Fatal Iatrogenic Hypoglycemia:
Falsely Elevated Blood Glucose Readings with a Point-of-Care Meter Due to a Maltose-Containing Intravenous Immune Globulin Product
Ann Reed Gaines, Ph.D., MT(ASCP)
Office of Biostatistics and Epidemiology (HFM-220)
Center for Biologics Evaluation and Research
L. Ross Pierce, M.D.
Office of Blood Research and Review
Center for Biologics Evaluation and Research
Patricia A. Bernhardt, B.S., MT(ASCP)
Office of In Vitro Diagnostic Device Evaluation and Safety
Center for Devices and Radiologic Health
INTRODUCTION
In July 2005, the Food and Drug Administration (FDA) received a case report of an elderly male diabetic patient who received a 10% maltose-containing intravenous immune globulin product (Octagam,1 Octapharma Pharmazeutika Produktionsges m.b.H., Vienna, Austria) and experienced hypoglycemic coma and irreversible neurological damage secondary to excessive insulin administration. His insulin dosing was guided by falsely elevated blood glucose measurements that were obtained from a point-of-care glucose meter (Accu-Chek Inform meter,2 Accu-Chek Comfort Curve test strips,2,3 Roche Diagnostics, Indianapolis, IN, U.S.). The glucose meter test strips used glucose dehydrogenase pyrroloquinolinequinone (GDH-PQQ) methodology,4 which may overestimate measurements3,4 when blood maltose levels exceed 0.9 mmol/L.3
Adverse events have been reported for immune globulin products that contain maltose,5-10 which functions as a protein stabilizer11-13 and an osmotic agent.14,15 Similar adverse events have been reported for Extraneal (Baxter International, Deerfield, IL), a peritoneal dialysis solution that contains icodextrin,5,6,8,16-19 which functions as an osmotic agent17-19 and is metabolized to maltose and other oligosaccharides.5-8,17-19 Other maltose-containing immune globulin products, parenteral galactose-containing, oral d-xylose-containing, and other intravenous maltose-containing products (e.g., parenteral nutrition products20) could theoretically yield overestimated GDH-PQQ glucose readings.1,3,4,14,21-23 However, we are unaware of similar adverse events involving other FDA-licensed maltose-containing immune globulin or d-xylose-containing products. We are further unaware of any currently FDA-licensed galactose- or other parentally administered maltose-containing products (which could, however, be developed in the future).
GDH-PQQ methodology is used in the test strips of several point-of-care blood glucose monitoring systems.4,8,9 Glucose oxidase, glucose hexokinase, glucose dehydrogenase nicotine adenine dinucleotide (GDH-NAD), and flavin adenine dinucleotide glucose dehydrogenase (FAD-GDH) methodologies are used in other devices or clinical laboratory instruments and do not yield falsely elevated blood glucose readings in the presence of maltose.18 (The glucose methodology used in any system is available in test strip professional package inserts and from device or instrument manufacturers.)
Following receipt of the index case, FDA conducted an investigation. This review represents the final report of that investigation.
METHODS
We communicated with staff of the hospital where the index patient was treated, manufacturers of the immune globulin and glucose meter products involved, manufacturers of other FDA-licensed maltose-containing immune globulin and glucose meter products, and foreign public health regulatory agencies. We reviewed medical records for the index patient, queried FDA databases for other serious adverse events for the product classes involved, reviewed the professional package inserts for these products, and searched the medical literature. (As defined by FDA, "serious" adverse events are life-threatening, require hospitalization, or necessitate medical intervention, among other criteria.25) Except for the index case, however, the adverse event case reports that were identified were not sufficiently complete to assess seriousness of the events. We therefore limited this review to adverse events that met this revised case definition: maltose-containing immune globulin product administration, falsely elevated GDH-PQQ blood glucose measurements, inappropriate hypoglycemic agent dosing based on those measurements, and iatrogenic hypoglycemia.
RESULTS
Adverse Event Case Report Summaries
We identified seven adverse event case reports from public health regulatory authorities, the literature,6,7 or FDA-licensed manufacturers26 that met the case definition. This seven-patient case series included the index patient, five foreign cases, and another U.S. patient. Two cases involved fatal outcomes.
Index Case
An 86-year-old diabetic male was admitted to the hospital with a 4-day history of cellulitis of the foot, which rapidly progressed to necrotizing fasciitis and sepsis. By hospital day 4, he experienced an above-the-knee amputation, probable pneumonia, and oliguria requiring dialysis. He received multiple blood component, blood derivative, and drug products, including intermittent subcutaneous (SC) regular insulin (range: 2-12 units) as needed for fluctuating blood glucose measurements (range: 3.6-16.3 mmol/L). His blood glucose levels were monitored with GDH-PQQ methodology (Accu-Chek Inform meter,2 Accu-Chek Comfort Curve test strips,2,3 Roche Diagnostics) and intermittently assayed with clinical laboratory instrumentation that used glucose oxidase methodology (SYNCHRON LXi 725 Systems,27 Beckman Coulter, Fullerton, CA). The blood glucose values with both GDH-PQQ and glucose oxidase methodologies corresponded closely through noon of day 4 (all times are approximate, Figure 1).
