

 |
 |
FDA
Home Page | Search FDA Site |
FDA A-Z Index | Contact FDA
![]()
 |
DEPARTMENT OF HEALTH AND HUMAN SERVICE |
Food and Drug Administration |
Rockville, MD 20857 |
||
December 31, 2004
Dear Colleague:
I am pleased to share with you the Food and Drug Administration (FDA), Office of Management (OM) Annual Performance Report for FY 2004. As in previous years, this report highlights OM/Agency accomplishments during the fiscal year, and is intended to let our customers and partners know what we have done and are doing to support the Agency's public health mission.
Fiscal Year 2004 was a year in which we continued to focus much of our effort toward the implementation of the President’s Management Agenda (PMA). We are providing Agencywide leadership on all of the PMA-related initiatives, and many of the accomplishments listed in this report are directly related to those initiatives.
We facilitated the start-up of the Office of Shared Services (OSS), both at headquarters and throughout the field, as well as the OSS Employee Resource and Information Center (ERIC), providing administrative services in the areas of Information Technology, Equal Employment Opportunity & Diversity Management, Buildings & Facilities, Acquisitions & Grants, and Travel & Finance.
For the sixth year in a row, the Agency’s financial statements received an unqualified or “clean” opinion from independent auditors with no material weaknesses. We continued to work with the Department and other HHS components on the development and implementation of the Unified Financial Management System (UFMS), which will help us meet Federal financial management systems requirements.
We spearheaded successful efforts resulting in a record appropriation for FY 2004, giving the Agency added resources to support our responsibilities under the Food, Drug, and Cosmetic Act. We also provided leadership in both the development and implementation of the financial management aspects of the Animal Drug User Fee Act (ADUFA), that was signed by the President in November, 2003.
It has been a privilege to work with the many talented and dedicated individuals who have contributed to FDA's accomplishments, both within the Office of Management as well as the Centers and Offices. If you have any questions or comments about this report, you can contact me via e-mail at jeff.weber@fda.gov.
Sincerely,
/s/
Jeffrey M. Weber
Associate Commissioner
for Management and Systems
Chief Financial Officer
Major Accomplishments in FY 2004
Planned Initiatives for FY 2005
![]()
In last year’s Annual Performance Report, the Office of Management (OM) identified several priority initiatives for Fiscal Year 2004 and beyond, including:
As a result of agencywide cooperation and the efforts of the entire FDA management team, we were able to achieve the majority of our FY 2004 goals in the areas listed above as well as several others. We are pleased to share our major accomplishments with you in this report.
In FY 2005 and beyond, OM must address additional challenges, while continuing to provide high quality management services. We will be utilizing many of the cost-saving and cost-avoidance initiatives currently underway, and will initiate further program improvements including:
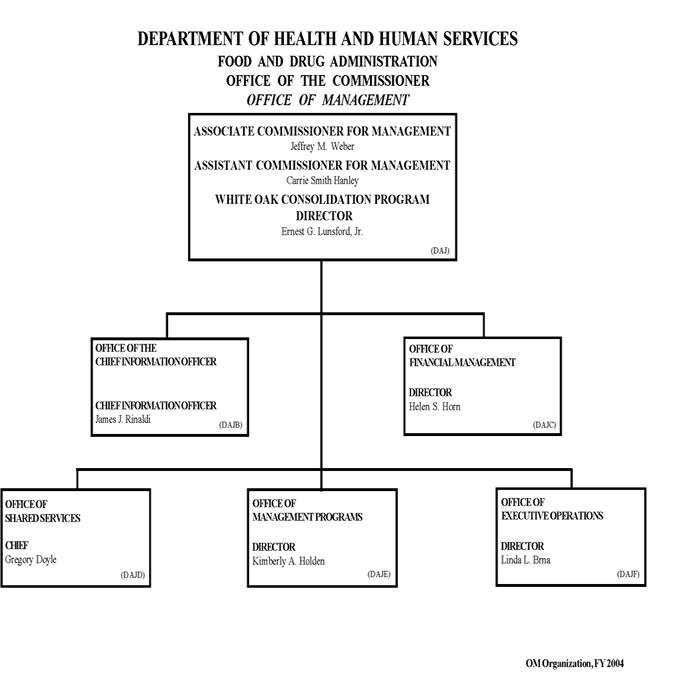
The Office of Management provides administrative services to the various programmatic components of the Food and Drug Administration and consists of the following components:
We are an organization of nearly 600 employees, including a professional staff of accountants, engineers, architects, administrators, and computer scientists who serve more than 10,200 agency employees nationwide. We are an integral part of the FDA management team and provide leadership, guidance, and solutions to a wide and varied number of management and resource issues facing FDA. We are customer-focused, supporting the management of the agency's resources in a collaborative, coordinated, and cost effective fashion.
Chief Information Officer: We provide agencywide leadership, guidance, and coordination for all information technology activities, including strategic planning, enterprise architecture, capital planning and investment control, project management, and security. Each of these activities is a key process enabling FDA to anticipate, and move seamlessly to, new technologies. We also provide a wide range of services to meet the day-to-day needs of FDA users. We build, buy, configure and maintain the systems that support mission area programs as well as critical administrative operations. We provide for those systems and individual users the underlying infrastructure, including telecommunications, network, server support, email, Internet and Intranet management, call center and asset management. We manage the IT Security program that facilitates the transmission of electronic data in a secure environment, protecting critical and vital information from attack and disruption. We also direct future IT investments and provide leadership for the FDA’s IT budget planning and execution in order to ensure the agency’s IT budget of nearly $200 million is used most effectively.
Executive Operations: We are a team of administrative and resource management specialists who ensure all Office of the Commissioner organizations effectively utilize the resources, information, and systems provided to them to accomplish their operational goals. As a staff of about 19 professionals, we provide advice and guidance on administrative and budget policies and procedures. We serve as the liaison between OC and functional offices in the Office of Shared Services, as well as the liaison with all of the administrative contacts in the Office of the Commissioner.
Financial Management: We provide resource management leadership and budgetary and financial management policy oversight to agency components, and serve as the focal point for FDA-wide financial management including budget planning, preparation, formulation, presentation, execution and control; user fees formulation, execution, and reporting; accounting and financial statement activities; and financial systems applications. We provide leadership during the development, monitoring, and tracking of an annual budget of $1.7 billion, which includes two direct appropriations and six separate and unique user fee activities. We manage FDA’s annual budget hearings with DHHS, OMB, and the House and Senate Appropriation Subcommittees. We advise, coordinate, and evaluate financial management responsibilities that were delegated to the Office of Shared Services to ensure that agency policy and procedures are being carried out appropriately; We manage and provide oversight for the annual financial statement audit, and we maintain oversight of FDA’s financial applications including FDA’s new financial system, a component of the DHHS Unified Financial Management System.
