![]()
Susan Thom, of Parma, Ohio, knows how important it is for people to know the number of calories from fat they eat each day.
As a registered dietitian, she counsels patients on the need to limit fat consumption to 30 percent or less of total daily calories. As a person with diabetes, and thus at increased risk for heart disease, she strives to do the same for herself.
But, in the past, obtaining that information from the food label has required some mathematical skill--namely, multiplying the total grams (g) of fat in a serving by 9, since 1 g of fat contains 9 calories.
"It does take time," Thom said. "But if you want to feed yourself well, you have to look at the label."
Help is on the way. For Thom and millions of other Americans who seek to restrict their fat intake to recommended levels, a new dietary component is being added to the food label--"calories from fat."
It's just one of many new items of diet-related information manufacturers are required to offer on their food products by 1994. There also will be information on saturated fat, cholesterol, dietary fiber, and other nutrients that relate to today's health concerns, such as heart disease, cancer, and other diseases linked, at least in part, to diet.
There will be more complete nutrient content information because almost all the required nutrients will have to be listed as a percent of the Daily Value. There will be more uniform serving sizes, too, which will make nutritional comparisons between foods easier. And, because nutrition labeling is now mandatory for almost all processed foods, there will be a lot more products with this important information.
"The new information is going to be very helpful for consumers," said Virginia Wilkening, a registered dietitian in FDA's Office of Food Labeling.
"Some of the nutrients--saturated fat and cholesterol--have been allowed on the label before but on a voluntary basis," she said. "Dietary fiber and sugars were not allowed in the nutrition label. With the new label, consumers will soon have information about these and other nutrients, which can help them choose their foods more wisely."
The new requirements for nutrition labeling are spelled out in regulations issued in January 1993 by FDA and the U.S. Department of Agriculture's Food Safety and Inspection Service (FSIS). FDA's regulations meet the provisions of the Nutrition Labeling and Education Act of 1990 (NLEA), which, among other things, requires FDA to make nutrition labeling mandatory for almost all processed foods. FSIS' regulations, which cover meat and poultry products, largely parallel FDA's. (Meat and poultry products were not covered by NLEA.)
FDA has set May 8, 1994, as the date by which food manufacturers must comply with the new nutrition labeling regulations. FSIS requires meat and poultry processors to relabel their products by July 6, 1994. However, some newly labeled products may begin appearing in grocery stores much sooner than the deadlines.
Dietary Components
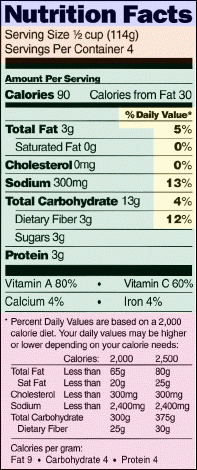 What can consumers expect? First, they will see a new name for the nutrition panel. It used to go by "Nutrition Information Per Serving." Now, it will be called "Nutrition Facts." That title will signal to consumers that the product is newly labeled according to FDA and FSIS' new regulations.
What can consumers expect? First, they will see a new name for the nutrition panel. It used to go by "Nutrition Information Per Serving." Now, it will be called "Nutrition Facts." That title will signal to consumers that the product is newly labeled according to FDA and FSIS' new regulations.
The new panel will be built around a new set of dietary components. The mandatory (boldfaced) and voluntary dietary components and order in which they must appear are:
These mandatory and voluntary components are the only ones allowed on the nutrition panel. The listing of single amino acids, maltodextrin, calories from polyunsaturated fat, and calories from carbohydrate, for example, may not appear on the label.
The reason, according to Wilkening, is to help consumers focus on nutrients of public health significance. "Too much additional information could clutter the label or mislead or confuse the consumer," she said.
Nutrients required on the label, she pointed out, reflect current public health concerns and coincide with current public health recommendations. She noted that the order in which the food components and nutrients are required to appear reflects their public health significance and the order in which they were specified in NLEA.
On the new food label, the listing of thiamin, riboflavin and niacin will not be mandatory. Under the old nutrition labeling program, these vitamins were required to be listed. But because deficiencies of these are no longer a public health problem in this country, listing them is now optional.
New Format
Consumers also will see a new format, one that calls for many of the macronutrients (such as fat, cholesterol, sodium, carbohydrate, and protein) to be declared as a percent of the Daily Value--a new label reference value. The amount, in grams or milligrams per serving, of these nutrients still must be listed to their immediate right. But, for the first time, a column headed "%Daily Value" will appear.
According to Wilkening, the percent declaration of the Daily Value offers an advantage over amount declaration: The percent Daily Values put the nutrients on an equal footing in the context of a total daily diet.
For example, she said, a food is low in sodium if it has less than 140 mg of sodium. "But people look at that number, 140, and think it's a tremendous amount, when it actually is less than 6 percent of the Daily Value."
On the other hand, she said, a food with 5 g of saturated fat could be construed as being low in that nutrient just because 5 is a small number. Actually, that food would provide one-fourth the total Daily Value of 20 g of saturated fat for a 2,000-calorie diet.
"People are affected by the size of numbers," she said. "That's why percentages are helpful. They put all of the nutrients on a level playing field."
The percent Daily Value listing will carry a footnote stating that the percentages are based on a 2,000-calorie diet and that a person's individual dietary goal is based on his or her calorie needs. Some nutrition labels--at least those on larger packages--will list daily values for selected nutrients for a 2,000- and a 2,500-calorie diet and the number of calories per gram of fat, carbohydrate and protein. The calorie conversion information is required as a general guide about the caloric contributions of fat, carbohydrate and protein.
The content of micronutrients--that is, vitamins and minerals--will continue to be expressed as a percent, although the term "Daily Value" will replace "U.S. Recommended Daily Allowance."
