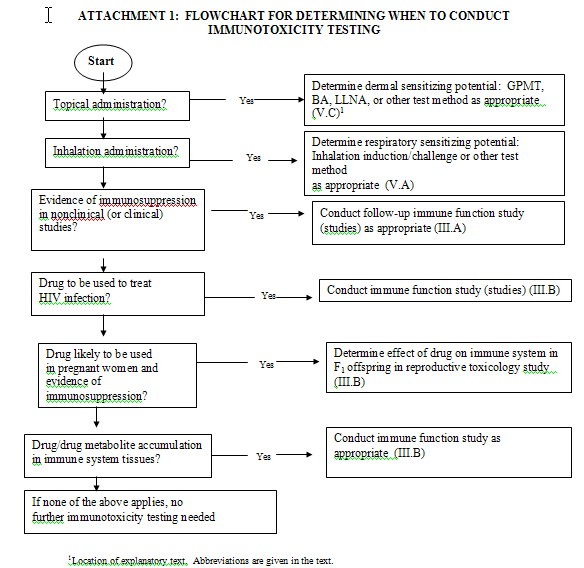
|
 |
 |
 |
 | |||||
| |||||||||
|
|
|
|
|
Guidance for Industry (PDF version of this document) U.S. Department of Health and Human Services October 2002 Guidance for Industry Additional copies are available from: U.S. Department of Health and Human Services October 2002 TABLE OF CONTENTS
I. INTRODUCTION
Guidance for Industry
INTRODUCTIONThis guidance makes recommendations to sponsors of investigational new drugs (INDs) on (1) the parameters that should be routinely assessed in toxicology studies to determine effects of a drug on immune function, (2) when additional immunotoxicity studies should be conducted, and (3) when additional mechanistic information could help characterize the significance of a given drug’s effect on the immune system. This guidance is intended for drug products and does not apply to biological products. Five adverse event categories are discussed in this guidance.
backgroundAssessment of potential adverse effects on the immune system is an important component of the overall evaluation of drug toxicity. Evidence of immunotoxicity usually can be observed in standard nonclinical toxicology studies, but in some cases additional studies are important. Observation of immune system effects may also suggest that more follow-up studies should be considered. IMMUNOSUPPRESSIONThe term immunosuppression refers to impairment of any component of the immune system resulting in decreased immune function (Descotes et al., 2000). Indicators of immunosuppression can be observed in standard nonclinical toxicology studies and include:
It is important to differentiate between unintended (adverse) immunosuppressive effects and intended (pharmacodynamic) effects. For example, many antitumor drugs are toxic to rapidly dividing cells. Immunosuppression due to bone marrow toxicity would be considered an adverse effect during the treatment of a solid tumor, but not necessarily during treatment of a hematologic malignancy. For drugs intended to be used for prevention of transplant rejection (e.g., cyclosporine), immunosuppression is the intended pharmacodynamic effect. Although this distinction appears to be relatively obvious, there are examples of drugs in which the relationship between immunosuppression and pharmacodynamic effects appears subtly, yet is important (e.g., nonsteroidal anti-inflammatory drugs, 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors) (Colville-Nash and Gilroy, 2001; Kwak et al., 2000). A. Detection of ImmunosuppressionAll investigational new drugs should be evaluated for the potential to produce immunosuppression. This is generally accomplished in repeat-dose toxicology studies using standard clinical and anatomic pathology methods, including determination of serum biochemical markers such as globulin levels, hematology (including differential), gross pathology findings, immune system-related organ weights, and histologic examination of immune system-related tissues (Basketter et al., 1995; Dean et al., 1998; De Jong et al., 1999; De Waal et al., 1995; International Collaborative Immunotoxicity Study, 1998; Richter-Reichhelm et al., 1995; Richter-Reichhelm and Schulte, 1998). Histology determinations should include examination of spleen, thymus, lymph nodes, and bone marrow. In addition, the lymphoid tissue that drains or contacts the site of drug administration (and therefore is exposed to the highest concentration of the drug) should be specifically examined (Kawabata et al., 1995b). These sites include the gut-associated lymphoid tissues (GALT) for orally administered drugs, bronchus-associated lymphoid tissues (BALT) for drugs administered by the inhalation route, nasal-associated lymphoid tissues (NALT) for drugs administered by the inhalation or nasal route, and the regional draining lymph nodes for drugs administered by the dermal, intramuscular, intradermal, or subcutaneous routes. For intravenously administered drugs, the spleen can be considered the draining lymphoid tissue. Methods to enhance detection of immunosuppression in standard toxicology studies have been described, including exact tissues that should be examined and effects that should be noted (Kuper et al., 1995, 2000). Although nonclinical studies designed to detect potential immunosuppressive effects usually have been conducted in rodents using daily administration for up to When effects indicative of immunosuppression are observed, such as depletion or hyperplasia in lymph nodes or splenic white pulp, changes in cortical (T-cell) or medullar (B-cell) areas should be noted. To better characterize such changes, a more quantitative histopathological assessment of lymphoid organs as well as immunohistochemical techniques might be useful (Kuper et al., 1995; Mitsumori et al., 1996; Ward et al., 1993). Decreases in serum globulin levels (often detected, where seen, as an increase in the serum albumin/globulin ratio) may indicate impairment of immunoglobulin production. However, decreased basal serum globulin level is a relatively insensitive indicator, because under normal circumstances the immune system should be challenged with antigen and a particular antibody response evaluated to detect immunosuppression. When decreased serum globulin level is observed, the protein components affected should be determined using appropriate assays (Duncan et al., 1994; Hall, 2001; Weingand et al., 1996). Other indicators of immunosuppression in nonclinical toxicology studies include treatment-related infections and lymphoproliferative type tumors (Burns-Naas et al., 2001). When treatment-related infections are observed in nonclinical toxicology studies, the cause of infections should be determined. Infections caused by weakly pathogenic organisms could be an important indicator of unintended immunosuppression. The relationship between immunosuppression and cancer is complicated and controversial (Luster et al., 1996; Penn, 1998; Trizio et al., 1988; Vial, 1992). Under most circumstances, when increased incidence of tumors is observed in standard 2-year rodent bioassays (or in other nonclinical toxicology studies), this effect is likely related to genotoxicity, hormonal effects, or other relatively well understood mechanisms. However, for some investigational drugs the cause of