|
NCI is establishing a new Clinical Trials Reporting Program (NCI CTRP), based on a June 2005 recommendation submitted by the Clinical Trials Working Group (CTWG), which was approved by the National Cancer Advisory Board.
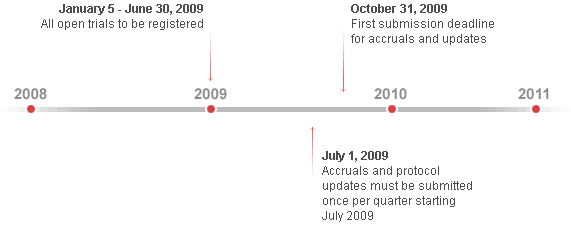
Even though NCI grantees are currently subject to trial reporting requirements, an upgraded set of mandatory reporting requirements will apply starting January 2009. In the long-term, the new reporting measures will help provide critical data to assist NCI in better coordinating research efforts to optimize our nation's investment in cancer research.
NCI clinical trials reporting will include up-to-date information about the status of all NCI-funded and/or sponsored clinical research, regardless of drug development phase, type of intervention or treatment, study design, or program through which funding is provided.
Live telephone support is available Monday to Friday, 8 a.m. to 8 p.m. Eastern Time, excluding federal holidays.
|