Management Programs: We serve as the focal point for many agency programs, which requires highly skilled experts dedicated to providing the best possible guidance, technical advice, and training that will enhance customers’ professional performance, while ensuring compliance with Federal laws and regulations. We administer FDA’s Delegations of Authority and organizational programs, serve as liaison for the Office of the Inspector General, conduct special studies and projects for the Office of the Commissioner, oversee FDA’s Internal Controls program, have responsibility for the FOI and Privacy Act programs, provide public access to FDA’s regulatory dockets, provide agency oversight for the Paperwork Reduction Act activities and records management, oversee the agency’s Competitive Sourcing and A-76 program, and manage the agency Ethics Program. We manage many of the agency’s human capital management and human resources (HR) development programs, such as Strategic Workforce Planning; Leadership Training and Development; Quality of Work Life; Reward & Recognition; and Performance Management, and serve as liaison to the Rockville HR Center. We also manage the agency Peer Review Program and Commissioned Corps Liaison activities
Shared Services: The Office of Shared Services (OSS) provides a ‘Portfolio of Services’ that is aligned with our customer’s needs. Services include communications, travel and finance, acquisitions and grants, buildings and facilities management, and equal employment opportunity and diversity management. The Employee Resource and Information Center (ERIC) serves as the first point-of-contact for these administrative and IT services to all FDA employees (customers). The Office of Acquisitions and Grants Services (OAGS) provides high quality support to FDA programs by managing contract and assistance agreements in a timely manner and at a reasonable cost. The Office of Equal Employment Opportunity & Diversity Management (OEEODM) leads the effort in developing policies and programs that ensure equal employment opportunities, promote inclusiveness, and foster a culture that values diversity and empowers individuals in the workforce. The Office of Field Finance and Acquisition Services (OFFAS) provides acquisition and financial services for the field activities within FDA. The Office of Financial Services (OFS) provides the day-to-day financial services related to accounts payable, travel support and payroll liaison, fleet and claims management. The Office of Real Property Services (ORPS) provides an efficient and effective program of nationwide support for FDA in the areas of real property management, engineering services, environmental, safety, and health and long range planning for the agency’s current and future facilities. This involves significant resources including annual lease costs of $135.5 million, related operational costs of $30 million, and owned facilities replacement value of $350 million. We manage 368 government owned or leased buildings and facilities nationwide, totaling 5.8 million square feet (leased and owned).
White Oak Consolidation Program: We integrate FDA’s technical, programmatic and facilities requirements into the overall budget and development plan for the agency’s consolidation at White Oak. This requires project development expertise, to direct the process by which Center requirements are incorporated into architectural design and to coordinate the overall design and construction process for the new campus with the General Services Administration. We evaluate and implement strategies that enable the agency to maximize efficiency through the consolidation of supportive and shared use functions. We monitor construction progress as individual projects proceed and coordinate necessary changes. We ensure meaningful and continuous communication with community leaders and associations, congressional representatives, other federal officials, State and local governments, and business leaders. In addition, we create and implement relocation plans needed to successfully transition the agency into its new location by coordinating various activities including the actual move, IT/telecom, security, safety and building operations.
> OM Accomplishments <
The immediate office/Office of Management accomplished significant OM and agencywide initiatives such as:
PDUFA:
Implemented the financial management aspects of the Prescription Drug User Fee Act (PDUFA) in FY 2004, including:
MDUFMA:
Implemented the financial management aspects of the Medical Device User Fee and Modernization Act (MDUFMA). During 2004 we:
ADUFA:
Provided leadership in both the development and implementation of the financial management aspects of the Animal Drug User Fee Act (ADUFA) that was signed by the President on November 18, 2003. Major accomplishments in FY 2004 include:
In FY 2004, FDA faced the considerable challenge of overhauling how it managed and applied IT. Up to then, IT budgets increased every year, competing more and more with mission needs. In FY 2004, the agency IT budget was reduced by $29 million. Yet, IT services also grew in importance, being institutionalized as necessary business tools. There also was momentum to move IT into a more strategic and service oriented arrangement, better aligned to business goals and objectives. Finally, HHS mandated consolidation beyond operating division boundaries. All of this created a challenge to streamline costs and reduce budgets without degrading service. However, the agency’s strategic goal of a Strong FDA (Ensure a world-class professional workforce, effective and efficient operations, and adequate resources to accomplish the mission of FDA), and the HHS goal of a “One HHS” provided a robust framework to achieve the IT accomplishments discussed below, all of which made significant contributions toward FDA’s long term goal to transform its IT organization into one of world-class stature.
Establish an integrated agency governance process. Governance, in the IT world, is the process for selecting and monitoring IT investments. This is best done by identifying stages in the system life cycle and using a set of artifacts produced at each stage to provide documented evidence of the continued feasibility and good health of the investment. In FY 2004 the OCIO:
Improve management and deployment of IT services and assets. Consolidation of IT resources provided FDA an opportunity to apply modern business techniques as a strategy for gaining efficiencies without affecting effectiveness. In FY 2004 the OCIO:
Further improve Project Management (PM) practices. In FY 2003, the OCIO commenced the Project Management initiative to improve and standardize how the agency managed projects, bringing it more in line with federal guidelines and requirements. In FY 2004, the OCIO continued to expand project management as a standard practice in the development and operation of IT systems and projects, accomplishing the following:
Develop standards to advance E-submission capability between FDA and Industry. By having the ability to receive all the pieces of an application in electronic form, through a single gateway, in a standardized way and format, the agency can then use its review resources more effectively, at the same time reducing time and cost. While efforts in the past have been made to advance this technology in piecemeal fashion, FY 2004 marked a turning point toward an agency solution, under OCIO coordination. Some of the FY 2004 accomplishments include:
Establish IT planning process to align IT resources and investments in support of the agency’s priority policy goals and objectives. Past efforts to implement new technologies were more effective from a technical rather than a support perspective. Technical issues were understood, while user needs were less clear, resulting in the timely roll out of technology, but with the performance gap never fully addressed. As a result, bridging that gap became a priority for the OCIO in FY 2004, leading to the following:
Research, evaluate and track emerging technologies. Introducing the appropriate new technology at the right time is more important than introducing new technologies simply because they are new. Effective planning can weigh need against risk and, coupled with an essential understanding of the capabilities of that technology, provide the best chance for success. In FY 2004 the OCIO:
Implement systems in support of FDA strategies and objectives. Building on improvements in the management and planning of IT, the OCIO began deploying systems as strategic tools in FY 2004, accomplishing the following:
In FY 2004, the OCIO strongly collaborated on HHS-wide projects in support of the HHS goal of “One HHS”, with collaboration ranging from being effective team members to taking on project leadership. Consolidation gains at the HHS level will provide increased efficiencies based on the buying power of a whole Department rather than just FDA’s purchasing base. Such efficiencies can be reinvested for strategic tools.
