|
 |
 |
 |
 |
FDA Home Page | CBER A-Z Index | CBER Search | Contact CBER | CBER Home Page |
||||
This guidance describes a nonclinical testing strategy for assessing the potential of a test substance to delay ventricular repolarization. The guidance includes information concerning nonclinical assays and integrated risk assessments. The assessment of the effects of pharmaceuticals on ventricular repolarization and proarrhythmic risk is the subject of active investigation. When additional data (nonclinical and clinical) are accumulated in the future, they will be evaluated and this guidance might be revised. FDA's guidance documents, including this guidance, do not establish legally enforceable responsibilities. Instead, guidances describe the Agency's current thinking on a topic and should be viewed only as recommendations, unless specific regulatory or statutory requirements are cited. The use of the word should in Agency guidances means that something is suggested or recommended, but not required.
The QT interval (time from the beginning of the QRS complex to the end of the T wave) of the electrocardiogram (ECG) is a measure of the duration of ventricular depolarization and repolarization. QT interval prolongation can be congenital or acquired (e.g., pharmaceutical-induced). When ventricular repolarization is delayed and the QT interval is prolonged, there is an increased risk of ventricular tachyarrhythmia, including torsade de pointes, particularly when combined with other risk factors (e.g., hypokalemia, structural heart disease, bradycardia). Thus, much emphasis has been placed on the potential proarrhythmic effects of pharmaceuticals that are associated with QT interval prolongation. Ventricular repolarization, determined by the duration of the cardiac action potential, is a complex physiological process. It is the net result of the activities of many membrane ion channels and transporters. Under physiological conditions, the functions of these ion channels and transporters are highly interdependent. The activity of each ion channel or transporter is affected by multiple factors including, but not limited to, intracellular and extracellular ion concentrations, membrane potential, cell-to-cell electrical coupling, heart rate, and autonomic nervous system activity. The metabolic state (e.g., acid-base balance) and location and type of cardiac cell are also important. The human ventricular action potential consists of five sequential phases:
Prolongation of the action potential can result from decreased inactivation of the inward Na+ or Ca2+ currents, increased activation of the Ca2+ current, or inhibition of one or more of the outward K+ currents. The rapidly and slowly activating components of the delayed rectifier potassium current, IKr and IKs, seem to have the most influential role in determining the duration of the action potential and thus the QT interval. The human ether-a-go-go-related gene (hERG) and KvLQT1 gene encode pore-forming proteins KCNH2 and KCNQ1 that are thought to represent the α-subunits of the human potassium channels responsible for IKr and IKs, respectively. These α-subunit proteins can form hetero-oligomeric complexes with auxiliary β-subunits (i.e. MiRP and MinK gene products), which have been speculated to modulate the gating properties of the channel proteins. The most common mechanism of QT interval prolongation by pharmaceuticals is inhibition of the delayed rectifier potassium channel that is responsible for IKr.
This guidance extends and complements the ICH guidance on S7A Safety Pharmacology Studies for Human Pharmaceuticals. This guidance applies to new chemical entities for human use and marketed pharmaceuticals when appropriate (e.g., when adverse clinical events, a new patient population, or a new route of administration raises concerns not previously addressed). Conditions under which studies are not called for are described in ICH S7A.
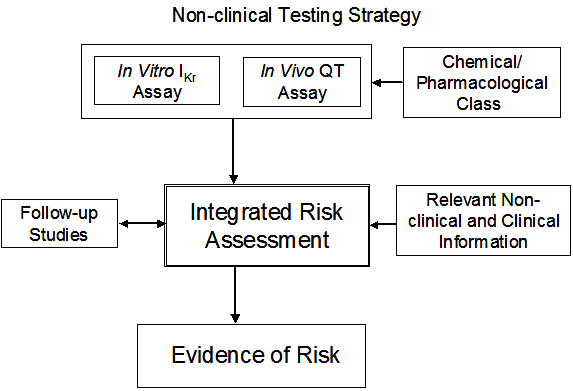
Principles and recommendations described in ICH S7A also apply to the studies conducted in accordance with the present guidance. In vitro IKr and in vivo QT assays described in sections II.C.1 and C.2 (2.3.1 and 2.3.2) when performed for regulatory submission should be conducted in compliance with good laboratory practice (GLP). Follow-up studies described in section II.C.5 (2.3.5) should be conducted in compliance with GLP to the greatest extent feasible. In vitro and in vivo assays are complementary approaches; therefore, according to current understanding, both assay types should be conducted. The investigational approach and evidence of risk should be individualized for the test substance, depending on its pharmacodynamic, pharmacokinetic, and safety profiles.