tumor findings in nonclinical studies might not be apparent. In those situations, the potential role of immunosuppression should be considered. B. Immune Function StudiesWhen warranted by observations in nonclinical general toxicology studies, additional studies to determine potential drug effects on immune function should be considered. Other considerations are important in determining if studies should be conducted to determine the potential adverse effects on immune function. Such considerations include (1) intended patient population, (2) known drug class effects (including structure-activity relationships), (3) observed pharmacokinetic effects (e.g., high concentrations of drug and/or metabolites in immune system tissues), and (4) effects suggestive of immunosuppression observed in clinical trials. If a drug is intended for treatment of HIV infection (e.g., nucleoside analogues, protease inhibitors), immune function studies should be conducted as part of the standard nonclinical assessment of safety, even when no signs of immunosuppression have been observed in the standard toxicology studies. If nonclinical pharmacokinetic studies indicate that the drug and/or metabolites concentrate in immune system tissues (e.g., macrophages), a study could be useful to determine the potential effect on immune function. In this situation, consideration should be given to the relationship between pharmacokinetics and pharmacodynamics. Certain drugs can be selected for clinical development because of the ability to concentrate in immune system cells such as macrophages (e.g., certain macrolide antibiotics) and immune function studies might not provide useful information. In other situations, concentration in immune system tissues might be an unintended effect (e.g., liposomal formulations of cytotoxic antitumor drugs), and determination of this effect on immune function might be informative. When signs consistent with immunosuppression are observed in clinical trials (such as a drug-related increase in incidence of infections), conduct of appropriate nonclinical studies to determine drug effect on immune function might be useful in understanding the clinical data. Also, developmental immunotoxicity should be assessed in some cases. If a drug has been shown to have immunosuppressive potential in adult animal studies, determination of potential developmental immunosuppression should be incorporated into an ICH Stage C to F reproductive toxicology study (ICH, 1994). At a minimum, this would include determination of clinical and anatomical pathology parameters indicative of immunosuppression (e.g. effect of maternal drug exposure on lymphoid system histology and hematology in the F1 generation offspring). Although methods have been proposed for assessing functional parameters of immunosuppression in neonatal animals (Ladics et al.., 2000), no recommendation is made concerning appropriate studies to determine the effect of fetal and/or perinatal drug exposure on immune function. If a drug is to be used to prevent perinatal transmission of HIV infection, determination of immunosuppressive potential should be included in an ICH Stage C to F reproductive toxicology study. If a drug belongs to a class known to cause immunosuppression, consideration should be given to conducting appropriate studies to determine potential effects on immune function. When immune function studies should be conducted, the most widely accepted general method is experimental determination of drug effect on immune response to a T-cell dependent immunogen (T-cell dependent antibody response). The antisheep red blood cell (SRBC) primary (IgM) antibody response assay (usually referred to as the plaque assay) was extensively evaluated by the National Toxicology Program (NTP) and was found to be useful in identifying immunosuppressant chemicals (Luster et al.,1988, 1992b, 1993). Modifications of the plaque assay are available that can be used to determine drug effects on both IgM and secondary (IgG) immune responses to SRBC (Holsapple, 1995). Other modifications of the plaque assay can be used to determine drug effects on immune response to T-cell independent immunogen (Holsapple, 1995). Techniques such as the enzyme-linked immunosorbent assay (ELISA) and the enzyme-linked immunospot (ELISPOT) can be used to quantitate antibody response and numbers of antibody-producing cells, respectively (Holsapple, 1995; Johnson et al., 2000; Kawabata, 1995a; Temple et al.,1993, 1995). Test methods have been developed using T-cell dependent immunogens other than SRBC (e.g., keyhole limpet hemocyanin, tetanus toxoid) (Exon and Talcott, 1995; Tryphonas et al., 2001). These immunogens have the advantage of being less variable, relatively standardized, and more readily obtained (as opposed to SRBC, an immunogen that has variable immunogenic potency and is not available as a standardized reagent). Antibody responses to these alternative immunogens are usually assessed using immunoassay techniques such as ELISA or ELISPOT. Assay designs have been developed to incorporate determination of drug effect on response to SRBC or other immunogens in standard nonclinical toxicology studies (Ladics et al., 1995). Integration of T-cell dependent antibody response determinations in standard nonclinical toxicology studies warrants more evaluation and is not recommended at this time. However, it is possible to conduct immune function assays in satellite group animals that otherwise can be used for pharmacokinetic and/or other determinations unlikely to be affected by experimental immunization (Wilson et al., 1999). The dose, duration, and route of administration in any immune function study should be consistent with the study in which an adverse effect was observed. Host resistance assays can be particularly valuable tools in assessing immunosuppression (Dean et al., 1981, 1982; Immunotoxicology Technical Committee, 1995; Wierda, 2000). Viral, bacterial, fungal, protozoal, and helminthic models (most using rodents) have been developed which can be used to assess the effect of drug exposure on resistance to infection (Burleson et al., 1995b; Thomas and Sherwood, 1995). Effect of drug on resistance to transplantable tumors could be useful in assessing the potential relationship between