Support HHS enterprise-wide initiatives. FDA provided expertise and resources, with special emphasis on HHSNet, Enterprise Email Support (EES), and e-Rulemaking. In FY 2004 the OCIO accomplished the following:
Support Secure One HHS. HHS established Secure One “to create an enterprise-wide secure and trusted IT environment in support of the overall HHS mission”. The OCIO supported this goal by constructing a comprehensive IT security program, consisting of aggressive security performance measures closely monitored by the FDA Chief Information Systems Security Officer. The program also characterizes and categorizes systems in order to develop the most effective security plans, which are then regularly reviewed and assessed. A comprehensive training and communication effort also increased user knowledge of the importance of good IT security practices. In FY 2004 the OCIO accomplished the following:
Special Projects:
All Hands Broadcasts: OEO coordinated four Commissioner all-hands broadcasts during FY 2004. This included purchasing satellite time, and arranging for the TV studio and speakers. Several topics were discussed, including an update on the Commissioner’s strategic goals, the new Office of Shared Services, an OM update on FDA administrative issues, and management changes in FDA.
OC Honor Awards: The OC Honor Awards Committee was chaired by the OEO Director, Linda Brna. Although delayed by the death of former President Reagan, a successful OC Honor Awards Ceremony was held on July 11. Dr. Lumpkin officiated, and Dr. Crawford presented the awards. The OC Awards Coordinator assisted the agency Awards Coordinator in distributing more than 500 congratulatory letters and memoranda to FDA Honor Award recipients and coordinated OC nominations for the 2004 DHHS Secretary’s Award for Distinguished Service.
Management Advisor Meetings: Management Advisor Meetings were held on a monthly basis, and three quarterly OC Administrative Contact meetings were also held. OEO hosted an appreciation reception for the AO Contacts at the end of the fiscal year. Topics for the various meetings included briefings on ERIC, E-Travel initiatives, simplified acquisitions, and Interagency Agreements. Other topics covered were administrative process issues in FDA, Travel Manager issues, recent changes in the policy for approving requests for outside activities, information on agency, OC, and Commissioned Corps awards, budget information, and IMPAC card policies.
Continuity of Operations Plan (COOP): As the OC COOP Coordinator, quarterly emergency telephone cascade tests were conducted, resulting in at least 87% participation with each test. The most recent test was the first conducted during non-duty hours with no notice. The test resulted in 89% participation. The OC COOP plan was exercised on September 22, which served to meet the requirements under FPC 65.
Q-12: OEO management worked with the FDA Training Office to coordinate OC manager training for the Q12 survey results. OEO had a response rate of 95%. A meeting was held with all OEO employees on January 22 to develop the OEO action plan.
A-76 Competitive Sourcing: The Project Officer for the Graphics Arts and Visual Information Services Most Efficient Organization (MEO) worked with the supervisor to develop a Quality Control Plan. The Quality Assurance Surveillance Plan was finalized in September.
OEO served as a member of the Performance Work Statement Team and the MEO Team for the Clerical A-76 process.
Performance Contracts: A member of the OEO staff administered the performance contract cascading policy to the SES, SES equivalent and GS-15 employees for OC as directed by the Department. We worked with Human Resources and administered the semi-annual call for pay adjustments, SES bonuses, and Presidential cash awards for the OC executives; and coordinated the effort to obtain SES employee signatures on an addendum to be attached to current year performance plans which added a required “exceptional” level to each plan.
Agencywide Employee Exit Clearance: OEO participated on the agency Exit Clearance Form workgroup to develop an on-line exit clearance process for employees who resign or retire from FDA.
Parking: Parklawn Building executive parking permits for use effective July 1 were distributed by the OC parking coordinator. We worked with the PSC Parking Office to determine the number of visitor block parking spaces for use by Centers not located in the Parklawn Building and to identify Center contacts to interact with the Parking Office for all other visitor block parking. OEO negotiated with the PSC Parking Office to obtain an additional 20 visitor block parking spaces, which were allocated among the Centers and ORA. The list of employees who have access to 5630 Fishers Lane parking was also reviewed.
Ethics: The listing of confidential filers was updated and submitted to OMP in August.
External Outreach: A very successful “Bring Your Children to Work Day” program was held on April 22 in conjunction with ORA. 90 children of OC and ORA employees participated.
Annual Reports: The annual Partnership for Administrative Quality (PAQ) submission and the preliminary and final Federal Managers’ Financial Integrity Act (FMFIA) report for OC were prepared.
Support for the Office of Shared Services :
OEO helped to coordinate and facilitate the arrival of the new OSS management, including space, furniture, equipment, phones, parking, and the hiring of support staff. Transition team meetings were held to address and resolve issues such as timekeeping, personnel actions and physical moves; establishing organization and property location codes; establishing the ERIC organization in EASE; and planning training for new OSS management on EASE and Travel Manager.
In conjunction with the Division of Budget Execution and Control, OEO worked to establish a plan to transfer funds and employees for the Office of Shared Services. Tracked the OSS budget, including IMPAC purchases, and distributed guidance to OSS administrative contacts on managing operating and payroll budgets.
Served as OC liaisons on all OSS functional office committees (ORPS, ERIC, OAGS, OFS, and OEEODM).
Financial:
OEO’s Resource Management Team created and distributed preliminary and final OC budget allocation scenarios for approval by the Associate Commissioner for Management and the Commissioner that calculated the distribution of payroll and operating funds to the OC non-OM offices. A new OC financial structure was outlined to obtain new accounting data for offices that moved in alignment with the FY 2004 reprogramming. On a bi-weekly basis, payroll reports were distributed to OC offices. Random timekeeping audits of OC offices were conducted to assure compliance with agency policies and procedures.
Travel Manager audits for various OC components were conducted. OEO staff worked with administrative contacts in OC to resolve routing and proxy list concerns. Travel Manager workshops for OC Offices were held, which provided system education and awareness to travelers and travel preparers.