The objectives of studies are to: (1) identify the potential of a test substance and its metabolites to delay ventricular repolarization, and (2) relate the extent of delayed ventricular repolarization to the concentrations of a test substance and its metabolites. The study results can be used to elucidate the mechanism of action and, when considered with other information, estimate risk for delayed ventricular repolarization and QT interval prolongation in humans.
Nonclinical methodologies can address the following:
As indicated above, these four functional levels can be investigated by in vitro and/or in vivo methods. Findings from the functional levels listed above are considered useful and complementary. In vitro electrophysiology studies can explore potential cellular mechanisms that might not be evident from in vivo data. Changes in other cardiovascular parameters or effects on multiple ion channels can complicate interpretation of data. Complementary assessments in other systems can address this issue. Although delay of repolarization can occur through modulation of several types of ion channels, inhibition of IKr is the most common mechanism responsible for pharmaceutical-induced prolongation of QT interval in humans. In vivo models that possess the full complement of molecular, biochemical, and physiological systems can also be informative with regard to the response in humans to the test substance. Carefully designed and conducted in vivo studies allow evaluation of parent substance and metabolites and can enable estimation of safety margins. In vivo ECG evaluations provide information on conduction properties and noncardiac influences (e.g., autonomic nervous system tone). Studies of action potential parameters provide information on the integrated activity of multiple ion channels in the heart.
The following sections describe a general nonclinical testing strategy for assessing risk for delayed ventricular repolarization and QT interval prolongation that is pragmatic and based on currently available information. The figure illustrates the component elements of the testing strategy, but not specific test systems or their designs.
Conduct of S7B nonclinical studies assessing the risk for delayed ventricular repolarization and QT interval prolongation prior to first administration in humans should be considered. These results, as part of an integrated risk assessment, can support the planning and interpretation of subsequent clinical studies.
Considerations for Test Systems (3.1) This section provides an overview of methodologies currently used to assess the potential for a test substance to delay ventricular repolarization and to prolong QT interval. The following should be considered in selecting the most appropriate test systems.
A sub-maximally effective concentration of a positive control substance should be used to demonstrate the responsiveness of in vitro preparations for ion channel and action potential duration assays and should be included in every study. In the case of in vivo studies, positive control substances should be used to validate and define the sensitivity of the test system, but need not be included in every study. For test substances belonging to a chemical/pharmacological class that is associated with QT interval prolongation in humans, the use of concurrent reference compound(s) (member(s) of the same class) in in vitro and in vivo studies should be considered to facilitate ranking the potency of the test substance in relation to its comparators.
In vitro electrophysiology studies can provide valuable information concerning the effect of a test substance on action potential duration and/or cardiac ionic currents. These assays have an important role in assessing the potential for QT interval prolongation and elucidating cellular mechanisms affecting repolarization. In vitro electrophysiology studies employ either single cell (e.g., heterologous expression systems, disaggregated cardiomyocytes) or multicellular (e.g., Purkinje fiber, papillary muscle, trabeculae, perfused myocardium, intact heart) preparations. Heterologous expression systems, where human ion channel protein(s) are expressed in noncardiac cell lines, are used to assess the effects of a test substance on a specific ion channel. Disaggregated myocytes are technically more challenging than the expression systems but have the advantage of being suitable for assessing effects on both action potential duration and ionic currents. Although single cell preparations are more fragile, they minimize diffusional barriers to the site of action. Multicellular preparations are stable test systems to study action potential duration. The analysis of parameters for each phase of the action potential such as Vmax for phase 0 (INa), APD30 or APD40 for phase 2 (ICa) and "triangulation" for phase 3 (IK) can be useful to investigate the effects on specific channels responsible for these phases. In addition, some parameters derived from the Langendorff preparation have been reported to provide information regarding proarrhythmic risk. Tissue and cell preparations for in vitro assays are obtained from different laboratory animal species including rabbit, ferret, guinea pig, dog, swine, and occasionally from humans. The ionic mechanisms of repolarization in adult rats and mice differ from larger species, including humans (the primary ion currents controlling repolarization in adult rats and mice is Ito); therefore, use of tissues from these species is not considered appropriate. Species differences in terms of which cardiac ion channels contribute to cardiac repolarization and to the duration of the action potential should be considered in selecting a test system. When native cardiac tissues or cells are used, the characteristics and source of the preparation should be considered because the distribution of ion channel types varies according to the region and type of cell. Test substance concentrations for in vitro studies should span a broad range, covering and exceeding the anticipated maximal therapeutic plasma concentration. Ascending concentrations should be tested until a concentration-response curve has been characterized or physicochemical effects become concentration-limiting. Ideally, the duration of exposure should be sufficient to obtain steady-state electrophysiological effects, unless precluded by the viability of the cell or tissue preparation. The duration of exposure should be indicated. Appropriate positive control substances should be used to establish the sensitivity of the in vitro assay system. Factors that can confound or limit the interpretation of in vitro electrophysiology studies include the following:
New technologies for potassium channel assays are being developed. Novel ion channel activity assays can be useful in preliminary screening of test substances to identify lead candidates. It is important to demonstrate concordance between conventional and new technologies before adopting new technologies for regulatory purposes. Competition binding protocols, in which test substances are studied for their ability to displace a radiolabeled hERG channel blocker from a cell line expressing hERG, are used. However, competition for radioligand-binding sites provides no information on agonistic or antagonistic effects of the test substance on IKr. Moreover, this assay will not identify test substances that bind to hERG at sites other than the radioligand binding sites. Based upon these potential limitations, this assay is not considered a substitute for voltage clamp assays described above.
