naming convention because it is used to order the submission in the correct sequence in CDRH’s document repository. Therefore, a file name should not be repeated or deviate from the sequential order by using decimals. When possible, the file name should be descriptive of its content and meaningful to the reviewers.
Note: Files size should determine when to create more than one file in a submission. If the file size is greater than 50MB, consider splitting the contents into multiple files and use the next number in the sequence. It is important to follow the file naming convention because it is used to order the submission in the correct sequence in CDRH’s document repository. Therefore, a file name should not be repeated or deviate from the sequential order by using decimals. When possible, the file name should be descriptive of its content and meaningful to the reviewers.
 If you have questions about complex submissions, please contact cdrhesub@cdrh.fda.gov prior to submission to ensure the electronic copy structure is acceptable.
If you have questions about complex submissions, please contact cdrhesub@cdrh.fda.gov prior to submission to ensure the electronic copy structure is acceptable.
Bookmarks and hyperlinks should be used to assist the reviewers in navigating through the content of the submission. If you used either bookmarks or hypertext links, consider the following:
![]() Important Note: Hyperlinks between individual PDF document files are not currently supported and any absolute links that reference across files will not work.
Important Note: Hyperlinks between individual PDF document files are not currently supported and any absolute links that reference across files will not work.
In general, for documents with a table of contents, provide bookmarks and hypertext links for each item listed in the table of contents including all tables, figures, publications, other references, and appendices. These bookmarks and hypertext links are essential for the efficient navigation through documents.
3. Optical Character Recognition
PDF documents produced by scanning paper documents are usually inferior to those produced from an electronic source document such as MS Word. Scanned documents are more difficult to read and do not allow the reviewers to search or copy and paste text for editing. The use of scanned documents should be avoided if at all possible. If scanning cannot be avoided, the following is highly recommended:
Quick Tip : Once a document has been scanned and completed optical character recognition, you may take the following steps to ensure that the text is searchable:
Figure 7: Selecting text in a PDF Document
If the source document is only available on paper, it should be scanned at resolutions that will ensure the pages are legible both on the computer screen and when printed. At the same time, remember to limit the file size to be less than 50MB. We recommend scanning at a resolution of 300 dots per inch (dpi) to balance legibility and file size. We discourage the use of grayscale or color because of file size. After scanning, avoid re-sampling to a lower resolution.
For files with images and photographs:
Also, when creating PDF files containing images, you should not resample images. Re-sampling does not preserve all of the pixels in the original. For photographs, the image should be obtained with a resolution of 600 dpi. If black and white photos are submitted, consider 8-bit gray scale images. If color photos are submitted, consider 24-bit RGB Color Model images. A captured image should not be subjected to non-uniform scaling (i.e., sizing).
Files with scanned images and photographs tend to be large in file size. Please do not exceed 50MB for a single file. Consider multiple files for these types of documents.
Note: Scanned tables and graphs cannot be extracted easily if scanned. Most OCR programs will distort the data in tables and graphs. Convert MS Word documents to PDF, as this method usually retains the formatting.
For a paper document with handwritten notes:
Paper documents containing handwritten notes should be scanned at 300 dpi. These handwritten notes should be made in black ink for clarity.
 If you have questions about creating electronic copies, please contact CDRH at cdrhesub@cdrh.fda.gov to ensure that the format is acceptable prior to developing your submission.
If you have questions about creating electronic copies, please contact CDRH at cdrhesub@cdrh.fda.gov to ensure that the format is acceptable prior to developing your submission.
An electronic copy CD/DVD can have one or multiple files. There is not a limitation of the total size of the submission, but each file should be limited to 50MB in file size. There are several ways to compress file size, including but not limited to: performing Optical Character Recognition, reducing file size in Adobe and creating logical section breaks.
 If you have questions about file size, please contact CDRH at cdrhesub@cdrh.fda.gov to ensure the file size is acceptable.
If you have questions about file size, please contact CDRH at cdrhesub@cdrh.fda.gov to ensure the file size is acceptable.
A: Choose a method for creating PDF documents that produces the best replication of a paper document. You can ensure that the paper copy is an exact duplicate of the electronic copy by using a content rendering software to print the Word document to a PDF version of the file. Once you have the final PDF version, simply print the PDF version for submission as the original paper copy.
The PDF files must be compatible with Adobe Acrobat 5.0, 6.0 and 7.0 without the use of additional plug-ins other than those provide by Adobe as part of Acrobat. There are features in Adobe Acrobat 8.0 that cannot be supported. If you would like to submit Adobe Acrobat 8.0 files, please contact CDRH at cdrhesub@cdrh.fda.gov to ensure you do not use a feature that is unsupported.1. Converting a MS Word Document to PDF using Adobe Acrobat 6.0 Professional
After you have completed your Word document (.doc format), you can easily create a portable document format (.pdf) file using the same process you use to print documents. In Microsoft Word, instead of printing your document to a printer, you can select the Adobe PDF printer option from the drop down print menu, and create a new file. (See sample in Figure 8.)