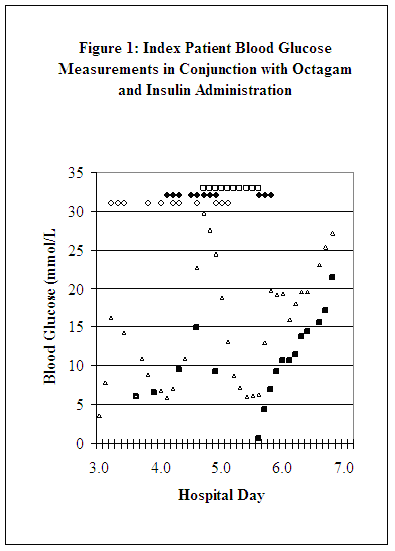

At that time, the patient, who was debilitated but responsive to verbal stimuli, was started on a 10-g dose (158.0 mg/kg) of Octagam at an infusion rate of 67 mL/hr (0.9 mg/kg/min) over 3 hr for treatment of sepsis (an off-label indication,1 Table 1). (All infusion rates were calculated from available data and are approximate.) At 7:00 PM, he was started on a 70-g dose (1105.8 mg/kg) of Octagam at an infusion rate of 200 mL/hr (2.6 mg/kg/min) over 7 hr, during which his GDH-PQQ blood glucose levels progressively increased. At 9:00 PM, the glucose meter reading was 23.3 mmol/L, and he received 20 units SC regular insulin. When an 11:00 PM GDH-PQQ glucose measurement was 29.7 mmol/L, he received 12 units/hr IV insulin, which was continued and titrated upward throughout day 5 (range: 14-24 units/hr).
The patient became increasingly unresponsive on day 5 and was completely unresponsive at 7:30 PM. The GDH-PQQ glucose reading obtained at 9:00 PM was 6.4 mmol/L; a concurrent glucose oxidase measurement was 0.7 mmol/L. He received 50% dextrose and insulin administration was temporarily stopped. However, he was continued on the 35-g dose (552.9 mg/kg) of Octagam that had been just been started at an infusion rate of 112.0 mL/hr (1.5 mg/kg/min) over 6 hr. He remained unresponsive during the remainder of his hospitalization, was diagnosed with irreversible neurological brain damage, and expired on day 65, following withdrawal of life support.
Figure 1 depicts patient blood glucose measurements in conjunction with Octagam and insulin administrations during days 3 through 6. Nearly simultaneous pre-Octagam administration blood glucose measurements showed equivalence between GDH-PQQ and glucose oxidase methodologies (e.g., 6.3 and 6.2 mmol/L). Post-Octagam administration, however, GDH-PQQ and glucose oxidase blood glucose measurements, although not precisely simultaneous, became increasingly but variably divergent (range of discrepancies: 2.8-15.4 mmol/L) and remained so throughout day 7. Not until 2 AM on day 8 (not shown in Figure 1), which was 48 hr following completion of the last Octagam infusion, did the GDH-PQQ and glucose oxidase blood glucose measurements converge.
Case 2
Reported to the United Kingdom Medicines and Healthcare Products Regulatory Agency in August 2002, this case involved a 50-year-old diabetic male with a failing pancreas-renal transplant who received Octagam as an anti-rejection agent (an off-label use28). On the basis of falsely elevated GDH-PQQ blood glucose measurements (Accu-Chek Advantage meter,29 Accu-Chek Comfort Curve test strips,3,29 Roche Diagnostics), he received inappropriate insulin dosing. He subsequently developed severe hypoglycemia, became comatose, was diagnosed with severe central nervous system depression, and died. Further information was unavailable.
Cases 3 and 4
These patients received a non-U.S.-licensed 10% maltose-containing intravenous immune globulin product (Intragam P,30 CSL Limited, New Zealand). Case 3 was reported to the Australian Therapeutic Goods Administration in December 2003 and involved a female patient of unknown age who received Intragam P for an unknown indication. Falsely increased blood glucose measurements were obtained with unspecified GDH-PQQ test strips. She was treated with an unspecified hypoglycemic agent and developed hypoglycemia. Follow-up inquiries yielded no further information.
Case 4 was initially reported in the literature6 in January 2004 and involved a 64-year-old diabetic female dialysis patient who received Intragam P for immune thrombocytopenic purpura (ITP, an off-label use [at that time]30). She developed falsely elevated GDH-PQQ blood glucose levels (Accu-Chek Advantage meter,29 Accu-Chek Comfort Curve test strips,3,29 Roche Diagnostics), received increased insulin, and developed hypoglycemia of 2.3 mmol/L, as measured with glucose oxidase methodology (not otherwise specified Precision meter, Precision Plus test strips, MediSense, Birmingham, UK). Despite follow-up inquiries, further information was unavailable.
Case 5
This case occurred in Brazil and was reported in the literature7 in August 2005 and to FDA in August 2006. The 34-year-old non-diabetic female patient, with a history of chronic renal failure and malignant hypertension, received Octagam for antibody desensitization (an off-label indication31) in preparation for a kidney transplant. Twelve hours after the Octagam infusion, she developed symptoms of mouth dryness and dehydration. Repeat blood glucose levels measured with GDH-PQQ methodology (Accu-Chek Advantage meter,29 Accu-Chek Comfort Curve test strips,3,29 Roche Diagnostics) were > 22.2 mmol/L. She received both SC and IV insulin dosing and subsequently exhibited signs and symptoms consistent with her 1.9 mmol/L blood glucose measurement (methodology unspecified). She recovered without sequelae following treatment with 50% dextrose solution.
