
Frequently asked questions about the NIDA Center for Genetic Studies
Q1. What is the NIDA Center for Genetic Studies (NCGS)?
A1. The NCGS is a NIDA-funded scientific resource for informing the human molecular genetics of drug addiction. The NCGS stores clinical data, diagnostic data, pedigree information, and biomaterials (including DNA, plasma, cryopreserved lymphocytes, and/or cell lines) from human subjects participating in studies that form the NIDA Genetics Consortium (NGC). The resource is for all investigators who have fulfilled the requirements for membership and agree to become members of the NGC. For more information about the NGC, please see FAQs about the NGC. For information about each study in the NGC, please see http://zork.wustl.edu. Click the NIDA icon, select public information, and then click on study information.
Q2. Will the data stored at the NCGS be made available to the scientific community?
A2. Yes. Data stored at the NCGS will be made available for sharing to the scientific community. Membership in the NGC is not required to access clinical data and DNA from the NCGS. The data will be made available to researchers other than the Principal Investigator either 18 months after the completion of the funding period for which the samples were obtained initially, upon publication, or through early release as defined by the principal investigator. Those researchers requesting access to data stored in the NCGS will go through an application procedure to assure appropriate use and reporting of the data [see Q5]. The sharing of data in the NCGS is done in strict accordance with the informed consent provided on each research subject.
Q3. What are the services of the NCGS?
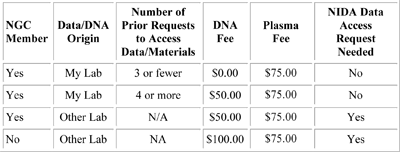
A3. The NCGS creates high-quality DNA, plasma (if consented), and cryopreserved lymphocytes (CPLs) on all samples submitted by NGC members. If samples do not have sufficient lymphocytes for cryopreservation, the sample will be made into an Epstein-Barr virus transformed cell line (from which DNA is extracted and stored). If DNA quantities become low, the CPLs are thawed and transformed into cell lines. The NCGS also provides a mechanism by which all databases containing clinical information and biomaterials (DNA) can be widely searched or distributed to qualified investigators in the scientific community. The services of the NCGS for shipping blood to Rutgers, processing the sample, producing cell lines at Rutgers if DNA is depleted, and storing clinical data are free to members of the NGC. Requests to access stored DNA samples (at 10 µg aliquots) may require a nominal fee depending on NGC membership status , the number of prior requests, and type of biomaterial being requested. Please refer to the fee structure below.
NO CELL LINE AVAILABLE:
CELL LINE AVAILABLE:
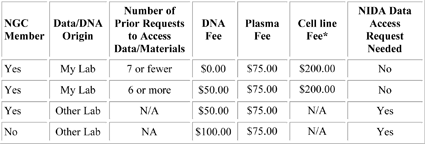
* The NIDA contract allows for cell lines to be made from the cryopreserved lymphocytes that are stored upon receipt of blood samples if the initial DNA stored from that sample has been depleted. If the DNA has not been depleted, but you would like to request that a cell line be made, you may do so directly through the Repository. This service will not be covered by the NIDA contract, but the Repository can invoice you directly. Further, the NIDA contract does not cover cell line distribution. Rutgers will invoice you directly if you request that cell lines be sent to you.
Q4. How may investigators gain access to submit samples to the NGC?
A4. The purpose of the NIDA contract that supports the NCGS is to provide funds for the processing of samples and storing them in the Repository for a variety of drug addiction research. The Repository has a limited budget and a maximum capacity, therefore some proposals may not be accepted into the Repository. If, for budgetary or capacity reasons the contract cannot cover samples to be place in the Repository, it is advised that the PI plan to include the addition of their samples into the Repository as if it were a sub-contract to Rutgers. If the PI includes this alternative method in the grant application, then the samples will be covered either by the contract or the grant if it is awarded. If the NIDA contract can cover the costs and has the capacity, then the budget allocated for that activity in the grant application will be administratively cut.
In the grant application, appropriate language could be, "We plan to request access to the NIDA Center for Genetic Studies for submitting our samples. If the study and the samples are approved, the NIDA contract will provide funds for receiving, processing, storing, and distributing the samples. If the study and samples are not approved, we provide budgetary justification for submitting the samples to the NIDA Repository using funds received through this grant application."
Q5. How may investigators gain access to data stored at the NCGS?