immunosuppression and tumor findings in rodent carcinogenicity bioassays (McCay, 1995). Depending on results observed in nonclinical toxicology studies, drug effects on other immune cell types or molecular systems could be informative. These include assays for drug effects on bone marrow progenitor cells (e.g., ex vivo colony-forming unit assays for erthythrocyte or granulocyte and/or macrophage precursors), macrophage or neutrophil function, or complement activation (Boorman et al., 1982; Burleson et al., 1995a, 1995b; Dean et al., 2001a; Hubbard, 1999). Although most methods used to assess drug-induced immunosuppression are conducted using standardized protocols (e.g., T-dependent immunogen assays usually specify 28 consecutive daily oral doses in mice or rats with immunogen challenge and study termination in the final week), the dose, duration, and route of administration used in functional assays should be consistent, where possible, with the nonclinical toxicology study in which an adverse immune effect was observed. This might call for modifications to standard protocols or use of alternative routes of exposure and/or different (usually higher) drug doses. Adaptations of immune function assays developed in rodents have been described using dogs and monkeys, which are species commonly used in routine drug safety evaluation studies (Jones et al., 2000; Tryphonas et al., 2001). Under most circumstances, immunological test methods can be appropriately modified. C. Immune Cell PhenotypingWhen a cause for concern has been identified, determination of potential drug effects on immune cell phenotypes may be useful (Gossett et al., 1999). Immune cell phenotyping can be accomplished by flow cytometry or immunohistochemical analysis. Cell surface phenotype determinations can be conducted using tissue obtained at necropsy (e.g., splenocytes, thymocytes, bone marrow, lymph node cells) or on circulating blood cells from animals on study or at necropsy. Analysis can include T-cell (e.g., CD3, CD4, CD8), B-cell, NK cell, and macrophage markers. Other cell types should be determined based on adverse immune effects observed in nonclinical toxicology studies and/or clinical trials. Where possible, immune cell phenotyping should be conducted using tissues and/or blood samples obtained under conditions in which immunosuppression was observed (e.g., species, dose, duration, route of administration). Although immune cell phenotype determination is not generally considered to be an adequate stand-alone test of drug effects on immune function (Immunotoxicology Technical Committee, 2001), this might be a useful indicator of immunosuppression for two reasons: (1) immune cell phenotype changes (as determined by flow cytometry) were significantly correlated with decreased host resistance against pathogens and/or tumors in studies conducted by the NTP (although the database was relatively small) (Luster et al., 1993), and (2) flow cytometry can be effectively used to monitor adverse effects in clinical trials (Selgrade et al.,1995). Both percentages and absolute cell counts can be determined by a single method (Cornacoff et al., 1995). Flow cytometric techniques have been developed that can be used to assess the effects of drugs on immune functional parameters (Burchiel et al., 1999). The optimum use of immune cell phenotype determination is in combination with tests of drug effect on immune function. An example would be the demonstration of an association between an adverse effect on immune function and a change in an immune cell phenotype (Luster et al., 1992a). Immune cell phenotyping could then be used as a method for assessing drug effect in clinical trials (Buhles, 1998). D. Evaluating Signs of ImmunosuppressionSigns of immunosuppression in nonclinical toxicology studies should be evaluated with respect to (1) statistical significance, (2) biological significance, (3) likely or demonstrated mechanisms, (4) relevance to other adverse drug effects, (5) intended use of the drug, and (6) potential role of stress. As with other toxicological parameters, a statistically significant change in a sign of immunosuppression does not necessarily indicate a biologically significant effect. A weight-of-evidence approach is recommended in which all adverse effects observed in nonclinical toxicology studies would be considered in determining if follow-up immune function studies should be conducted, including treatment parameters (dose, duration, route of administration), degree of change in immunological parameters, numbers of studies and different species in which adverse effects were observed, and number of concurrent immune-related adverse effects. Results of animal studies suggest that, at least for certain drugs, immunosuppression exhibits relatively predictable dose-response characteristics using host resistance models as indicators of biologically relevant effect (Keil et al., 1999; Lebrec et al., 1994; Luster et al., 1992b). However, it is likely that changes in some immunological parameters exhibit threshold characteristics, requiring more than a statistically significant effect to result in biologically significant immunosuppression (Biagini, 1998; Luster et al., 1992a). Thus, small but statistically significant changes in some parameters might not be cause for concern. Methods such as drug effect on T-cell dependent antibody response have been shown to be sufficiently predictive of adverse effects in humans to allow for both risk assessment as well as hazard identification (Vos and Van Loveren, 1998). It is likely, therefore, that statistically significant changes observed using these methods would indicate biologically significant effects. Other methods, such as drug effects on in vitro blastogenesis responses, appear to be useful only as hazard identification methods, and statistically significant effects might not indicate biological significance. Identification of a biomarker or biomarkers of immunosuppression that could be used in clinical trials is an important potential result of nonclinical toxicology studies. Although it is difficult to determine the degree of change in clinically observable immune parameters that would constitute an adverse drug effect, there are known relevant examples. In humans, a decrease of more