Case 6
In this Belgian case reported to FDA in August 2006, a male patient of unknown age was treated with Octagam in "preparation for a renal transplant" (an off-label indication31). While under anesthesia, his GDH-PQQ blood glucose measurement (Accu-Chek Sensor meter,29 Accu-Chek Sensor Comfort test strips,3,29 Roche Diagnostics) was "very high," and he was treated with insulin. Upon re-testing, the glucose meter reading was 5.8 mmol/L, while a glucose oxidase measurement (not otherwise specified Glucocard meter, A. Menarini Diagnostics, Florence, Italy) was 1.1 mmol/L. The hypoglycemia was "diagnosed and controlled in time," and the patient suffered no other complications.
Case 7
As reported to FDA in August 2006, an adult male diabetic patient received unspecified insulin doses for gradually increasing GDH-PQQ blood glucose results (range: 7.5-17.2 mmol/L, Accu-Chek Advantage meter29 Accu-Chek Comfort Curve test strips,3,29 Roche Diagnostics) concurrent with Octagam administration for an unreported indication. At a later time, the glucose meter value was 7.5 mmol/L although the patient was "experienc(ing) hypoglycemic symptoms," which he self-treated by drinking a cola beverage. He received no other medical intervention and recovered without complication.
DISCUSSION
Blood glucose measurement considerations
Blood glucose measurements reflect the physiologic/metabolic state of the patient but also artifactual and analytical factors that may result in falsely increased or decreased measurements.3,32,34-38 Factors that may affect blood glucose measurements include diminished peripheral blood flow (e.g., hypovolemia, peripheral vascular disease), hematocrit (e.g., anemia vs. polycythemia), blood specimen (e.g., capillary vs. venous, whole blood vs. plasma), glucose methodology (e.g., GDH-PQQ vs. glucose oxidase), and interfering substances of exogenous and endogenous origin (e.g., maltose, lipids, acetaminophen, uric acid).
GDH-PQQ methodology and cause of overestimated blood glucose readings in the presence of maltose
GDH-PQQ methodology involves the oxidation of glucose by glucose-dehydrogenase-pyrroloquinolinequinone and measurement of reaction end-products that are directly proportional to the glucose concentration; the reaction site is the free reducing group of the glucose molecule.18,19 Accordingly, as a 2-glucose molecule, and if present in sufficient concentration, maltose may yield overestimated blood glucose levels with GDH-PQQ methodology.3,18,19
Pharmacokinetics of maltose metabolism
The falsely increased GDH-PQQ blood glucose readings in Case 1 reflect both endogenous glycemia and maltosemia from the exogenous Octagam. Judging by differences between the GDH-PQQ and glucose oxidase blood glucose measurements, maltose clearance in this patient required 48 hr after completion of the last Octagam infusion. However, based solely on the data available, we cannot deduce the maltose half-life or pharmacokinetics because of his medical complications and the insulin dosing. Any pharmacokinetic inferences derived from his records would be confounded and not generalizable to other patients.
For Cases 2 through 7, we had no relevant pharmacokinetic data. Therefore, we could not assess the time period following maltose-containing immune globulin product administration during which falsely elevated GDH-PQQ blood glucose measurements would persist.
We were able to estimate maltose clearance from a literature report that involved individuals with normal renal function who received 10% maltose at infusion rates of 0.2 and 0.4 g/kg/hr over a 2-hr time period.20 On average, their serum maltose levels exceeded 0.9 mmol/L, which is the threshold for overestimation with GDH-PQQ blood glucose measurements, within 15 min of the start of the infusion, peaked at 2 hr, and decreased below the threshold level 6 hr following completion of the infusion. Extrapolating these data to patients receiving maltose-containing immune globulin products, time periods of ≥ 6 hr may be required for blood maltose concentrations to decrease below the threshold. However, maltose dose, infusion-related variables, and physiologic/metabolic factors could affect maltose peak blood concentrations, pharmacokinetics, and elimination rates. Thus, caution should be exercised in applying these estimated data to other patients.
FDA-approved maltose-containing immune globulin products and GDH-PQQ glucose testing systems
Table 1 lists the five FDA-licensed maltose-containing immune globulin products, their maltose concentrations, indications for use, and licensure dates. This list is believed current at this time but is subject to change if formulations are modified or as new products are developed, licensed, and marketed.