A5. Investigators must submit a NIDA Genetic Data Access Request to the NIDA Genetic Data Access Request Committee. Requests should consist of one original plus one copy of the following:
- Cover Letter. A cover letter containing the name, mailing address, e-mail address, fax number, and telephone number of the applicant principal investigator. This letter should be written on the letterhead of the sponsoring institution at which the research is or will be conducted.
- Biographical Sketches. The biographical sketches of the applicant principal investigator and all co-investigators (NIH format).
- Resources and Environment (NIH format).
- Full Disclosure of Funding. Declaration of all sources of public and private funding, periods of support, active/pending status, and relationships with any public or private agency conducting work related to the proposed research. In lieu of the above information, the "Other Support" pages from a NIH format application may be attached.
- Study Description. A description of the study design that includes the following sections:
- Abstract. Summarize the study in no longer than one-half page.
- Specific Aims. Clearly state the hypotheses to be tested.
- Background and Significance. Justify that the hypotheses are of interest to the scientific community and that the resources of the NCGS are essential to the study.
- Method. Provide a clear description, justification, and timetable of the research to be conducted including the DNA (number and amount) and data requirements, the markers to be used and the general methods of analysis. In addition, present evidence of a clear understanding of the limitations, strengths, and feasibility of using the dataset to address the proposed study aim(s).
- Statistical Analysis. Indicate which analytical techniques will be applied, including, when relevant, power calculations to justify the amount of DNA requested.
- Statement of Public Health Good. Identify the public health benefits and risks of the proposed research, and describe how the findings from the proposed research would be used to promote public health.
- Dissemination of Research Findings. Clearly specify the plans to disseminate findings from the proposed research.
- Human Subject Approval. Applicants should provide documentation of IRB review and either approval of the proposed project or exemption of the proposed project from the need for further IRB review, according to the requirements of the Department of Health & Human Services Office for Human Research Protections (OHRP). Although IRB approval will not be required at the time the Access Request is submitted, neither data nor DNA can be released until evidence of IRB approval has been received. Applicants should indicate to their IRB that all data and materials in the repository were stripped of all personal identifiers before they were submitted to the repository, such that no identifying information is associated with the samples.
- A completed Distribution Agreement. See the following web page:
http://www.nida.nih.gov/about/organization/genetics/DistPol.html
- A completed List of Requested DNA and Associated Data. See the bottom of the following web page:
http://www.nida.nih.gov/about/organization/genetics/GeneticDistPol.html
Q6. Where should NIDA Genetic Data Access Requests be sent?
A6. Requests should be sent to:
Joni L. Rutter, PhD
Chair, NIDA Genetic Data Access Request Committee
NIH/NIDA
Genetics and Molecular Neurobiology Research Branch
Division of Neuroscience and Behavioral Research
6001 Executive Blvd.
Rm 5227, MSC 9555
Bethesda, MD 20892-9555
Rockville, Maryland 20852 (for courier/overnight mail service)
Phone: 301-443-1887
Fax: 301-594-6043
E-Mail: jrutter@mail.nih.gov
Q7. What is the NIDA Genetic Data Access Request Committee?
A7. The NIDA Genetic Data Access Request Committee, selected by NIDA staff, is a panel composed of one bioethicist and four other individuals with expertise in one or more areas including human genetics, genetics of complex traits, statistical analysis, molecular genetics and drug addiction research. NIH Program and Review staff members are excluded from membership on the Committee. The Committee has several charges:
For requests to join the NGC and submit samples to the NCGS:
- Does the investigator have a funded grant to cover the sample collection?
- Does the study fill a scientific gap area?
- Is there sufficient power within the sample for the research question to be addressed?
- Is the NIDA contract for the Repository able to cover the costs to add the samples?
- Should the samples/study be granted access to the Repository resource? If yes, should this study take high priority?
For requests to access samples/data:
- Is investigator qualified? (see below)
- Does the investigator have funding for the work proposed?
- Is there an appropriate plan for depositing the data obtained back into the Repository?
Can investigator address research question with samples requested?
The following criteria will be used to determine whether an investigator is qualified:
- Experience and qualifications of the applicant principal investigator and co-investigator(s)
- Adequacy of the research environment(s)
- Ethical considerations
- Applicant principal investigator's familiarity with the characteristics, limitations, and strengths of the dataset
- Adequacy of proposed research design, statistical methods, and timetable of proposed project
- Adequacy of the applicant principal investigator's funding resources to support the proposed study
- Benefit to public health
Q8. What happens after Access Requests are approved?