than 40 percent in total lymphocytes (Hannet et al., 1992; Luster et al., 1993) or 75 percent in granulocyte counts (Johansen, 1983) are known to be clinically significant. Ultimately, clinically relevant immunosuppression could be detectable, in appropriately designed clinical trials, as immune-related adverse effects such as increased infections (Biagini, 1998; Buhles, 1998). Determining the mechanism of immunosuppression can be important in understanding the clinical relevance of observed adverse effects. For example, changes in blood cellular elements can suggest immunosuppression, but evaluation can be complex. Blood dyscrasias can be associated with effects ranging from direct bone marrow toxicity to hemolysis caused by drug-induced anti-erythrocyte antibodies (Bloom and Brandt, 2001). Differentiating direct bone marrow toxicity or direct drug-mediated intravascular hemolysis from immune-mediated cytolysis can be difficult. Direct bone marrow toxicity is usually determined by cytologic examination. Several ex vivo methods (e.g., colony-forming unit assays) can be used to determine the bone marrow progenitor cell targets of cytotoxicity (Deldar et al., 1995). Direct intravascular hemolysis is frequently accompanied by increases in white cell counts, increased spleen weight, hemosiderosis of various tissues, and reticulocytosis (Bloom and Brandt, 2001). Drug-mediated hemolysis can sometimes be confirmed by in vitroassay (incubating the drug with erythrocytes and determining release of hemoglobin) (Reilly and Aust, 1999). Detection of cell-bound antibodies can indicate whether the immunosuppressive effect has an autoimmune or antidrug antibody component (Bloom and Brandt, 2001). This mechanism of immunosuppression, however, is rarely observed in standard nonclinical toxicology studies. The timing of the onset of any blood dyscrasia should be carefully evaluated. Cell loss in circulation resulting from damage to bone marrow cells follows a time course that reflects the half-life of the cell type. For example, with damage to an early stem cell, granulocytopenia is likely to be observed first, followed by thrombocytopenia (Bloom and Brandt, 2001). Anemia will appear much later, reflecting the long lifetime (approximately 120 days in humans) of red blood cells (Bloom and Brandt, 2001). If the loss of a cell type is inconsistent with bone marrow damage, direct attack on mature cells might be indicated. As an example, cytotoxic cancer chemotherapeutic drugs are often bone marrow toxins and are likely to produce adverse effects such as neutropenia (Chabner et al., 1996). Follow-up immune function studies might not be useful in this case, since neutropenia itself is an adverse immunological effect and is likely predictable based on pharmacokinetic parameters. However, if neutropenia is observed in nonclinical studies where the effect is not related to drug pharmacodynamic activity, it may be helpful to conduct follow-up studies to determine the likely mechanism (Lorenz et al., 1999). Potential immunosuppressive effects should be evaluated in terms of both dose and, when data are available, systemic exposure. Dose comparisons to clinical use should be based on relative body surface areas. Other considerations include (1) the relationship of the dose at which immunosuppressive effects were seen to doses causing other toxicities, (2) the doses at which pharmacological activity was observed, and (3) the reversibility of immunosuppressive effects. In laboratory animals, certain environmental conditions, such as crowding, isolation, temperature, food or water deprivation, alteration of light-dark cycle, immobilization, handling, and drug administration procedures, are known to have an effect on the immune system (Ader and Cohen, 1993). Such stress‑related changes are often reversible with repeated dosing and might not be dose‑related. There are methods for determining the contribution of stress to an immunosuppressive response. For example, determination of stress-related blood hormone levels (e.g., corticosterone) and comparison with systemic drug exposure could be helpful in understanding the role of stress in drug-induced immunosuppression (Pruett et al., 1999, 2000). The pharmacological effects of the drug should be considered (e.g., where adverse immune changes result indirectly from effects of the drug on the central nervous system or the hypothalamic-pituitary-adrenal axis). When examination of immunosuppressive effects does not suggest a stress reaction or does not appear to be related to the pharmacological properties of the drug, the possibility exists that the drug has a direct adverse effect on the immune system. Even when there are potential indirect mechanisms for alterations in immune parameters, the patterns should be carefully evaluated to determine whether additional immune function studies would be useful. IMMUNOGENICITYDrug immunogenicity refers to the ability of a drug to induce an immune response. Drugs can be grouped into two major classes with respect to potential immunogenicity: (1) polypeptides or proteins with molecular weights $ 10,000, and (2) low molecular weight compounds (# 1,000). Polypeptides and protein drugs with molecular weights $ 10,000 are usually immunogenic if administered to a mammalian species in which the molecule does not naturally occur. Smaller peptides or proteins in the 5,000 to 10,000 range also may be immunogenic, although immune responses to these drugs may be fairly weak. Immunogenicity is unpredictable for compounds in the 1,000 to 5,000 range (De Weck, 1974). Low molecular weight compounds are immunogenic only if covalently bound to proteins to form hapten-protein complexes. Examples of low molecular weight drugs that can be immunogenic include penicillin and sulfonamides. There are two major concerns associated with drug immunogenicity: (1) drug allergenicity, and (2) the ability of antidrug immune responses to alter the biological activities of the drug (pharmacokinetics, pharmacodynamics, and/or toxicities). Allergenicity refers to either (1) protein allergens, or (2) small molecular weight drugs that become allergens when bound to proteins (discussed in Section V). Evaluation of protein drugs for allergenic potential is difficult in nonclinical