Table 1--FDA-licensed maltose-containing immune globulin products that may result in falsely increased glucose readings with GDH-PQQ glucose monitoring systemsa,b
Manufacturer |
Trade Name |
Proper Name |
Maltose Concentration (%) |
Licensed Indication(s) for Use |
Licensure Date |
|---|---|---|---|---|---|
|
Talecris Biotherapeutics |
Gamimune N 5% |
Immune Globulin Intravenous (Human) |
9-11 |
Primary humoral immunodeficiency, idiopathic thrombocytopenic purpura, bone marrow transplantation, pediatric HIV infection (14)
|
2/28/86 |
| Octapharma Pharmazeutika Produktionsges m.b.H. | Octagam | Immune Globulin Intravenous (Human) | 10 |
Primary immune deficiency diseases 1 | 5/21/04 |
| Cangene Corporation | WinRho SDF Liquid | Rh o (D) Immune Globulin Intravenous (Human) | 10 |
Immune thrombocytopenic purpura, suppression of Rh isoimmunization 55 | 3/31/05 |
| Cangene Corporation | (none) | Vaccinia Immune Globulin Intravenous (Human) | 10 |
Prevention and treatment of specified smallpox vaccine complications c,78 | 5/2/05 |
| Cangene Corporation | HepaGam B | Hepatitis Immune Globulin (Human) | 10 |
Acute exposure to blood containing HBsAg, perinatal exposure of infants born to HBsAg-positive mothers, sexual exposure to HBsAg-positive persons, household exposure to persons with acute HBV infection 56 | 1/27/06 |
| a This list is believed to be current at this time but is subject to change should the formulations of these products be modified or as new therapeutic products are developed, licensed/approved, and marketed. | |||||
| b Recommended doses of Gamimune N 5%, Octagam, and Vaccinia Immune Globulin (Human) would likely result in falsely elevated blood glucose readings with GDH-PQQ methodology. Calculations suggest that when WinRho SDF Liquid and HepaGam B are administered at the recommended doses, peak blood maltose concentrations are unlikely to interfere with GDH-PQQ blood glucose testing. However, off-label uses of WinRho SDF Liquid and HepaGam B at higher than recommended doses could result in blood maltose concentrations sufficient to yield overestimated GDH-PQQ blood glucose readings. | |||||
| c Eczema vaccinatum, progressive vaccinia, severe generalized vaccinia, vaccinia infections in individuals with skin conditions such as burns, impetigo, varicella-zoster, or poison ivy or eczematous skin lesions because of either the activity or extensiveness of such lesions; aberrant infections induced by vaccinia virus that include its accidental implantation in eyes (except in cases of isolated keratitis), mouth, or other areas where vaccinia infection would constitute a special hazard. | |||||
A comprehensive list of FDA-approved and U.S.-marketed GDH-PQQ blood glucose monitoring systems is not provided because any such list would be misleading. Multiple systems that are currently on the market are distributed under multiple trade and proper (generic) names. Regulations do not require manufacturers to notify FDA of the trade names under which approved systems are marketed. In addition, new systems continue to be introduced on an ongoing basis into this market, which has undergone enormous and sustained growth in recent years.39-41 Furthermore, manufacturers of GDH-PQQ systems currently on the market may subsequently change to non-GDH-PQQ methodology; however, those systems would remain on the previously published list of GDH-PQQ systems. Even if FDA could provide a list that were comprehensive and current at this time, the list would become increasingly out-of-date over time and, if used as a reference, would serve as a source of misinformation. Therefore, instead of relying on lists of GDH-PQQ systems, we urge healthcare providers and patients to refer to test strip professional package inserts or consult device or instrument manufacturers to confirm the glucose methodology in any system that is to be used for monitoring patients receiving maltose-containing immune globulin products.
Partial lists of GDH-PQQ blood glucose monitoring systems have been previously published.4,8,21,22 The list issued by Roche Diagnostics4 remains current at this time. However, the list published in the ISMP Medication Safety Alert8 contains incomplete and inaccurate information. Not all GDH-PQQ Accu-Chek systems (Roche Diagnostics) are listed. Precision Xtra Test Strips (Abbott Diagnostics, Alameda, CA) were incorrectly identified as GDH-PQQ methodology;8,9 the methodology is GDH-NAD.9,42 In addition, although correct at the time, Bayer HealthCare subsequently changed Ascensia Contour Test Strips (Ascensia Contour meter, Bayer HealthCare, Mishawaka, IN) to FAD-GDH methodology.24 (Although no longer distributed by the manufacturer, GDH-PQQ Ascensia Contour or Microfill Blood Glucose Test Strips may remain in use.) Even the list initially posted by FDA21,22 incorrectly listed GDH-NAD methodology as subject to blood glucose measurement overestimation in the presence of maltose.
Increasing use of point-of-care glucose meters in monitoring patient blood glucose levels
Strategies and protocols for tight glycemic control are being increasingly adopted, no doubt stimulated by diabetes standards and guidelines that have been adopted by the American Diabetes Association, American College of Endocrinology, American Association of Clinical Endocrinologists, and others who advocate tight glycemic control for patients.32,33,44-47,49,50 The intensive blood glucose monitoring required by these approaches has shifted reliance from clinical laboratory instruments to point-of-care devices for various reasons (e.g., convenience, turnaround time).32,33,38,39,44-46,50 Accordingly, glucose meters are increasingly prevalent in hospitals and other point-of-care settings.32,33,38,39,40,41,44-46,50
This targeting approach is expected to increase the prevalence of hypoglycemic events, which have been cited as the primary limitation of and major complication in diabetes management.32,33,44,47,49,50 Patients receiving maltose-containing immune globulin products whose blood glucose levels are monitored using GDH-PQQ systems are at even more risk than other diabetic patients for life-threatening or fatal hypoglycemia because overestimated GDH-PQQ blood glucose measurements may obscure recognition of insulin-induced hypoglycemia, delay medical intervention, and result in morbidity or mortality.
Incidence of insulin-induced hypoglycemia in response to falsely elevated blood glucose measurements
Why have so few reports of this potentially serious adverse event been received by FDA? Explanations could include a low incidence rate, lack of recognition when it occurs, or limited reporting to manufacturers or FDA. The frequency of this adverse event in the U.S. is unknown. FDA uses primarily passive surveillance in its MedWatch adverse event reporting system. Among the notable limitations inherent in MedWatch is underreporting of adverse events to both manufacturers and FDA, which is influenced by various factors (e.g., reliance on voluntary reporting, how long a product has been marketed, seriousness of adverse event).51-53 Although not examined specifically for MedWatch, serious adverse events that are reported to passive surveillance systems likely represents only a limited proportion of those that occur.51-53 Furthermore, we have no means to estimate the frequency with which this serious adverse event occurs but is unrecognized.