A8. After the NIDA Genetic Data Access Request has been granted approval, an electronic data file, documentation, and pedigree drawings will be sent to the applicant principal investigator. Based upon the information on these forms, the principal investigator must e-mail or fax a list comprised of the identification number for each subject for which DNA is requested. This list must be submitted to the Secretary of the NIDA Genetics Access Request Committee. This list will be forwarded to the NCGS, which will contact the applicant principal investigator regarding shipping and payment. Payment by check or wire transfer is required before samples are processed for shipment.
Q9. I am a member of the NGC and have contributed data to the NCGS. Do I need to submit a NIDA Genetic Data Access Request to receive my own clinical data and biomaterials?
A9. No. You need only to provide a written or email request to the RUCDR web portal if you wish to access samples that you provided. Contact Dr. Joni Rutter (jrutter@mail.nih.gov) for more information. In the written request you should provide the number of samples, the amount of DNA requested, and provide the identification number for each subject for which DNA is requested. For payment, please refer to the fee structure (above).
Q10. I am a member of the NGC and have contributed data to the NCGS, but I wish to access materials provided by another investigator. Do I need to submit a NIDA Genetic Data Access Request?
A10. Yes. If you would like access to available samples provided by another investigator, you will need to submit a NIDA Genetic Data Access Request form to Dr. Joni Rutter (jrutter@mail.nih.gov) for expedited review. For payment, please refer to the fee structure (above).
Q11. I am NOT a member of the NGC and I have NOT provided data to the NCGS. What is the procedure and cost of accessing the data stored at the NCGS?
A11. Investigators seeking access to the data that they did not contribute to the NCGS must gain access through the NIDA Genetic Data Access Request Committee. For payment, please refer to the fee structure (above).
Q12. How can I find out what studies are participating in the NGC and contributing clinical data and biomaterials to the NCGS?
A12. This information can be found at the NCGS website, which is maintained at Washington University (http://zork.wustl.edu/). Click on the NIDA icon, select public information, and then click on study information.
Q13. I have been told that investigators belonging to the NGC used nested informed consents in which research participants agreed to different levels of access to data stored at the NCGS. Is this true and how does this affect access to data stored at the NCGS?
A13. NIDA and the NCGS must abide by and implement the wishes of research participants as indicated in their nested informed consents. These nested consent forms vary from study to study, but typically include three levels of consent. These levels of consent may restrict access to data to the scope of the original study, to addiction and related medical disorders, or to any genetics study. NIDA will provide the access status of the samples on the NCGS website (http://zork.wustl.edu/).
Q14. How soon can qualified scientists from around the world gain access to data stored at the NCGS?
A14. Access to DNA and anonymized clinical data is dependent upon the data sharing plan of the submitting PI. In general, access can occur if one of the following conditions are met: either18 months after the ending date of the grant period for the first competitive award (the proprietary period), upon initial publication of the sample set, or upon early release as defined by the investigator. Requests for access will start sometime in 2006. For information on availability of samples, the NCGS website (http://zork.wustl.edu/).
Q15. How can I get access sooner than the end of the proprietary period?
A15. You can access the data stored in the NCGS sooner than the end of the proprietary period through collaboration or agreement with the PI by obtaining written approval from the principal investigator who originally submitted the data to the NCGS. This letter must be countersigned by the business official at the principal investigator's institution. The requester must have IRB approval or a waiver of IRB approval from the local IRB to analyze the data. Once written approval has been obtained, a NIDA Access Data Request must be submitted as described above.
Q16. To gain access to data stored at the NCGS, must the Recipient have IRB approval?
A16. Yes. As stated above, applicants should provide documentation of IRB review and either approval of the proposed project or exemption of the proposed project from the need for further IRB review. The IRB approval will not be required at the time the Access Request is submitted. However, no data can be released from the NCGS until evidence of IRB approval has been received.
Q17. Must the Recipient follow the conditions for use of Biomaterials, Clinical Data, and Genetic Analysis Data as approved by the Institutional Review Board (IRB) of their own institution and the IRB of the NIDA contractor and subcontractor that operate the NCGS?
A17. The Recipient must follow the conditions for use of Biomaterials, Clinical Data, and Genetic Analysis as approved by the Institutional Review Board of their own institution and of the IRB of the NIDA contractor and subcontractor that operate the NCGS in accordance with the United States Department of Health and Human Services regulation at 45 CFR Part 46. Some IRBs may waive IRB approval for studies concerned with secondary analysis of data.