Intact animal models allow investigation of ventricular repolarization or associated arrhythmias where integrated effects on the full complement of ion channel and cell types are assessed. Also, potential neuronal and hormonal influences on the pharmacodynamic effect of the pharmaceuticals are present in animals. The QT interval of the ECG is the most commonly used endpoint to gauge effects of a test substance on ventricular repolarization. In specialized electrophysiology studies, information regarding the ventricular repolarization (e.g., monophasic action potential duration and effective refractory period) can also be obtained from in vivo models. Additional safety parameters of interest, including blood pressure, heart rate, PR interval, QRS duration, and arrhythmias, can be assessed simultaneously. The QT interval and heart rate have an inverse, nonlinear relationship, which varies among species and between animals within a species. Thus, a change in heart rate exerts an effect on QT interval, which can confound the assessment of the effect of the test substance on ventricular repolarization and the QT interval. There are two important situations where there is variability in heart rate among animals: one is due to difference in autonomic tone, and the other is due to effects of test substances on heart rate. Therefore, the interpretation of data from in vivo test systems should take into account the effect of coincident changes in heart rate. Ideally, QT interval data obtained after administration of a test substance should be compared with control and baseline data at similar heart rates. When the heart rate variability is not due to the test substance, it can be reduced by acclimatization, or the use of anesthetized animal models. When the effects are due to a test substance, the most common approach is to correct the QT interval for heart rate (QTc) using formulae such as Bazett or Fridericia. The choice of heart rate correction formula should be justified with data from the test system. When differences in heart rate between treatment and control are large, the correction formulae may not be effective for assessing risk of QT interval prolongation. An alternative approach is to maintain a constant heart rate using cardiac pacing. An analysis of QT/RR relationship, including correction of the QT interval using formulae for individual animals, may be more appropriate. Laboratory animal species used for in vivo electrophysiology studies include dog, monkey, swine, rabbit, ferret, and guinea pig. The ionic mechanisms of repolarization in adult rats and mice differ from larger species, including humans (the primary ion currents controlling repolarization in adult rats and mice is Ito); therefore, use of these species is not considered appropriate. The most appropriate in vivo test systems and species should be selected and justified. The dose range should be in accord with that discussed in ICH S7A and, whenever feasible, should include and exceed the anticipated human exposure. The dose range can be limited by animal intolerance to the test substance (e.g., emesis, tremor, or hyperactivity). For studies designed to relate the extent of delayed ventricular repolarization to concentrations of the parent test substance and its metabolites, controlled exposure via constant intravenous infusion can be used. Monitoring exposure to the test substance and metabolites (see ICH S3A) provides opportunities to interpret dose- and concentration-response data and to design follow-up studies, if appropriate. Factors that should be considered in conducting studies and interpreting the results include the following:
The precise relationship between test substance-induced delay of ventricular repolarization and risk of proarrhythmia is not known. Directly assessing the proarrhythmic risk of pharmaceuticals that prolong the QT interval would be a logical undertaking. Indices of proarrhythmic activity (e.g., electrical instability, temporal and/or spatial dispersion of refractoriness, reverse use-dependency, changes in action potential configuration) and animal models might have utility in assessing proarrhythmia. Interested parties are encouraged to develop these models and test their usefulness in predicting risk in humans.
1 This guidance was developed within the Expert Working Group (Safety) of the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) and has been subject to consultation by the regulatory parties, in accordance with the ICH process. This document has been endorsed by the ICH Steering Committee at Step 4 of the ICH process, May 2005. At Step 4 of the process, the final draft is recommended for adoption to the regulatory bodies of the European Union, Japan, and the United States. 2 Arabic numbers reflect the organizational breakdown in the document endorsed by the ICH Steering Committee at Step 4 of the ICH process, May 2005.
|