Also unknown is the number of patients treated with maltose-containing immune globulin products. Although FDA requires immune globulin product manufacturers to submit data about the quantity of product distributed in the U.S.,25 those data allow only estimates of the patients treated, given that dosage regimens vary by indication and patient weight. Without absolute numbers of both this adverse event and the patients treated with maltose-containing immune globulin products in the U.S., we cannot estimate the incidence rate (e.g., occurrences per 100,000 patients) or a reporting rate (e.g., reports per 1,000,000 prescriptions).
Recommended doses of Gamimune N 5% and Octagam would likely result in falsely increased readings by GDH-PQQ methodology (Table 1). Gamimune N 5% (Talecris Biotherapeutics, Research Triangle Park, NC) was licensed in the U.S. in 1986 (Table 1) and also marketed in other countries for years. Within the U.S., this product has not been manufactured since December 2005, and there is no in-date product in distribution.54 However, the product remains in distribution in other countries.54 Octagam was only FDA-approved in May 2004. However, Octagam was initially licensed in 1994 in Europe and is currently distributed in many other countries. Thus, both Gamimune N 5% and Octagam have been distributed worldwide over a significant enough time period that some patients who received these products would presumably have been diabetic, hospitalized, and subject to GDH-PQQ blood glucose monitoring. However, we are unaware of other related serious adverse events involving either of these products.
We have received no serious adverse event reports of this type for WinRho SDF Liquid, Vaccinia Immune Globulin (Human), or HepaGam B (Cangene Corporation, Winnipeg, MB, Canada), which were only licensed in the U.S. in 2005 and 2006 (Table 1). Although recommended doses of Vaccinia Immune Globulin (Human) would likely result in falsely elevated GDH-PQQ blood glucose readings, peak blood maltose concentrations of WinRho SDF Liquid and HepaGam B are unlikely to do so when administered at recommended doses. Off-label uses at higher than recommended doses could result in peak blood maltose concentrations sufficient to yield overestimated GDH-PQQ blood glucose readings, however.
The market distribution of blood glucose meters represents another factor that may influence the frequency of this adverse event. FDA has no precise market share figures to indicate what proportion of glucose meters in the U.S. market rely on each glucose methodology. Furthermore, FDA has no distribution data about which glucose monitoring system(s) predominate in hospitals and other point-of-care settings where patients would be likely to receive maltose-containing immune globulin products. Data that are representative of glucose monitoring system usage in these specific healthcare settings are further unavailable from FDA-accessible proprietary databases or from the market research report publishers and hospital-based consortia that we contacted during this investigation.
Regulatory and risk communication efforts
A healthcare provider from the hospital where the index case occurred was instrumental in the initial health alert publication in September 2005.8,9 Subsequent FDA initiatives included professional package insert revisions for the affected products;1 issuance of "Dear Healthcare Provider" letters4,23,57,58 in conjunction with those manufacturers; Internet health alert postings;21,22,59 an advisory committee presentation;60 a Web cast;61 and outreach to diabetes organizations, healthcare professionals, and patient advocacy groups. Those efforts, in turn, generated secondary risk communications.62-75
CONCLUSION
FDA encourages physicians, other health care professionals, and patients to submit adverse event reports, particularly for serious events, for any FDA-approved, -licensed, or -regulated product, including maltose-containing immune globulin and glucose meter products. Adverse events can be reported to manufacturers or distributors of these products, who are required by federal regulation to submit those to FDA.25,76 Contact information for manufacturers or distributors is generally available in professional package inserts or on manufacturer- or distributor-sponsored Web sites. Alternatively, adverse event reports can be submitted directly to FDA.77
Acknowledgments
The authors wish to particularly thank the health care providers who reported the index case to FDA. The authors would also like to express appreciation to Robert Wise, Office of Biostatistics and Epidemiology and Nisha Jain, Charles Maplethorpe, Office of Blood Research and Review, Center for Biologics Evaluation and Research, FDA and Steve Gutman, Courtney Harper, Office of InVitro Device Evaluation and Safety, Center for Devices and Radiologic Health, FDA for their review of this final report. We would also like to acknowledge the contributions of the following individuals who provided input and participated in various aspects of the investigation and risk communication efforts that resulted from the index case: Miles Braun, Robert Wise, Tina Khoie, Karen Lee, Office of Biostatistics and Epidemiology; Basil Golding, Toby Silverman, Charles Maplethorpe, Nannette Cagungun, John Finlayson (retired), Office of Blood Research and Review; Toni Stifano, formerly of Office of Compliance and Biologics Quality; Center for Biologics Evaluation and Research, FDA and Steve Gutman, Carol Benson, Alberto Gutierrez, Office of InVitro Device Evaluation and Safety; Quynh Hoang, Patricia Kingsley, Robert Fischer, Office of Surveillance and Biometrics; Matthew Tarosky, Office of Compliance; Mark Barnett, Office of the Center Director; Center for Devices and Radiologic Health; FDA.
References
- Octagam, Immune Globulin Intravenous (Human) 5% Solvent/Detergent Treated, Octapharma Pharmazeutika Produktionsges m.b.H., Vienna, Austria [professional package insert], March 2007.
- Roche. Accu-Chek Inform system [product detail]. Available at: http://www.roche-diagnostics.com/products_services/accuchek_inform.html (accessed: April 14, 2008).
- Accu-Chek Comfort Curve Test Strips, Roche Diagnostics, Indianapolis, IN, [professional package insert], 2004.