Q18. How soon must the Recipient provide NIDA with any Genetic Analysis Data derived from data stored at the NCGS and received under the conditions of the Distribution Agreement?
A18. The Recipient agrees to provide NIDA, through the NCGS, with any and all Genetic Analysis Data derived from data stored at the NCGS and received under the conditions of the Distribution Agreement within 18 months after receipt of such data or upon publication of the research, whichever comes first.
http://www.nida.nih.gov/about/organization/genetics/DistPol.html
Q19. When must the Recipient destroy Biomaterials, Clinical Data, and Genetic Analysis Data received from the NCGS?
A19. The Recipient must notify NIDA and provide written certification of the destruction of Biomaterials, Clinical Data, and Genetic Analysis Data received from the NCGS in accordance with any applicable laws and/or accepted safety procedures upon completion of the project or within 3 years after receipt of clinical data and DNA.
Q20. What happens if the Recipient fails to comply with the terms in the distribution agreement?
A20. Failure to comply with any of the terms may result in disqualification of the Recipient from receiving additional Biomaterial, Clinical Data, and Genetic Analysis from NIDA, as provided by the NCGS. This disqualification may be posted on the NIDA web site and in the NIH Guide.
Q21. Is there any obligation by the Recipient to collaborate with investigators that contributed data and biomaterials to the NCGS?
A21. There is no obligation by the Recipient to collaborate with the investigators that contributed clinical data and biomaterials to the NCGS. However, the Recipient or Principal Investigator will acknowledge the contribution of scientists who generated Genetic Analysis Data received from the NCGS, in any and all oral and written presentations, disclosures, and publications resulting from any and all analyses of these Genetic Analyses of Data.
Q22. Who is liable for demands, damages, expenses, and losses arising out of the Recipient's use for any purpose of Biomaterials, Clinical Data, and Genetic Analysis Data received from the NCGS?
A22. The Recipient is liable. Under the Distribution Agreement the Recipient agrees to hold the United States Government, its contractor and subcontractor that operate the NCGS, Submitters, and other Recipients providing Genetic Analysis Data to the NCGS harmless and to indemnify all such parties for all liabilities, demands, damages, expenses, and losses arising out of the Recipient's use for any purpose of Biomaterials, Clinical Data, and Genetic Analysis Data received from the NCGS.
Q23. Is the Recipient forbidden from any effort to discover the identity of anonymized subjects from Clinical Data and DNA received from the NCGS?
A23. Yes. Under the Distribution Agreement the Recipient will not make any effort to discover the identity of anonymized subjects from Clinical Data and DNA received from the NCGS.
Q24. What safeguards are in place to prevent disclosure of subject identities whose biomaterials and clinical data are sent and stored at the NCGS?
A24. Several safeguards are in place to protect subject confidentiality.
First, biomaterials and clinical data sent to the NCGS are stripped of all subject identifiers and given a code by the Principal Investigator sending the samples. The NCGS then provides a new identifier number for the sample and informs the Principal Investigator of the new identifier number that corresponds to the code given by the Principal Investigator. Any samples containing subject identifiers submitted to the NCGS are returned immediately to the investigator.
Second, a Certificate of Confidentiality also covers the NCGS. This protects the NCGS from court orders and subpoena to release information from the NCGS in most cases.
Third, the NCGS must operate in accordance with the United States Department of Health and Human Services regulation at 45 CFR Part 46 concerning human subjects and its local institutional review board.
Fourth, anyone given access to clinical data and DNA is forbidden to attempt to discover the identity of the research participants from DNA and biomaterials obtained from the NCGS. This is stipulated as a term and condition in the Distribution Agreement.
Q25. What assurance to the investigator and their subjects is there that NIDA will handle the clinical data and biological materials (DNA) as indicated in the data-sharing plan?
A25. The data-sharing plan becomes part of the notice of grant award (NGA). The NGA is a contractual agreement between the investigator's institution and NIDA. The NGA cannot be changed except by mutual agreement between the investigator's institution and NIDA. NIDA is also legally bound to follow the United States Department of Health and Human Services policy regarding protection of human subjects.
Q26. Has there ever been a breach of confidentiality by the NCGS?
A26. No. There has never been a breach of identity of research participants in over 20,000 samples for all projects collected as of Jan 1, 2005.
|