Note: All figures in this section are using Adobe Acrobat 6.0. If you are using another version of Adobe Acrobat, the figures may be slightly different.
Figure 8: Selecting the Adobe PDF option under the Print Function
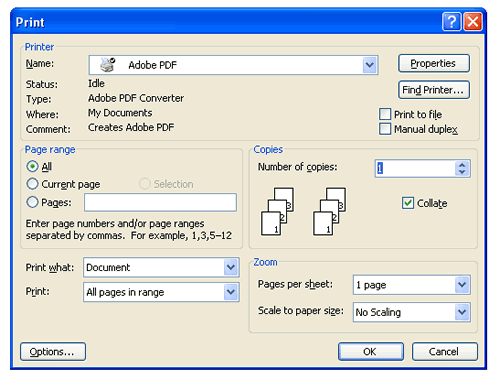
After you select Adobe PDF and press OK, you are prompted to save your document as a .pdf file and to select a location to save it. (See Figure 9.) Remember to use the correct naming convention. The PDF document will be stored in the location in which it was saved.
Figure 9: Saving your Word Document to a specified location as a PDF file

2. Fonts
PDF viewing software automatically substitutes a font to display text if the font used to create the text is unavailable on the reviewer’s computer. Font substitution can affect a document’s appearance and structure, and in some cases it can affect the information conveyed by a document. We cannot guarantee the availability of any one font. Therefore, you should embed all fonts you are using in the PDF files to ensure that those fonts will always be available to the reviewer. When embedding fonts, all characters for the font should be embedded (not just a subset of the fonts being used in the document). One problem associated with embedding fonts is that embedding requires additional computer storage space. Three techniques to help limit the storage space taken by embedding fonts:
Resizing a document because the contents are too small to read is inefficient. We believe that Times New Roman, 12-point font, is adequate in size for reading narrative text. This is the preferred font. Although sometimes tempting for use in tables and charts, fonts smaller than 12 points should be avoided whenever possible. We recommend the use of a black font color. Blue font may be used for hypertext links. If a font color other than black is used, avoid light colors that do not print well on grayscale printers. It is advised that you test the color reproduction prior to submission by printing sample pages from the document using a grayscale printer. In addition to font colors, keep formatting simple in tables. When extracting a table from the PDF document, the use of light or white font color will not allow the transfer of text back into a Word document.
3. Page Orientation
Pages should be properly oriented. For example, you should set the page orientation of landscape pages to landscape prior to saving the PDF document in final form to ensure correct page presentation. Landscape pages (including tables) should be oriented such that the header of the document aligns with the left edge of the page and the footer of the document aligns with the right edge of the page.
4. Page Size and Margins
The print area for pages should fit on a sheet of paper that is 8.5 inches by 11 inches. You should allow a margin of at least 1 inch on all sides to avoid obscuring information if the pages are subsequently printed and bound.
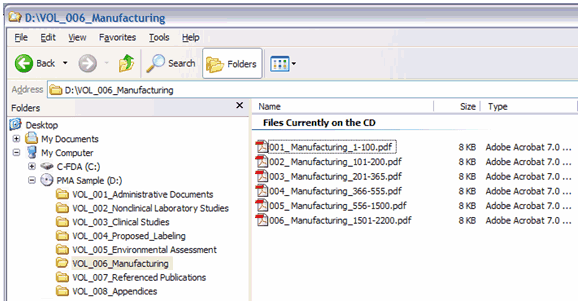
If a submission includes files divided into more than one PDF document, such files should have contiguous pagination. To simplify navigation, please make page numbers for the paper document and the PDF file the same. In order to ensure that the reviewer can locate information by using the page numbers as a reference (see Figure 10), consider naming the files something that reflects the pages involved (e.g., 001_Manufacturing_1-100.pdf should precede a file named 002_Manufacturing_101-200.pdf).
This will also ensure that the pagination is consistent throughout the submission. For submissions organized in volumes, it is suggested that each volume start its pagination with page 1.
Figure 10: Example of a PMA submission with Volumes and using page numbers in the file names
7. Document Properties - Description Tab
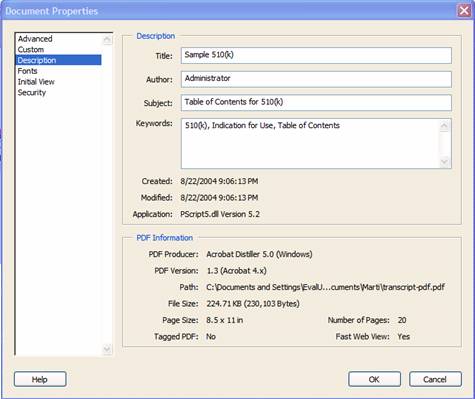
Document properties are used to search for individual documents and to identify the document when found. To modify document properties, from the tool bar navigate to File | Document Properties. Figure 11 shows the Document Properties Description tab, the keywords can be added to the Keyword field.