- Roche. Safety alert [ACCU-CHEK Blood Glucose Monitoring Systems - Reminder of Potential for Falsely Elevated Blood Glucose Readings due to Drug Interferences], September 7, 2006. Available at: http://www.accu-chek.com/en_US/pdf/06_205POC_MaltoseSafetyAlert.pdf (accessed: April 14, 2008).
- Medicines and Healthcare Products Regulatory Agency. Medical Device Alert. Available at: http://www.mhra.gov.uk/Publications/Safetywarnings/MedicalDeviceAlerts/CON2031807. July 19, 2007 (accessed: April 14, 2008).
- Kannan S, Rowland CH, Hockings GI, Tauchmann PM, Blackwell EA. Intragam can interfere with blood glucose monitoring [letter]. MJA. 2004; 180(5):251-252.
- Souza SP, Castro MCR, Rodriques RA, Passos RH, Ianhez LE. False hyperglycemia induced by polyvalent immunoglobulin [letter]. Transplantation. 2005, 80(4):542-543.
- ISMP Medication Safety Alert. Be aware of false glucose results with point-of-care testing. 2005, 10(18):13. Available at: www.ismp.org/Newsletters/acutecare/articles/20050908.asp?ptr=y (accessed: April 14, 2008).
- ISMP Errata re: Medication Safety Alert. Be aware of false glucose results with point-of-care testing. 2005, 10(19):3. Available at: www.ismp.org/Newsletters/acutecare/articles/20050908.asp?ptr=y (accessed: April 14, 2008).
- Australian Red Cross, CSL Bioplasma. Safety Alert: Intragam P, CMV Immunoglobulin, tetanus immunoglobulin (for intravenous use): interaction with capillary blood glucose measurements. December 23, 2003.
- Ochs HD, Fischer SH, Wedgwood RJ. Modified immune globulin: its use in the prophylactic treatment of patients with immune deficiency. J Clin Immunol. 1982; 2(2 Suppl):22S-30S.
- Ochs HD, Buckley RH, Pirofsky B, Fischer SH, Rousell RH, Anderson CJ, Wedgwood RJ. Safety and patient acceptability of intravenous immune globulin in 10% maltose. Lancet. 1980; 2(8205):1158-1159.
- McCue JP, Hein RH, Tenold R. Three generations of immunoglobulin G preparations for clinical use. Rev Infect Dis. 1986; 8(Suppl 4):S374-381.
- Gamimune N 5%, Immune Globulin Intravenous (Human), Talecris Biotherapeutics, Inc. Research Triangle Park, NC, [professional package insert], October 2005.
- Octagam, 4th generation intravenous immunoglobulin (IVIG), Octapharma, Ltd. [summary of the product characteristics], May 2005. Available at: http://217.160.163.235/content/04_plasma_products/01_product_information/02_octagam/01_octagam.php (accessed: August 16, 2006).
- TGA News. Australian Government, Department of Health and Ageing, Therapeutic Goods Administration. Interference between Extraneal Peritoneal Dialysis Solution and Advantage II Blood Glucose test strips for Accu-Chek and Accu-Trend blood glucose monitors; April 2005. Available at: www.tga.gov.au/docs/html/tganews/news46/iris.htm (accessed: April 14, 2008).
- Riley SG, Chess J, Donovan KL, Williams JD. Spurious hyperglycaemia and icodextrin in peritoneal dialysis fluid. BMJ. 2003; 327(7415):608-9.
- Wens R, Taminne M, Devriendt J, et al. A previously undescribed side effect of icodextrin overestimation of glycemia by glucose analyzer. Peritoneal Dialysis International. 1998; 18:603-609.
- Lage C. Effect of icodextrin on laboratory measurements. PDServeConnection. 2004; 8(2):1-3. Available at: http://www.pdserve.com/pdserve/pdserve.nsf/Content/PDServe+Connection (accessed: April 14, 2008).
- Motton G., Ricci F, Arienzo A, et al. Clearance of maltose administered at different infusion rates. It J Surg Sci. 1987; 17(2):153-159.
- FDA. MedWatch Safety Alert - FDA reminds healthcare professionals about falsely elevated glucose levels. November 9, 2005. Available at: http://www.fda.gov/medwatch/SAFETY/2005/safety05.htm#maltose and www.fda.gov/cdrh/oivd/news.html#110905 (accessed: April 14, 2008).
- FDA. MedWatch Safety Alert - Important safety information on interference with blood glucose measurement following use of parenteral maltose/parenteral galactose/oral xylose-containing products; November 9, 2005. Available at: http://www.fda.gov/medwatch/SAFETY/2005/safety05.htm#maltose and www.fda.gov/cber/safety/maltose110405.htm (accessed: April 14, 2008).
- Octapharma. Important Drug Warning [Octagam]; October 19, 2005. Available at: http://www.octapharma.com/USA/documents/FINAL%20Maltose%20WARNING%20Letter%2019-10-5.pdf (accessed: April 14, 2008).
- Ascensia Contour Blood Glucose Test Strips, Bayer HealthCare, Mishawaka, IN, [professional package insert], September 2006.
- Reporting of adverse experiences. Code of Federal Regulations. 2006 ed. Title 21, Pt. 600-799, §600.80-600.81, 16-20. Available at: http://ecfr.gpoaccess.gov/cgi/t/text/text-idx?sid=b87d41d6f6f60d08ce3462d1a200289f&c=ecfr&tpl=/ecfrbrowse/Title21/21cfrv7_02.tpl (accessed: April 14, 2008).
- FDA Adverse Event Reporting System, Rockville, MD, unpublished data.