Facilities/Renovations:
Several renovations and moves for OC offices occurred in FY 2004 that were coordinated by OEO, including:
Began planning for acquisition of space in the 14C wing for the Office of Science and Health Coordination, the Office of Counterterrorism Policy, and the Office of Policy and Planning. Planning also began to move the OC Training room from the 10 th floor to the 4 th floor and to build a new conference room for OC use which will accommodate nearly 50 people.
The Commissioner’s office in the Switzer Building was expanded to include swing space for senior staff when they need to work downtown. Furniture, computer equipment, and telephones were installed.
Information Technology:
Certification and accreditation of 9 OC systems (FIRST, FURLS, EASE, AIMS, MDI, Internet, Intranet, ASSET, PRISM) was completed.
A completion rate of 98 percent of OC personnel for the “Improve Security Awareness” training was achieved for FY 2004.
Structure Changes for a New Direction in Financial Management
At the beginning of FY 2004, FDA transferred its processing of financial transactions (commercial payments, travel, payroll, etc.) from the Office of Financial Management (OFM) to the Office of Shared Services. The Office of Shared Services (OSS) was created to provide administrative services for all FDA staff in the centers, field, and headquarters using the “shared services” model to achieve savings through management efficiencies and cost effective service delivery. OFM retained the functions related to policy, reporting, systems application management, budgetary formulation and execution.
OFM created the User Fees Team to better manage the execution, reporting and accountability of the FDA’s user fee programs, in addition to the information provided for the budget formulation process. These programs include the Prescription Drug User Fee Act (PDUFA), Medical Device User Fee and Modernization Act (MDUFMA), Animal Drug User Fee Act (ADUFA), Mammography Quality and Standards Act (MQSA), and Export Certification user fees. The User Fees Team is also responsible for implementing the new user fee systems to administer user fee transactions and assist in the development of the financial reports required by Congress for PDUFA, MDUFA and ADUFA.
OFM will focus on providing strategic resource leadership to the Commissioner and senior management; leading FDA’s implementation of the HHS Unified Financial Management System and specific financial applications; improving the financial infrastructure for FDA user fees programs; coordinating the financial statement audit and follow-up; and serving and facilitating the information needs for various parties, such as DHHS, OMB, and Congressional appropriation subcommittees, involved in the budgetary formulation, execution and accounting processes.
Continued Excellence with Financial Statements
FDA received its sixth clean audit opinion on its FY 2003 financial statements, reinforcing the steady progress made over the past seven years to improve its financial management performance, through changes to its organization, policies, processes, and procedures. As a result, FDA went from not having an unqualified opinion with three material weaknesses and five reportable conditions in FY 1997 to having an unqualified opinion with no material weakness and one reportable condition in FY 2003.
FDA has strong internal controls for financial reporting and continues to reconcile data to ensure the correct balances are reported to Treasury and OMB. FDA prepares monthly and quarterly reconciliations to ensure the balances reported in financial reports are accurate, and quarterly financial statements to the Department. These strong internal controls have contributed to the agency not having any material weaknesses for internal control deficiencies related to financial reporting in its audits during the past four years.
Financial Systems
To comply with Federal Financial Management Improvement Act (FFMIA) and the Secretary’s directive to consolidate information technology programs, FDA is working with HHS and other HHS components in the implementation and development of the Unified Financial Management System (UFMS). The UFMS project replaces five legacy accounting systems currently used across the Operating Divisions and replaces these systems with one new system that will meet federal financial management systems requirements. Through preparing for the UFMS implementation, the agency has improved its compliance and anticipates that the completion of UFMS will lead to full compliance with FFMIA and provide HHS leaders with a more timely, reliable and consistent financial management information to make better informed decisions regarding agency operations.
The FDA responsibility for implementation of UFMS is led by OFM. The team, comprised of staff from all components of the agency, will perform business analysis, technology analysis and change management activities to determine and carry forward FDA’s business requirements. This effort includes more than 50 staff, excluding contractors, to implement. In FY 2004, FDA entered into the developmental phase of the UFMS project that entailed specific preparatory work including ensuring software met FDA needs, testing the system, determining user training needs, and performing data clean-up. This phase is part of the agency’s implementation of Financial Enterprise Solutions (FES).
UFMS and FES create new opportunities to improve financial management services at Headquarters and at ORA regions by using the latest web-based technology to integrate systems. UFMS replaces FDA’s 30-year old legacy systems and provides real-time access to financial information. This improves response time and financial reporting to OMB, HHS and to FDA components. For employees currently using existing financial and accounting systems, the new system will enhance daily job functions.
FES comprises a set of FDA financial systems that are integrated with HHS’s Unified Financial Management System (UFMS). The UFMS and FES financial efforts involve four phases beginning in FY 2002 and are scheduled to conclude in FY 2005. FDA’s implementation, schedule and activities for FES and UFMS are as follows:
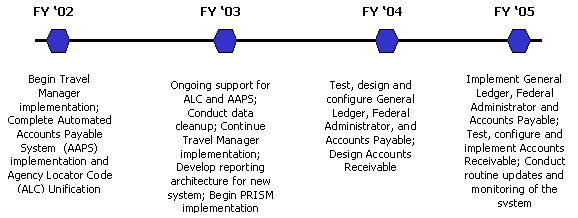
An update on the progress of some of the FES applications includes:
Travel Manager . OFM is scheduled to complete the implementation of Travel Manager for the entire agency by the end of 2004. As an effort to improve service, OFM began automating Sponsored Travel (348s) by modifying NIH’s 348 Travel Manager Module for FDA’s accounting interface. The Travel Manager team completed the pilot of 348 Sponsored Travel module with the Center for Devices and Radiological Health allowing for the complete automation of sponsored travel related forms. OFM and the Office of Financial Services are part of the core team preparing for the E-Travel initiative in FY 2006.
PRISM / I-Procurement. The Purchase Request Information System (PRISM) involves developing a system to automate FDA’s procurement functions. The system is intended to automate FDA’s requisitions and award processes within the simplified acquisitions and contract functions. The agency selected the Oracle I-Procurement module to be FDA’s requisitioning system and anticipates its implementation by April 2005. The I-Procurement module includes requisitioning, training, visa card purchases and receiving. It also includes electronic funds control and electronic approval of all purchases.
Animal Drug User Fee Act (ADUFA). OFM designed and implemented a system to administer user fee transactions for ADUFA. This system includes an interface to: obtain applicant data, track ADUFA billing and collection, and provide financial reports of ADUFA activities. The ADUFA implementation included modifying the Accounts Receivable System by capturing initial ADUFA receipts and transitioning ADUFA receipts to the Accounts Receivable module of the new financial system.