Figure 11: Sample of Document Properties
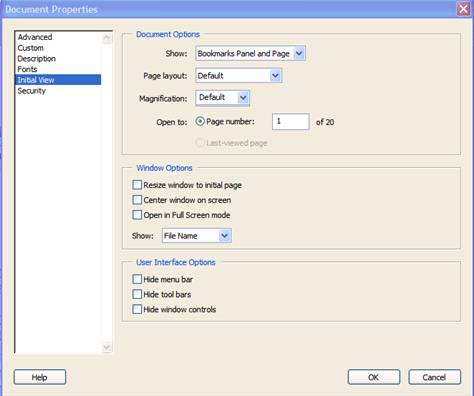
8. Document Properties – Initial View Tab
In the Document Properties box, the Initial View tab can also be found. (See Figure 12.) In the Document Options section, all PDF files should set the Show field as Bookmarks Panel and Page. If there are no bookmarks, set the initial view as Page only. Set the Page Layout and Magnification fields to default.
Figure 12: Set Document Properties to always open with Bookmarks and page view
9. Document Properties – Security
PDF files are stored as original documents and will not be altered from their original form. If any security settings, read-only, or password protection is used on the files, the FDA may need to :
10. Indexing PDF Documents
Full text indexes are used to help find specific documents and/or to search for text within documents. When a document or group of documents is indexed, all words and numbers in the file and all information stored in the Document Information fields are stored in special index files that are functionally accessible using the search tools available in Acrobat. Portions of a document that are imaged are not indexed. Even if the document only contains images, the text in the Document Information fields of the file will be indexed. All PDF files should be full-text searchable prior to submitting to the FDA.
11. Plug Ins
It is acceptable to use plug-ins to assist in the creation of a submission. However, the review of the submission should not require the use of any plug-ins other than any provided by Adobe as part of Acrobat, and must be compatible with Adobe Acrobat 5 or greater.
A: Submitting an electronic copy of your application makes that copy available to review staff more quickly, enables additional editing, saves resources as well as provides navigational and searching tools. However, it should be understood that submission of an electronic copy does not provide preferential treatment or change the order in which applications are reviewed.
A: An EUA request may be submitted with an electronic copy. To obtain further information regarding EUA requests, we encourage you to refer to the Guidance entitled “Emergency Use Authorization of Medical Products,” which may be found on the FDA website at http://www.fda.gov/oc/guidance/emergencyuse.html and http://www.fda.gov/oc/bioterrorism/emergency_use.html
A: Many of the electronic copies submissions received to date have not followed all of the instructions presented on this webpage.
| Common mistakes | Suggested solution |
|---|---|
| Cover letter does not state that the electronic copy is an exact duplicate of the original paper submission. | Put the following statement in your cover letter, “Per the instructions accessed at http://www.fda.gov/cdrh/elecsub.html, an electronic copy is being provided with this submission and it is an exact duplicate of the original paper submission.” |
Files and folders do not follow the numbering convention. For example:
|
Files should be named with the following convention: XXX_ plus name extension. The XXX represents a numeric sequence (i.e., 001, 002, 003, etc) for which the files should be ordered in the table of contents. For example:
|
Inappropriate use of folders. For example:
|
Folders should only be used to manage the content of a volume. Folders should be placed at the root (in the example below that is “IDE Sample”) of the CD/DVD and only be named as follows: Vol_001 thru Vol_999 or Vol_001 Description thru Vol_999 Description. For example:
If only one file exists for a volume, it is suggested that you do not use volumes to organize your submission. |
| Subfolders within the Volume folders. | Subfolders are not to be placed within a volume for any reason. If a section needs to be organized further, consider creating a new volume for that section. |
| File sizes greater than 50MB (i.e., 250MB) | The submission content should be organized in volumes or by logical headings to reduce the overall size of each file. Always check file size before submitting your electronic copy. |
 Please contact the CDRH Office of Information Technology at cdrhesub@cdrh.fda.gov regarding any technical details of the construction of the PDF file.
Please contact the CDRH Office of Information Technology at cdrhesub@cdrh.fda.gov regarding any technical details of the construction of the PDF file.
Any questions regarding FDA regulations or guidance should be directed to the appropriate division point of contact listed below.
Any questions regarding FDA regulations or guidance should be directed to the appropriate division point of contact listed below.
ODE Divisions and Contacts
ODE Program Operations Staff
(240) 276-4040
Division of General, Restorative, and Neurological Devices
Assistant Director of Program Operations (ADPO): (240) 276-3737
Division of Cardiovascular Devices
Project Manager : (240) 276-4080
Division of Ophthalmic and ENT Devices
ADPO: (240) 276-4200
Division of Reproductive, Abdominal, and Radiological Devices
Project Manager: (240) 276-3636
OIVD Contacts
Sousan Altaie: (240) 276-0378
Patricia Beverly: (240) 276-0379
Updated October 22, 2007
![]()
CDRH Home Page | CDRH A-Z Index | Contact CDRH | Accessibility | Disclaimer
FDA Home Page | Search FDA Site | FDA A-Z Index | Contact FDA | HHS Home Page
Center for Devices and Radiological Health / CDRH