- SYNCHRON System(s), Beckman Coulter, Brea, CA [chemistry information sheet: glucose]; June 3, 2007. Available at: http://www.beckmancoulter.com/customersupport/ifu/ifu_display_products.asp?lang=en&productline=DXC&acronym=GLUCm&system=DXC&&acronym_desc=GLUCM (accessed: April 14, 2008).
- Octagam, Normal Intravenous Immunoglobulin, Octapharma Ltd., Coventry, UK [summary of the product characteristics]. May 4, 2005. Available at: www.octapharma.com/documents/spc/Octagam_20050504_spc_840_88 71.pdf (accessed: April 14, 2008).
- Roche. Accu-Chek Advantage/Accu-Chek Sensor system [product detail]. Available at: http://www.roche-diagnostics.com/products_services/accuchek_advantage.html (accessed: September 13, 2007).
- Intragam P, Human normal immunoglobulin for intravenous administration, CSL Limited, Parkville 3052 VIC, Australia [consumer medicine information], August 2004.
- Thomas Schleis, Octapharma, personal communication, 8-22-06.
- Sacks DB, Bruns DE, Goldstein DE, et al. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Clin Chem. 2002, 48(3):436-472.
- Clement S, Braithwaite SS, Magee MF, et al. Management of diabetes and hyperglycemia in hospitals. Diabetes Care. 2004; 27:553-591.
- FDA. Center for Devices and Radiologic Health - Home healthcare medical devices: blood glucose meters; February 29, 2008. Available at: http://www.fda.gov/cdrh/cdrhhhc/bgm.html (accessed: April 14, 2008).
- FDA. Center for Devices and Radiologic Health - Home-Use Tests - Glucose; February 29, 2008. Available at: http://www.fda.gov/cdrh/oivd/homeuse-glucose.html (accessed: April 14, 2008).
- Lock JP, Szuts EZ, Malomo KJ, Anagnostopoulos A. Whole-blood glucose testing at alternate sites. Diabetes Care. 2002; 25(2):337-341.
- Diabetes Insight. Controlling blood glucose - blood glucose testing; March 4, 2001. Available at: http://www.diabetic.org.uk/lwd/management/control/DI_bg_testing.asp (accessed: September 13, 2007).
- Tang Z, Du X, Louie RF, et al. Effects of drugs on glucose measurements with handheld glucose meters and a portable glucose analyzer. Am J Clin Pathol. 2000; 113:75-86.
- Lewandrowski K, Lee-Lewandrowski E. Capillary blood glucose testing at the point of care-clinical applications and the evaluation of diagnostic technologies. Business Briefing: Global Healthcare. 2002; 3:76-79. Available at: www.wma.net/e/publications/pdf/2002/lewandrowski.pdf (accessed: April 14, 2008).
- Enterprise Analysis Corporation. 2004 U.S. Hospital Point-of-Care Blood Glucose Survey; November 2004. Excerpt available at: http://www.researchandmarkets.com/reportinfo.asp?report_id=295447 (accessed: April 14, 2008).
- TriMark Publications. Blood Glucose Testing and Diabetes Management; August 16, 2006. Excerpt available at: http://trimarkpublications.ecnext.com/coms2/summary_0230-4461_ITM (accessed: April 14, 2008).
- Precision Xtra Blood Glucose Test Strips with TrueMeasure Technology, Abbott Diagnostics Care, Alameda, CA, [professional package insert], June 2005.
- Ascensia Contour or Microfill Blood Glucose Test Strips, Bayer HealthCare, Mishawaka, IN, [professional package insert], September 2005.
- American Diabetes Association. Standards of medical care in diabetes-2006. Diabetes Care. 2006, 29(S1):S29-S32.
- Ogedegbe HO. Diabetes mellitus: a clinical laboratory perspective. Labmedicine. 2006, 37(5):292-297.
- Van Den Berghe G, Wouters P, Weekes F, et al. Intensive insulin therapy in the critically ill patient. N Engl J Med. 2001; 345:1359-1367.
- American College of Endocrinology and American Diabetes Association. Consensus Statement on Inpatient Diabetes and Glycemic Control. Diabetes Care. 2006; 29(8):1955-1962 and Endocr Pract. 2006; 12(4):458-68. Also available at: http://www.aace.com/meetings/consensus/IIDC/IDGC0731.pdf (accessed April 14, 2008).
- Patel AH, Pittas AG. Does glycemic control with insulin therapy play a role for critically ill patients in hospital? CMAJ. 2006, 174(7):917-918.
- Pittas AG, Siegel RD, Lau J. Insulin therapy for critically ill hospitalized patients, a meta-analysis of randomized controlled trials. Arch Intern Med. 2004; 164:2005-2011.
- American College of Endocrinology. Position statement on inpatient diabetes and metabolic control. Endocr Pract. 2004; 10(Suppl 2):4-9.
- Bortnichak EA, Dai WS. Epidemiologists and adverse event data-a challenge to the field. Pharmacoepidemiol Drug Saf. 1999; 8:457-461.
- Goldman SA. Limitations and strengths of spontaneous reports data. Clin Ther. 1998; 20(suppl C):C40-C44.
- Trontell AE. How the US Food and Drug Administration defines and detects adverse drug events. Curr Ther Res. 2001; 62:641-649.
- Robin Huff, Talecris Biotherapeutics, personal communication, 8-15-06.
- WinRho SDF Liquid, Rho(D) Immune Globulin Intravenous (Human), Cangene Corporation, Winnipeg, MB, Canada R3T 5Y3 [professional package insert], April 2006.