In partnership with the Center for Veterinary Medicine, workgroups were conducted with a multitude of subject matter experts and consultants to develop policy, procedures and a computerized system of billing and collecting for ADUFA. This state-of-the-art system will also serve as the basis for the implementation of additional FDA user fees.
Budget and Performance Integration
OFM worked closely with the Office of Planning (OPL) to develop budget guidance for program components, analyze budget and performance submissions from FDA components, and prepare budget requests and performance plans.
In November 2003, OFM and OPL met with the budget contacts in the Centers, OC, and ORA to discuss the Congressional justification in regards to changes necessary for the FY 2006 performance budget submission. For the Congressional submission, OFM briefed the budget contacts on the changes that would infuse the budget document with performance related information. These changes included providing an expanded background section to give a greater context on the scope of the regulatory authority imposed on industry and/or the public health impact on the American public; providing program increases by FDA strategic and performance goals, incorporating success stories to show how resource funding is linked to performance results; and inserting charts and pictures to provide variety and improve flow in the document. The FY 2005 Performance Plan was linked to the budget request by describing the use of the funding but left the specific performance implications to the annual performance plan. The request included examples of specific performance goals throughout the document to justify the request by describing performance targets attributable to these resources.
For FY 2006, FDA has produced two performance based budget submissions. Performance information was placed throughout the document that linked funding with performance targets. Other performance related information was also provided that included performance analysis on all of the program’s goals, the full cost presentation for each of the FY 2006 performance goals, and a conscious linking of the existing traditional budgetary outputs along side of GPRA performance targets.
Under this new methodology, the traditional budget presentation is now coupled with performance information presenting a complete resource and performance picture. The presentation order in the FY 2006 performance budget is: base activities (justification), p rogram activity data (PAD), and performance targets. This request funds base activities that in turn support the accomplishment of discrete workload outputs, as shown in the PAD and in performance goal targets. Each of these measures contribute to the achievement of long-term public health outcomes and strategic goals. Since the annual performance plan is subsumed into the performance budget, the remaining parts of the plan are included in the budget’s appendix sections.
FDA Follow-up on the OMB’s Program Assessment Rating Tool (PART). Based on the results of its FY 2004 PART evaluation, FDA committed to a series of changes raised by OMB. FDA developed outcome and efficiency goals, reduced the number of performance goals, and embraced the improvements envisioned in the President’s Management Agenda (e.g., in the areas of competitive sourcing, workforce planning, organizational de-layering, electronic government, and improved financial performance). When OMB decided to re-evaluate FDA as a single entity program in the summer of FY 2003, the agency was able to show significant progress and was recognized by OMB with a rating of moderately effective.
Building on this success, in FY 2004, FDA proceeded to make progress in achieving these PART and PMA goals. FDA leadership established the Strategic Planning Council to monitor progress and make changes to the way FDA conducted business. In January 2004, the council agreed to establish a performance framework that systematically linked an array of program activities, outputs, and outcomes to support and demonstrate progress in meeting the long-term outcome goals. This council has also directed that the agency should prepare for the FY 2006 PART process with DHHS and OMB to demonstrate FDA’s progress and to facilitate the making of performance and resource decisions for the upcoming budget cycle. In May 2004, FDA met with DHHS and OMB officials and provided evidence of progress in achieving long-term outcome goals.
FY 2004 Appropriation. On January 23, 2004, the President signed the Consolidated Appropriation Act, 2004, which provides FDA with a FY 2004 program level budget of $1.7 billion. These resources will support our responsibilities under the Federal Food, Drug, and Cosmetic Act to ensure that new products are safe and effective for consumers. This legislation allows for $286.5 million for user fees, including $249.8 million for Prescription Drug User Fees, $31.6 million for Medical Device User Fee and Modernization Act, and $5 million in newly appropriated user fees authorized by the Animal Drug User Fee Act. Indefinite appropriations for MQSA, Color Certification and Export Certification provide an additional $23.2 million in user fee resources.
Provided for in the appropriation is $23.3 million for pay inflation, $2.4 million for CDER’s move to White Oak, $20.5 million for food safety, $1.15 million for UFMS, $6.0 million for medical device review, $3.0 million for patient safety involving human drugs and devices, $.65 million for over the counter drugs, $8.0 million for generic drugs, and $4.0 million to implement the Best Pharmaceuticals for Child Act as well as reductions of $28 million in management and other savings, $29.1 million in IT consolidations, and $.95 million for Buildings and Facilities.
Budget Execution. In the financial management area, Budget Execution completed projects relating to the structure changes in the agency. These include:
Development of the initial budget policies related to the establishment of the Office of Shared Services, enabling FDA to establish interim budgets and charge costs to its users as appropriate. The FDA Management Council approved the budget for OSS in January, 2004.
OFM coordinated development of initial spending and operating plans, with CVM and ORA, for the use of new Animal Drug User Fees, and successfully implemented the collection and allocation of these fees during the first year of operation of this program.
Worked with CVM, CDRH, CDER, and OC staff to begin implementation of the Memorandum of Agreement which establishes funding arrangements for the new animal facilities program at the FDA facilities at White Oak.
Improved communication of resource issues and mission priorities with Departmental, OMB, and Congressional staff and facilitated informational briefings and visits to FDA facilities to improve understanding of FDA’s mission and increased responsibilities.
Responded more quickly to Congressional inquiries, including the annual questions and answers, which follow the Budget Hearings. OFM also provided more background materials and electronic transmission of FDA program announcements to this staff. This included internal briefing to the staff and site visits of our facilities.
Human Resources Consolidation - DHHS “40 to 4” Consolidation . Served as liaison with the Rockville Human Resources Center to prepare for stand-up on January 25, 2004. In preparation, coordinated migration of staff and functions, including the coordination of physical space moves, establishing client contacts, review and approval of Service Level Agreements and coordination of service delivery.
Federal Managers’ Financial Integrity Act (FMFIA) Activities.
Delegations of Authority (DOA) Activities.
Office of Inspector General (OIG) Program .
Organization Program.
Freedom of Information Act (FOIA) Activities.
Ethics Program Activities.
Paperwork Reduction Act/OMB Team (PRAT) .
Records Management and Information Dissemination (RID) Team .