toxicology. Although immunogenicity is an important property of protein allergens, not all protein immunogens are allergens (Kimber et al., 1999). Nonclinical methods have been developed that could be used to evaluate the allergenic potential of protein drugs, although these have not been extensively validated with respect to drug development (Karol et al., 1985; Kawabata et al., 1996; Wierda etal., 2001). Although demonstrating immunogenicity in an animal model does not necessarily predict adverse effects in humans, there are other reasons why it might be important to assess antidrug immune responses (Wierda et al., 2001). These responses could complicate interpretation of findings in repeat-dose nonclinical toxicology studies. Antidrug antibody responses can neutralize drug activity and alter drug clearance, plasma half-life, and tissue distribution. Pharmacodynamic and/or pharmacokinetic parameters such as these may thus be altered so that effects observed in nonclincial studies may not indicate the true pharmacologic and/or toxic potential of the drug. Evaluation of protein drug immunogenicity in nonclincial studies also allows for the development of drug immunoassays that could be useful in clinical trials. HYPERSENSITIVITY (DRUG ALLERGY)Hypersensitivity refers to antigen-specific immunological reactions that have adverse effects (i.e., drug allergy). The classification system discussed below includes four types of hypersensitivity responses (Coombs and Gell, 1975):
The methods discussed in this section are intended to apply to the safety assessment of small molecular weight drugs (although some principles and methods also apply to protein drugs). Small molecular weight drugs can become allergenic if they covalently bind to proteins as the parent drug or as metabolites. Immunogenic drug-protein conjugates may or may not be allergenic. Induction of a hypersensitivity reaction by a drug-protein conjugate depends on many factors, such as (1) degree of immunogenicity of the conjugate (e.g., hapten density), (2) the route of administration (oral, intramuscular, intravenous, topical), the (3) pharmacokinetics and metabolism of the drug, (4) host genetic factors, and (5) the type of drug-specific T-cell and/or antidrug antibody produced (IgE, IgG, IgM). Assays for these types of reactions are discussed in the following sections. A. Type IType I hypersensitivity reactions are mediated by IgE in humans. With respect to safety evaluation of drugs, there are two general subtypes of Type I reactions: systemic hypersensitivity (e.g., anaphylaxis, urticaria) and respiratory hypersensitivity (e.g., asthma) (Kay, 2001a, 2001b). Methods have been developed that could be used to detect drug-induced IgE (or biologically similar antibody) production following systemic or inhalation exposure. Although route of exposure appears to be an important consideration in interpretation of findings using these assays, demonstration of drug-specific IgE (or anaphylactic antibody) production should be taken as an indication of hazard for both systemic and inhalation hypersensitivity (Briatico-Vangosa et al., 1994; European Centre for Ecotoxicology and Toxicology of Chemicals, 1993; Kimber et al., 1996). Three methods have been used extensively to detect induction of drug-specific anaphylactic antibody: (1) the passive cutaneous anaphylaxis (PCA) assay, (2) the active cutaneous anaphylaxis (ACA) assay, and (3) the active systemic anaphylaxis (ASA) assay. These assays have been used to detect allergenic proteins but have not proven to be useful in identifying small molecular weight allergens (with the possible exception of highly reactive compounds) (Chazal et al., 1994; Verdier et al., 1994). If serum collected from animals immunized with a small molecular weight drug produces a reaction in the PCA or ACA assay, the drug might have sensitizing (allergenic) potential. However, a negative result in either the PCA or ACA assay does not necessarily indicate that a small molecular weight drug lacks sensitizing potential, especially when biotransformation would be important for production of potential haptens (Choquet-Kastylevsky and Descotes, 1998). The ASA assay has been used to determine the ability of a drug to induce anaphylaxis in an animal following immunization with the drug or drug-protein conjugate. As with the PCA and ACA assays, this method detects the ability of proteins and protein-reactive compounds to produce signs of anaphylaxis (Chazal et al., 1994). Like the PCA and ACA assays, however, the ASA assay might not be appropriate for determining the sensitizing potential of nonreactive small molecular weight drugs (where biotransformation might be important for production of hapten), and negative findings should not be interpreted to indicate that an experimental drug cannot produce anaphylactic reactions (Choquet-Kastylevsky and Descotes, 1998). The usefulness of this assay for the safety assessment of drugs is thus considered limited. The PCA, ACA, and ASA assays are not recommended for the routine safety evaluation of INDs. Any drug that will be administered by the inhalation route should be evaluated for potential to induce Type I hypersensitivity reactions (DeGeorge et al., 1997). Adaptations of assays used to identify drugs that have the potential to induce Type IV hypersensitivity reactions have been used for hazard identification of respiratory sensitizers. For example, methods have been developed to determine the IgE response in mice following dermal exposure to the test compound (Hilton et al.,1995; Manetz and Meade, 1999). Serum cytokine patterns induced by topical exposure in mice have also been used to detect respiratory sensitizers (Dearman et al., 1995, 1996). These methods, conducted in tandem with the murine local lymph node assay (LLNA, discussed under Type IV reactions), might be useful in detecting drugs that could induce respiratory hypersensitivity (Kimber et al.,1996; Vandebriel et al., 2000). However, these methods have not been demonstrated to detect IgE production with relatively nonreactive drugs, especially where biotransformation appears to be important for production of hapten(s). In addition, the relationship between cytokine patterns and type of chemically-induced immunopathy remains controversial (Lebrec, et al., 2001; Ulrich et al., 2001a). A tiered method for identifying respiratory sensitizers using guinea pigs has been proposed (Sarlo and Clark, 1992). This method uses sequential analysis of the test compound for (1) structural alerts (SAR), (2) in vitro covalent binding to proteins, B. Type II and IIIType II and III immunopathies tend to occur simultaneously and are commonly associated with systemic or organ hypersensitivity reactions (Adkinson, 1998). Type II and III immunopathies are the result of IgG and/or IgM antibody responses to drugs or drug metabolites. The associated pathologies are due to antibody-dependent cellular cytotoxicity (ADCC) and/or complement-mediated lysis of somatic cells (Type II) or immune complex formation, deposition, and complement activation with local tissue destruction (Type III). Type II and III immunopathies include anemia, leukopenia, thrombocytopenia, pneumonitis, vasculitis, lupus-like reactions, or glomerulonephritis, and are often indistinguishable from autoimmune reactions (Adkinson, 1998; Park et al., 1998). Type II and III immunopathies appear to be rarely modeled in animals and signs of these immunopathies are most commonly indicative of direct, nonimmune-mediated drug toxicity. Although there are examples of drugs that are associated with Type II and III hypersensitivity reactions, there are no standard nonclinical methods for predicting these effects (Park et al., 1998). There are instances, however, when follow-up studies should be considered to determine if immune mechanisms are involved in these pathologies. In the case of anemia, a positive direct Coombs test could indicate an immune-mediated hemolytic anemia (Verdier et al., 1997). In the case of tissue damage, such as vasculitis, immunohistochemical demonstration of antibody or complement in the affected tissue could suggest immunopathy (Andrews et al., 1994). Demonstration of immune complex formation with peptide and protein drugs in animal studies does not directly predict the potential for immune complex disease in humans. Such findings, however, should be carefully considered, especially when immune complex deposition leads to pathological effects. The consequences of immune complex formation can also include neutralization of drug activity and changes in pharmacokinetics. Certain classes of drugs that appear to produce Type II and/or III immunopathy have been shown to induce metabolite-specific antibodies that could be useful as biomarkers. For example, the inhalation anesthetic halothane is known to cause severe liver damage in rare instances, and this effect appears to have an immunologic basis (Pohl et al.,1988). Antibodies reactive with liver metabolites of halothane are associated with halothane hepatitis (Hubbard et al.,1988; Kenna et al.,1984), and these metabolites have been identified as trifluoroacetylated proteins (Pohl et al.,1989). Compounds that are chemically related to halothane can be administered to guinea pigs to determine the formation of hepatic trifluoroacetylated proteins (Clarke et al.,1995). This biomarker might be useful for indirectly assessing the sensitizing potential of chemicals related to halothane (Furst et al.,1996). C. Type IVType IV immunopathies are T-cell mediated and most commonly occur as delayed-type hypersensitivity skin reactions (contact dermatitis). When a drug is intended for topical administration, the sensitizing potential of the drug should be determined using an appropriate assay as part of nonclinical safety evaluation. The classic nonclinical studies use sensitization (induction) and challenge (elicitation) and are typically conducted in guinea pigs (Klecak, 1996). Although numerous assays have been developed, the most common methods for evaluating the dermal sensitizing potential of drugs have been the Buehler assay (BA) and the guinea pig maximization test (GPMT) (Botham et al.,1991). These methods are considered reliable and have demonstrated a high correlation with known human skin sensitizers (Kligman and Basketter, 1995). These methods, along with the split adjuvant technique and the Draize test, are currently accepted by CDER for determining the sensitizing potential of drugs intended for topical use. Other methods (such as the optimization assay) have also been used for the nonclinical evaluation of topical drugs and have been accepted by CDER. Histologic examination of induced skin lesions for basophil infiltrates has been used to differentiate Type IV and Type I immunopathies, but this method has not been adequately evaluated for recommendation (Graziano et al., 1983). Techniques using mice, rather than guinea pigs, have also been developed. The mouse ear-swelling test (Gad et al.,1986, 1987) uses an induction and challenge pattern similar to the traditional guinea pig tests. This method has not been extensively used in drug safety evaluation. Experimental techniques that detect the induction phase of delayed-type hypersensitivity reactions may be useful in drug development. One technique in particular, the murine LLNA, has been the subject of several studies with known contact sensitizers (Basketter et al.,1991; Kimber et al.,1991; 1995; Loveless et al., 1996; Scholes et al.,1992). The test is designed to detect in situ lymphoproliferation. Studies have indicated that results obtained with the LLNA correlate well with traditional guinea pig tests (Basketter and Scholes, 1992; Basketter et al.,1993; Dean et al., 2001b; Edwards et al.,1994; Haneke et al., 2001; Kimber et al.,1990, 1998; Sailstad et al., 2001). The LLNA may have advantages over guinea pig tests. The results are quantitative rather than essentially subjective; Freund's adjuvant is not used; and colored products can be accurately assayed. Alternative assays have been developed using methods other than radiolabel incorporation for detection of lymphoproliferation, but these techniques have not been extensively evaluated (Ulrich et al., 2001b). Results obtained with the murine LLNA can be used to support the safety of proposed clinical trials with topical drug products. When a murine LLNA is conducted to support the safety of clinical trials, the sensitizing potential of the drug substance, clinical excipient, and clinical formulation should be evaluated. In addition, a