- HepaGam B, Hepatitis Immune Globulin (Human), Cangene Corporation, Winnipeg, MB, Canada R3T 5Y3 [professional package insert], April 2007.
- Baxter Healthcare Corporation and Cangene Corporation. Important Drug Warning [WinRho SDF Liquid; letter], December 5, 2005.
- Bayer HealthCare, Important Drug Warning [Gamimune N 5%; letter], November 8, 2005.
- FDA reminders for falsely elevated glucose readings from use of inappropriate test method; November 9, 2005. Available at: http://www.fda.gov/cdrh/oivd/news/glucosefalse.html (accessed: April 14, 2008).
- FDA. Serious adverse effects following falsely elevated glucose measurements resulting from administration of an IGIV product containing maltose, Blood Products Advisory Committee [transcript]; November 4, 2005. Available at: http://www.fda.gov/ohrms/dockets/ac/05/transcripts/2005-4190t2.rtf (accessed: April 14, 2008).
- FDA Patient Safety News. Avoiding glucose monitoring errors in patients receiving other sugars; February 1, 2006. Available at: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/psn/transcript.cfm?show=48#4 (accessed: April 14, 2008).
- Beckley ET. FDA issues glucose meter safety warning. DOC News. 2006, 3(1):7.
- eMedicineHealth - Consumer First Aid and Health Information. False elevations of glucose levels with Maltose, galactose, or xylose [drug recalls and alerts - 2005]. Available at: http://www.emedicinehealth.com/script/main/art.asp?articlekey=60270&page=4, November 9, 2005 (accessed: April 14, 2008).
- Maltose, Galactose, and Xylose May Yield Falsely Elevated Glucose Readings. Diabetes in Control Newsletter. 2005; 286. Available at: http://www.diabetesincontrol.com/modules.php?name=News&file=article&sid=3258 (accessed: April 14, 2008).
- Florida Hospital Clinical Laboratories and Florida Pathology Lab. Alert: the Accuchek Inform meters used for bedside glucose testing at Florida Hospital are affected by sugars other than glucose and falsely elevated results may occur. Laboratory Lines. 2006; 11.1. www.floridapathology.com/LabLines/Lab_Lines_Feb_2006.pdf. (accessed: April 14, 2008).
- National Guideline Clearinghouse. FDA WARNING/REGULATORY ALERT. Available at: www.guidelines.gov/summary/word.aspx?doc_id=3612&stat=1&string= - Supplemental Result (accessed: April 14, 2008).
- University of California San Francisco. Department of Laboratory Medicine. WARNING - SPURIOUS BLOOD GLUCOSE VALUES; April 2006. Available at: http://labmed.ucsf.edu/labmanual/mftlng-mtzn/tips/labupdates.html (accessed: April 14, 2008).
- Drug Information Service, University of Utah Hospitals & Clinics. Falsely Elevated Glucose Results With Galactose, Maltose, and Xylose Products; November 14, 2005. Available at: http://uuhsc.utah.edu/pharmacy/alerts/122.html (accessed: April 14, 2008).
- Agency for Health Care Administration. Important Safety Information on Interference With Blood Glucose Measurement Following Use of Parenteral Maltose/Parenteral Galactose/Oral Xylose-Containing Products; August 17, 2006. Available at: http://ahca.myflorida.com/blood_glucose_safety_info.shtml (accessed: April 14, 2008).
- Nursing Center. Beware of falsely elevated glucose readings. Nursing Critical Care. 2006; 1(2):54-55. Available at: http://www.nursingcenter.com/library/journalarticleprint.asp?Article_ID=645197 (accessed: September 13, 2007).
- Bureau of Laboratory Improvement, Utah Department of Health. Safety. UDOH Laboratory Bulletin. February 2006. Available at: http://health.utah.gov/lab/labimp. (accessed: August 9, 2006).
- American Society of Health-System Pharmacists. Check Glucose Testing Method, FDA Urges. Available at: http://www.ashp.org/news/ShowArticle.cfm?id=13216 (accessed: April 14, 2008).
- Prescription Solutions. MedWatch Drug Safety Alerts [11-9-2005]. Available at: http://www.rxsolutions.com/c/rxnews/rxnews_view.asp?Article=641&type=18 (accessed: April 14, 2008).
- American Diabetes Association. Managing Your Blood Glucose-Possible Interference with Blood Glucose Measurements from Certain Medical Products. Available at: http://www.diabetes.org/type-1-diabetes/blood-glucose.jsp (accessed: April 14, 2008).
- About.com. Interference With Blood Glucose Measurement After Use of Parenteral Products. Available at: http://diabetes.about.com/b/a/220034.htm. Accessed: August 17, 2005.
- Medical Device Reporting. Code of Federal Regulations. 2006 ed. Title 21, Pt. 800-1299, §803.10, 43-44. Available at: http://ecfr.gpoaccess.gov/cgi/t/text/text-idx?sid=b87d41d6f6f60d08ce3462d1a200289f&c=ecfr&tpl=/ecfrbrowse/Title21/21cfrv8_02.tpl (accessed: April 14, 2008).
- FDA. Reporting Adverse Experiences to FDA; October 20, 2006. Available at: http://www.fda.gov/medwatch/how.htm (accessed: April 14, 2008).
- Vaccinia Immune Globulin (Human). Cangene Corporation, Winnipeg, MB, Canada R3T 5Y3 [professional package insert], January 4, 2006.