Dockets Management
The Division of Dockets Management continues to receive electronic submissions that provide an easy way for the public to submit both general comments on specific issues and specific comments on proposed rules, guidances, notices of meetings, workshops, and other activities. More submissions are being submitted electronically than via paper, since the implementation of electronic submissions in 1998.
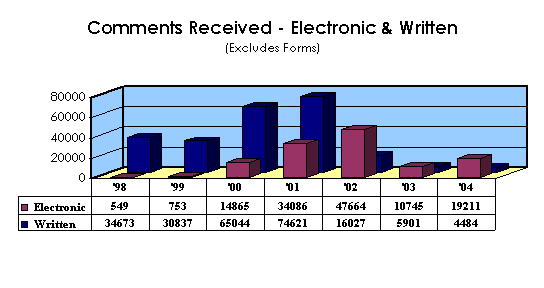
Reorganization:
Reward & Recognition Program
Strategic Workforce Planning
Quality of Worklife Initiatives
Leadership Training & Development
In early FY 2004, the Office of Shared Services was created to provide high quality administrative services from a single organization. The implementation of OSS, along with de-layering initiatives and competitive sourcing activities, contributed to the agency achieving a cost savings of $2.6 million. Furthermore, FDA met the FY 2004 administrative staff reduction of 7.5 percent identified in the President’s budget. The following table details OSS initiatives and accomplishments for FY 2004.
Initiatives |
Summary of Accomplishments |
|---|---|
Implemented ‘Shared Services’ organization |
|
Implemented CALL Center (ERIC) |
|
Awarded the IT Infrastructure Services Contract |
|
Purchase Request Information System (PRISM) |
|
Consolidation of Document Control Room & Records Management Services |
|
Financial Enterprise Solutions/Unified Financial Management System Logistical Support |
|
Department Acquisition Consolidation |
|
E-Grants Initiative |
|
Small Business Goals |
|
Business Category |
Accomplishment * |
DHHS Goals |
|||
|---|---|---|---|---|---|
% |
$ in 000’s |
|
% |
$ in 000’s |
|
Small Business |
37.02 |
80,162 |
30.32 |
95,572 |
|
8(a) Awards |
10.91 |
23,621 |
5.56 |
17,526 |
|
SDB |
21.99 |
47,611 |
11.12 |
35,051 |
|
Women-Owned |
6.40 |
13,856 |
5.05 |
15,918 |
|
HUBZone |
4.02 |
8,716 |
3.03 |
9,551 |
|
Service Disabled Veteran |
.01 |
11 |
3.00 |
9,456 |
|
EEO General Administration |
|
EEO Compliance |
|
Diversity Management |
|
Conflict Prevention & Resolution (CPR) |
|
OFFAS Organization |
|
MEO Activities - Office of Field Financial and Acquisition Services |
|
MEO Activities – Office of Real Property Services |
|
Travel Card Delinquency Letters |
|
Move Management Services |
|
Invoice Payments |
|
Bio-Safety Level 3 Laboratories |
|
Decommissioning |
|
Construction, Space Acquisition & Renovation |
|
Life Sciences Laboratory: On December 11, 2003, a dedication ceremony was held for the Life Science Laboratory, a state of the art biological science, chemistry and animal research facility. As the first new building to open on the site, the laboratory provides approximately 124,000 gross square feet (gsf), for 120 employees from CDER and CDRH.
CDER Office Building: Construction of this building began on November 15, 2002, and has progressed on schedule for occupancy in spring 2005. This 560,000 gsf modern office facility will accommodate the Office of New Drugs which is comprised of approximately 1,700 medical review and support staff. The facility also includes a 60,000 gsf modern document storage center, mail room and associated support space.
Central Shared Use I Building (CSU): Design has been completed on the CSU, and the construction contract was awarded in September 2004. When fit-out is completed, this building will provide employees and visitors with a cafeteria, conference and training center, credit union, fitness center, health unit, central library and R&W store, along with housing the agency security command center, central data center and NTEU offices.
CDRH Engineering/Physics Laboratory: Began review of technical proposals for this laboratory, which is expected to be awarded by the end of 2004. When ready for occupancy, it will provide approximately 140,000 gsf of high tech laboratories engaged in evaluating electromagnetic and medical devices, radiological instruments and consumer appliances generating radiological signals. The laboratory contains numerous vibration isolation slabs, electromagnetic shielding, and anechoic chamber and laser devices specifically supportive of the scientific program. This facility is scheduled for occupancy in 2006.
CDER Office Expansion Building: Design of this building has progressed to the 15 percent level. When construction is complete, it will provide approximately 290,000 gsf that will accommodate the Center Director’s office and the balance of the CDER scientific and support staffs. This is a uniquely designed office building that features a raised floor air distribution system. This design change provides for more offices benefiting from direct outside daylight, taller windows and more efficient distribution of air and electricity along with IT/Telecom and security wiring. Award is expected in the summer of 2005.
Community, Employee and Official Presentations: Conducted briefings and tours for Administrative and congressional staff including a tour for the Senate Appropriations Committee. Tours were regularly provided to FDA managers, NTEU officials, employees and members of the local community and private industry.
Relocation Planning and Coordination for White Oak Consolidation: Initiated the planning required to occupy the second building to be completed at the FDA consolidation site in White Oak. Through detailed scheduling and coordination with multiple FDA components and needed vendors, the relocation is planned to start in April 2005. The preparation and coordination involves the following:
The OM team is proud of FDA’s FY 2004 managerial accomplishments and looks forward to the challenges ahead. In FY 2005, OM will continue to work with all organizational components of the FDA management team to position the agency to meet Departmental goals and objectives with state-of-the-art technology, re-designed cost management systems, streamlined business processes, and innovative approaches while addressing our customer’s needs. A discussion of some of the high priorities on which OM plans to focus in FY 2005 follows.
> Chief Information Officer <
Continue increasing the efficiency and effectiveness of agency management by:
Continue transitioning Information Technology from an enabler to a strategic tool by:
The OCIO will support the “One HHS” approach to management by:
> Executive Operations <
Address outstanding property inventory issues in OC by implementing mandatory training for OC Property Custodial Officer’s and reviewing/revising current OC property procedures.
Prepare, implement and enforce procedures for GSA-leased OC shared-use vehicles
Schedule and facilitate four quarterly All-Hands Broadcasts with Commissioner and CDRH TV Studio.
Manage registration process for EEO compliance training for OC managers and supervisors.
Coordinate Gallup survey process for OC employees.