concurrent positive control should be used, and individual animal data should be provided. Photoallergy is a special case of Type IV hypersensitivity in which photoactivation of a drug results in a covalent-binding metabolite (hapten), which then acts as a sensitizer. Animal models may be useful for evaluating photoallergenic potential (Gerberick et al.,1989; Scholes et al., 1991, Ulrich et al., 1998), but the predictive value of these models for human effects is uncertain. For this reason, nonclinical testing for photoallergenic potential is not routinely expected by CDER. Other determinations could be valuable in assessing the sensitizing potential of experimental drugs. Although covalent binding to proteins should not be considered a predictor of allergenic potential, in certain situations it could be useful to determine if a drug has this potential (Park and Kitteringham, 1990). For example, if an investigational drug belongs to a class known to produce hypersensitivity reactions through covalent binding (e.g., b-lactams, sulfonamides), demonstration of in vitro and/or in vivo covalent binding to proteins could be taken as a biomarker of sensitization potential (Dewdney and Edwards, 1992; Sarlo and Clark, 1992). D. Pseudoallergic (Anaphylactoid) ReactionsA pseudoallergic reaction can result from activation of inflammatory or anaphylactic mechanisms independent of antigen-specific immune responses. Pseudoallergy is known to have several causes, including but not limited to direct histamine release and complement activation (Descotes, 1986; Szebeni, 2001). This reaction is likely to be dose-related. If signs of anaphylaxis are observed in animal studies, follow-up studies should be considered. Anaphylactoid reaction can be differentiated from true IgE mediated anaphylaxis by various methods, including in vitro testing (e.g., drug-induced histamine release using a mast cell line) (Baxter et al., 1993; Toyoguchi et al., 2000). Biochemical markers of an anaphylactoid reaction can be observed in nonclinical toxicology studies (e.g., detection of serum anaphylactic complement products in animals showing signs of anaphylaxis) (Szebeni, 2001). Careful evaluation of these reactions has resulted in valuable information on biochemical markers used in clinical trials. AUTOIMMUNITYAutoimmunity refers to a pathological process in which the immune system responds to self-antigens. Autoimmune targets include functional membranes (such as the renal glomerulus), protective membranes (such as myelin), or receptors (such as thyroid stimulating hormone or acetylcholine receptors). Glomerulonephritis, lupus-like syndrome, hemolytic anemia, and vasculitis are among the most common pathologies that may have an autoimmune basis (Rose and Bhatia, 1995). The effectors of autoimmunity can include antibodies or T-cells specific for self-antigens. The consequences of autoimmunity include direct tissue damage, immune complex deposition with complement activation, or stimulation of target function. Type II and III hypersensitivity reactions often have autoimmune components, and drug-associated autoimmunity can originate as a drug-specific hypersensitivity reaction (Dansette et al., 1998; Descotes, 1990; Kammüller et al., 1989; Knowles et al., 2000). Immune stimulation due to immune reactions (e.g., hyperthyroidism due to stimulatory IgG) is a type of autoimmunity. There are no standard methods for determining the potential of experimental drugs to produce autoimmune reactions. The popliteal lymph node assay (PLNA) and various adaptations of it have been proposed to test for autoimmunity induction by drugs (Albers et al., 1999; Bloksma et al., 1995; Descotes and Verdier, 1995; Goebel et al., 1996; Pieters and Albers, 1999a; Shinkai et al., 1999; Vial et al., 1997). Although at least one extensive evaluation of the PLNA has been published (Pieters and Albers, 1999b), no study has been reported that would support general use in drug development. Adaptations of the PLNA and the LLNA have been described, which can be used to detect the potential for drugs to produce both autoimmune reactions as well as systemic hypersensitivity (Gutting et al., 1999; Kimber, 2001; Pieters, 2001). Other methods have been proposed, such as determining markers of Th2 activation in rodents prone to develop autoimmune reactions (Bagenstose et al., 1999). Screening for autoantibody production in nonclinical toxicology studies has also been proposed and has been reported to predict clinical effects associated with certain protein drugs (Verdier et al., 1997; Wierda et al.,2001). ADVERSE IMMUNOSTIMULATIONAdverse immunostimulation refers to any antigen-nonspecific, inappropriate, or uncontrolled activation of some component of the immune system. Chronic inflammation can result from adverse immunostimulation, although it is more likely to be associated with products such as implanted medical devices and vaccine adjuvants than with drug products (Anderson and Langone, 1999; Verdier and Morgan, 2002). Unintended nonspecific immunostimulation appears to be an unusual adverse effect associated with drugs. In some respects, this class of immunotoxicity overlaps with pseudoallergy and, in fact, the distinction is subtle. Compounds with this type of activity are often proposed for use as immune stimulants (e.g., adjuvants), and in this instance, adverse immunostimulation would be considered exaggerated pharmacodynamic activity (Del Giudice et al., 2002). Cytokine release syndrome is another type of adverse immunostimulation that has been associated with certain types of therapeutic monoclonal antibodies (Winkler et al., 1999). The clinical manifestations of adverse immunostimulation pose a diagnostic challenge because of the variety of cells and tissues that could be affected. A common manifestation of adverse immunostimulation is leukocytic infiltration of tissues (Van Luyn et al., 2001). Adverse immunostimulation can be difficult to identify because the observed effect may not be in an immune system component. For example, the limiting toxicity of the immunostimulant interleukin-2 at high doses is diffuse capillary leakage (Winkelhake and Gauny, 1990). No general method is available for assessing the potential for a drug to produce adverse immunostimulation. SAFETY