Modifications
Some foods will carry a variation of this format. For example, the label of foods for children under 2 (except infant formula, which is exempt from nutrition labeling under NLEA) will not carry information about calories from fat, calories from saturated fat, saturated fat, polyunsaturated fat, monounsaturated fat, and cholesterol.
The reason, according to Wilkening, is to prevent parents from inadvertently assuming that infants and toddlers should restrict their fat intake, when in fact, they should not. Fat is important during this life stage, she said, to ensure adequate growth and development.
The labels of food for children under 4 cannot include percentages of Daily Values for macronutrients, except protein, nor any footnote information, including the lists of Daily Values for selected nutrients. The reason: Other than protein, FDA has not established Daily Values for macronutrients for this age group. The percent Daily Values for vitamins and minerals is allowed, however. The content of the other nutrients must be expressed as an amount by weight in a separate column to the right of the macronutrients.
Other foods may qualify for a simplified label format. This format is allowed when the food contains insignificant amounts of seven or more of the mandatory dietary components, including total calories. "Insignificant" means that a declaration of "zero" could be made in nutrition labeling or, for total carbohydrate, dietary fiber, and protein, a declaration of "less than 1 g."
For foods for children under 2, the simplified format may be used if the product contains insignificant amounts of six or more of the following: calories, total fat, sodium, total carbohydrate, dietary fiber, sugars, protein, vitamins A and C, calcium, and iron.
When the simplified format is used, information on total calories, total fat, total carbohydrate, protein, and sodium--even if they are present in insignificant amounts--must be listed. Calories from fat and other nutrients must be listed if they are present in more than insignificant amounts. Nutrients added to the food must be listed, too.
Serving Sizes
Whatever the format, the serving size remains the basis for reporting each nutrient's amount. However, unlike in the past, serving sizes now will be more uniform and closer to the amounts that many people actually eat. They also must be expressed in both common household and metric measures.
Metric Conversion Chart
Units as they will appear for serving sizes on label:
| Household Measure | Metric Measure |
|---|---|
| 1 tsp | 5 mL |
| 1 tbsp | 15 mL |
| 1 cup | 240 mL |
| 1 fl oz | 30 mL |
| 1 oz | 28 g |
| tsp = teaspoon tbsp = tablespoon fl oz = fluid ounce oz = ounce mL = milliliter g = gram |
|
The uniformity also is important, she said, for giving consistency to health claims and words describing nutrient content, such as "high fiber" and "reduced fat."
FDA and FSIS define serving size as the amount of food customarily eaten at one time. It is based on FDA- and USDA-established lists of "Reference Amounts Customarily Consumed Per Eating Occasion."
These reference amounts, which are part of the new regulations, are broken down into 139 FDA-regulated food product categories, including 11 groups of foods for children under 4, and 23 USDA meat and poultry product categories. They list the amounts of food customarily consumed per eating occasion for each food category, based primarily on national food consumption surveys. FDA's list also gives the suggested label statement for serving size declaration.
For example, the category "breads (excluding sweet quick type), rolls" has a reference amount of 50 g, and the appropriate label statement for sliced bread is "__ piece(s) __ (g)" or, for unsliced bread, "2 oz (56 g/__ inch slice)."
The serving size of products that come in discrete units, such as cookies, candy bars, and sliced products, is the number of whole units that most closely approximates the reference amount. For example, cookies have a reference amount of 30 g. The household measure closest to that amount is the number of cookies that comes closest to weighing 30 g. Thus, the serving size on the label of a cookie package in which each cookie weighs 13 g would read "2 cookies (26 g)."
If one unit weighs more than 50 percent but less than 200 percent of the reference amount, the serving size is one unit. For example, the reference amount for bread is 50 g; therefore, the label of a loaf of bread in which each slice weighs more than 25 g would state that a serving size is one slice.
For food products packaged and sold individually, if an individual package is less than 200 percent of the applicable reference amount, the item qualifies as one serving. Thus, a 360-milliliter (mL) (12 fluid-ounce) can of soda is one serving because the reference amount for carbonated beverages is 240 mL (8 fluid ounces).
However, if the product has a reference amount of 100 g or 100 mL or more and the package contains more than 150 percent but less than 200 percent of the reference amount, manufacturers have the option of deciding whether the product is one or two servings.
For example, the serving size reference amount for soup is 245 g. So a 15-ounce (420 g) can can be listed as either one or two servings.
Presentation
There also are rules governing how the nutrition information is displayed. Under existing FDA regulations, nutrition information must appear on the information panel to the immediate right of the principal panel. Thus, on boxed foods, for example, in which the principal panel is on the front of the box, the nutrition information appears on the right side of the box. Packages whose area to the immediate right is too small or not suited for such labeling may provide information on the next panel to the right.
FSIS allows nutrition information to be listed on the principal or information panels.
The new food labeling rules call for one additional variation: For packages that are 40 square inches or less, the nutrition information may be placed on any label panel.
The rules also address size and prominence of the typeface. For example, the heading "Nutrition Facts" must be set in the largest type on the nutrition panel and be highlighted in some manner, such as boldface, all capital letters, or another graphic to distinguish it from the other information. Such highlighting also is required for headings such as "Amount per serving" and "%Daily Value" and for the names of dietary components that are not subcomponents--that is, calories, total fat, cholesterol, sodium, total carbohydrate, and protein.
Exceptions and Exemptions
In some instances, special provisions exist for providing nutrition information. For example:
But, there will be plenty of other foods carrying the new nutrition information. Dietitian Susan Thom sees that as a plus.
"We'll all know exactly what we're putting in our mouths," she said. "So there'll be little room for excuses."
Paula Kurtzweil is a member of FDA's public affairs staff.