> Financial Management <
Congressional Outreach
Continue the long-term process of strengthening communications with the appropriations subcommittees, as well as Office of Management and Budget and DHHS staff to increase their knowledge of the agency’s mission, goals, and public health initiatives. This will be accomplished by: 1) refining the agency’s performance budget submissions; 2) conducting program briefings and facilities tours; 3) providing timely and accurate background information; 4) integrating IT resources and planning into the process; and 5) coordinating with the Office of Legislation to ensure appropriations and authorizations committees receive consistent information.
Budget
Develop the FY 2007 Performance Budget, through a planning process that focuses on critical needs and carefully examines base funded programs. Refine the integration of the FY 2007 budget submissions to more directly link the budget and performance integration.
Work with CFSAN to monitor funds in the Revolving Fund for Certification of Colors and develop a proposal for a change in the fees charged under this program to keep the program running at its current level.
Assure that funds are provided in FY 2005 for the costs of occupying additional facilities at the new White Oak, Maryland campus, primarily for the CDER office buildings to be occupied during 2005.
Work with the Centers, ORA, OC, and the Office of Shared Services, to complete and implement budget allocations for the Office of Shared Services for the inclusion of Field and NCTR shared services during FY 2005.
User Fees
Finalize the upgrade of the PDUFA and MDUFMA billing and collection system. This will enable FDA to generate reports and have the consistent look and features for all user fees. It allows industry to go to one central site for drug, animal drugs, biologics, and medical devices user fee payments and applications. It will allow OFM to track and more easily record receipts and the billing and collection system will be interfaced with the UFMS.
Complete reporting requirements and begin implementation of an Activity-Based Management and Activity-Based Costing system that will: 1) enable better process for good managerial costing; 2) take advantage of data available through UFMS; 3) support managers’ ability to allocate scarce resources to most effectively perform mission-critical functions; and 4) provide an agencywide activity accounting and reporting process.
Provide annual PDUFA Financial Reports to Congress, manage the fee-exceeds-the costs waiver provisions, and issue Federal Register notices for new fees every August.
Accounting
Maintain an unqualified audit opinion for the FY 2004 audit while at the same time meeting the accelerated deadlines. This will be accomplished by preparing monthly reconciliations throughout the year and by closing the General Ledger sooner using the new procedure for preparing financial statements. This new procedure will save time and allow us to meet the increased reporting requirements, as well as the accelerated dues dates.
Produce the FDA Annual Financial Report within the designated time frames established by the Department and the OIG by ensuring advanced coordination and communication with all of the component contributors.
Resolve the reportable condition with FDA’s financial analyses and reporting controls by preparing timely reconciliations and analyses on fund balance with Treasury, undelivered orders, accounts payable, and accounts receivable.
Improve the close-out process for open obligations by closely monitoring monthly reports and working with components on timely deobligations.
Financial Systems
Launch the second phase of FDA’s implementation of the Department’s Unified Financial Management System during April 2005. In the interim, as a part of FDA’s Financial Enterprise Solutions, develop the five interfaces required by FDA to work within UFMS. Continue to conduct UFMS awareness sessions and training in agency components.
Implement the Oracle I-Procurement module and provide training as FDA’s requisitioning system with a target date of April 2005. The I-Procurement module provides electronic funds controls and approvals for purchases.
Implement a sponsored travel module and a travel reporting module using Oracle and Business Objects. The approval process for travel will be streamlined by requiring only signatures of the travelers.
Begin planning for the consolidation of property.
Begin preparations with OFS to migrate to E-Travel in FY 2006.
Begin implementation of an activity-based costing solicitation for CDRH, the Office of Shared Services, and Office of Information Technology Shared Services.
> Management Programs <
Federal Managers’ Financial Integrity Act
Office of Inspector General (OIG) Program
HR Programs Staff
> Shared Services <
Implement a web-based Service Provider Resource Center to ensure that OSS staff has the appropriate tools to enhance the effectiveness of business operations.
Establish and manage business processes to increase efficiency and effectiveness of our mission-critical functions, including partnerships with other FDA components to consolidate common processes, increase efficiency, cross systems analysis, automation of process, and increase return on investment, etc.
Develop and implement business process improvements to assure overall service performance by improving productivity, high-customer satisfaction, and cost-efficiency.
Develop and implement a Clerical Services Management Plan.
Establish career training plans to provide employees with the necessary tools to perform their work.
Develop OSS directives to establish and document systems of internal controls for administrative and management activities, and to serve as a template to review and improve internal administrative functions.
Develop and implement plans, including aggressive customer service, to improve communication both internally and externally by engaging with employees, customers and stakeholders.
Present an open forum where OSS Service Providers and customers can discuss services and activities, including the development and revisions of Service Agreements.
Collaborate with other HHS OPDIVs to maximize on existing ERIC Call Center infrastructure. Currently reviewing adding e-Travel and UFMS into the ERIC portfolio of services.
Establish outreach programs with additional Government agencies and private industry to share information and review best practices.
Competitive Sourcing
Continue to support the President’s Management Agenda and agency goals by conducting cost comparison studies under OMB Circular A-76. OAGS will support the initiative by conducting competitions and contract functions for the agency’s designated FY 2005 A-76 activities. During FY 2005, OAGS will conduct a competition for the clerical support function. Approximately 350 FTEs will be included in this study.
Strategic Sourcing Initiatives and Enterprise Spend Management
Continue to participate in the Department’s strategic sourcing initiatives. The strategic sourcing initiatives are part of the on-going "One Department" initiative within HHS to reduce operating costs and consolidate administrative functions.
Department Consolidation
Continue to participate in the Acquisition Consolidation Work Group sponsored by the Office of the Secretary, DHHS.
E-Grants Initiative
Continue to work with agency IT representatives and NIH staff to achieve full migration of FDA grants operations to the IMPAC II system. In addition, OAGS will proceed with training and the examination of the functions of each IMPAC II module.
FDA will also continue participating in the Awarding agency Grants Administration Manual (AAGAM) workgroup, the Grants Streamlining Committee, the Electronic Grants Subcommittee in support of Public Law 106-107, and the expansion of electronic government.
Continue to consolidate the Animal Husbandry Services into one contract and the anticipated savings from this consolidation are 10 percent of present day costs.
Continue to partner with OFM to support the agency’s implementation of an automated requisitioning tool, iProcurement, that will streamline business processes, eliminate duplication of data entry, and further enable our management of the demand for supplies and services.
Rollout PRISM to contract specialists working on large contracts.
EEO Activities
PRISM
Implement PRISM rollout to all OFFAS locations in the first quarter of FY 2005. The simplified acquisitions module will roll out in October and to contract specialists working on large contracts in November.
Partner with OAGS and the Office of Financial Management to support the agency’s implementation of the automated requisitioning tool.
Implement processes to improve vendor payment and meet the FDA requirement for consistent procedures for processing vendor payments for contracts and simplified acquisitions.
Payment Digital Imaging
Implement the payment digital imaging to expedite invoice payment and meet the FDA requirement for consistent procedures for processing vendor payments for contracts and simplified acquisitions.
Significantly Reduce Travel Card Misuse & Delinquency
Significantly reduce misuse and delinquency of the travel card program by expanding travel, training, and management awareness. In addition, will increase travel card awareness through quarterly US Bank training awareness session.
Bio Safety Level 3 Laboratories
Complete and certify required Bio Safety Level 3 laboratories at the ORA Facilities in Cincinnati, OH; CFSAN facilities in Laurel, MD and Chicago, IL, and CBER facilities in Bethesda, MD.
Construction, Space Acquisition, and Renovations
Implementation of Executive Orders
Laboratory Decommissioning
Continue and/or complete decommissioning of the following facilities: ORA Southeast Regional Laboratory (Crawford Building and Annex 1 in Atlanta, GA); CFSAN FB-8 laboratory in Washington, DC; CDRH 12709 Twinbrook Parkway laboratory in Rockville, MD; and CFSAN laboratory in Chantilly, VA.
Implementation of PMA Real Property Management Initiative
Develop and implement plans to address the new real property management initiatives in the President’s Management Agenda.
> White Oak Consolidation Program <
During FY 2004, OM employees contributed greatly to the accomplishment of the agency mission through their continued commitment, dedication, and hard work. The efforts of many of these employees were recognized at FDA’s Honor Awards Ceremony, with the presentation of numerous individual and group awards. The following is a listing of OM Honor Award recipients for FY 2004.
Carrie S. Hanley
FDA/Booz Allen Hamilton Shared Services Team
|
Richard R. Alvarez |
John D. Gugliotti |
Jennifer M. Newell |
|
For outstanding commitment to and collaborative support of the many activities related to the design and implementation of a new service delivery model in FDA. |
||
A-76 Study Teams |
||
Gail E. Becker |
William R. Harris |
Ronald J. Loube |
For untiring effort in the development of comprehensive Performance Work Statements and cost effective Most Efficient Organizations which resulted in six successful competitions . |
||
ERIC Development and Implementation Team |
||
Ronnie D. Conner |
Lynn K. Pellegrino |
Joyce M. Washington |
For outstanding service in establishing the FDA Employee Resource and Information Center. |
||
AWARD OF MERIT |
||
Kimberly A. Holden |
||
For outstanding leadership during a time of uncertainty and change as a result of the consolidation of HR functions in the Department. |
||
GROUP RECOGNITION AWARD |
||
Shared Services Steering Committee |
||
Mary L. Babcock |
Carrie S. Hanley |
Joan K. Jappa |
For significant involvement in and commitment to the successful design and implementation of a new service delivery model in FDA, the Office of Shared Services. |
||
FDA Benefits Staff |
||
Fatmata P. Bah |
David R. Leffler |
Sandra S. Rogers |
For sustained outstanding performance and commitment in processing over 200 buyout and early out retirements. |
||
OUTSTANDING SERVICE AWARD |
||
Arlene S. Karr |
||
For exceptional management and facilitation of the process leading to the selection of the OSS leadership team. |
||
|
|
|
Alfonzo Hilliard |
|
|
For exemplary performance in managing the FDA Labor Relations Program. |
||
|
|
|
COMMISSIONER’S AWARD OF EXCELLENCE |
||
Katherine A. Busch |
|
|
|
|
|
For outstanding performance in furthering the implementation of the President’s Management Agenda and related DHHS and FDA Initiatives. |
||
|
|
|
Congressional Liaison Team |
||
Stacy M. McBride |
Thomas B. O’Brien |
|
|
|
|
For outstanding commitment to assist FDA in its effort to secure funding for new initiatives and defend current mission-related initiatives. |
||
|
|
|
COMMISSIONER’S SPECIAL RECOGNITION AWARD |
||
CDER Therapeutics Facility Renovations and Moves Team |
||
Marc J. Bloom |
Carla P. Forehand |
Mark S. Schmall |
David E. Dwyer |
John P. Long |
Laurie A. Whalen |
For outstanding performance in providing space and facilities support to allow for the timely completion of the Commissioner’s Initiative to consolidate Therapeutics work in CDER. |
||
|
|
|
Irvine Facility Contract Support Team |
||
Patricia G. Calhoun |
Clyde L. Messerly |
Kathleen S. Smith |
Lorena M. Forgosh |
Mark S. Schmall |
|
|
|
|
For successful negotiation and award of the contracts for the Irvine facility. |
||
|
|
|
Dockets Management Group, Office of Management Programs |
||
Michelle Bigesby |
Delores I. Johnson |
Gloria M. Ortega |
|
|
|
For providing exemplary dockets management services to FDA components and the public. |
||
Agency Information System Security Officers (ISSO’s) |
||
Ruth Bandler |
Matthew Scholl |
|
For establishing and overseeing the FDA IT security program and the successful implementation of an agencywide IT security training and awareness curriculum. |
||
COMMISSIONER’S ADMINISTRATIVE MANAGEMENT AWARD |
||
Valerie Young |
||
For extraordinary effort in producing a consistent, well-designed set of pages and content for the ERIC Portal, the focal point for the new Shared Services organization. |
||
Bradford K. Joyce |
||
For exceptional effort in the requirements analysis and contract negotiations while ensuring migration of FDA’s website from former host UUNet to the current host ATT. |
||
OC Travel Manager Team |
||
David T. Caines |
Michael B. Fullem |
Paula B. Searle |
For successful implementation of Travel Manager through FDA. |
||
COMMISSIONER’S ADMINISTRATIVE MANAGEMENT AWARD |
||
Jane M. Peterson |
||
For providing outstanding administrative support to the organizations that she serves within the Office of the Commissioner. |
||
COMMISSIONER’S COMMUNITY SERVICE AWARD |
||
Virginia Boline |
||
For extraordinary personal effort and sacrifice in leading the Office of Executive Operations staff in supporting a needy family during the holiday season. |
||
The OMS team is pleased to work with all FDA personnel as we strive to protect, promote, and enhance the health of the American people. For more information about OMS services and systems, please contact:
Additional information about OMS programs is available on FDA’s Internet
at PDUFA, Dockets
Management, and the
Yellow Book.
![]()