ConsiderationsNonclinical immunotoxicology studies for assessing safety or for exploring mechanisms of immunotoxicity are summarized in Attachment 1. As the flowchart indicates, additional immunotoxicology studies to complement the standard repeat-dose toxicology studies are expected when the drug is administered by topical or inhalation routes. For drugs administered by these routes, the sensitizing potential of the drug should be screened using an appropriate test such as the GPMT, the BA, the murine LLNA, or the guinea pig inhalation induction and challenge assay. Alternative assays can be used if appropriate. After determining whether testing is warranted based on the route of administration, the nonclinical toxicology studies conducted to support the safety of a drug in clinical trials should be carefully examined. If evidence of drug-induced immunosuppression is found, additional follow-up studies may be appropriate. Depending on the intended use of the drug, these follow-up studies may not be essential for assessing drug safety. They can, however, be useful in providing information for the risk and/or benefit assessment. For further evaluation of immunosuppressive effects, two assays in particular should be considered: (1) drug effect on T-cell dependent antibody response (e.g., the plaque assay), and (2) immune cell phenotyping (e.g., flow cytometry, immunohistochemistry). Other follow-up assays may be useful in determining the mechanism of immunosuppression (e.g., methods to determine the mechanism of blood dyscrasia). In addition to immunosuppression findings in nonclinical (or clinical) studies, accumulation of drug and/or metabolites in immune system tissues could indicate that follow-up studies on associated immune function would be useful. If the drug is intended for the treatment of HIV infection, potential to produce unintended immunosuppression should be assessed in an appropriate immune function assay (e.g., effect on response to a T-cell dependent immunogen). This immune function testing will provide additional safety assurance for subjects in whom drug-induced immunosuppression could have serious consequences. If a drug is expected to be used in pregnant women and has been shown to induce immunosuppression in adults, incorporation of immunotoxicology in the ICH Stage C to F reproductive toxicology study should be considered. Ideally, the effect of maternal drug exposure on lymphoid system organ weights, histology, and hematology in the F1 generation offspring should be included in the terminal examination. When adverse reactions suggestive of drug-induced hypersensitivity are observed in toxicology studies, additional immunotoxicity testing might be useful for clarifying the immune system’s role. For example, when anemia is present, a Coombs test could indicate whether immune-mediated hemolytic anemia is the cause. Likewise, to explore tissue damage, such as vasculitis, demonstration of immune complex deposition in the affected tissue would indicate an immunopathologic mechanism. Follow-up studies can also differentiate anaphylactoid reactions from true IgE mediated anaphylaxis. For example, drug-induced histamine release from cells or complement activation following in vitro or in vivo drug exposure would indicate an anaphylactoid reaction. Drug-induced autoimmunity suspected in toxicology studies is difficult to confirm with current methods. Nonetheless, the popliteal lymph node assay and biomarker assays might provide insight into potential autoimmune mechanisms. The final indication of whether to undertake additional immunosuppression testing is tumorigenicity. If chronic toxicology studies or rodent bioassays indicate carcinogenic potential, determination of a potential role for drug-induced immunosuppression could be helpful. Tumor host resistance models may be useful for evaluating the potential role of immunosuppression in carcinogenicity findings. Immunosuppression does not appear to be a common finding with investigational drugs. If a potentially valuable therapeutic agent has significant immunosuppressive activity in nonclinical toxicology studies, this activity would warrant careful attention in clinical trials. For instance, cancer therapeutic agents that are potent myelotoxins can be used if appropriate prophylactic measures are used to avoid infections. Certain combinations of drugs can be contraindicated when both drugs are human immunosuppressants. When submitting a marketing application, the sponsor should describe how it has addressed safety considerations for immunotoxic potential of the therapeutic agent, whether or not immunotoxicity studies have been conducted. Relevant information on immunotoxicity should be included in the product labeling. SUMMARYThe immune system consists of a diverse and complex set of cells and organs that have complicated interactions with each other and with other physiological systems. These complexities make the detection and evaluation of drug-induced immunotoxicity in animal models difficult. Nonetheless, regulatory considerations for immunotoxicologic effects discovered during the development of a drug are no different than for other adverse effects. Sponsors should submit any information on the immunotoxicology evaluation of drugs whenever such information becomes available. Immunotoxicologic findings could suggest additional follow-up studies to investigate the nature and mechanism of the immunotoxic effects. Any further testing should depend on (1) the intended use of the drug, (2) whether immunotoxicity is an expected or tolerable side effect, and (3) whether results from additional testing would alter the clinical development plan, including potential risk and/or benefit considerations. Modifications in clinical trials could be indicated by immunotoxicity findings (e.g., certain immune parameters might be monitored). Immunotoxicity findings could be included in the investigator's brochure or in the product label. Although immunotoxicity findings could indicate that a drug is unsafe for some types of clinical investigations or certain indications, these findings appear to be rare. Immunotoxicology is a rapidly advancing field and new methods are constantly being developed and evaluated. It is anticipated that new methods (e.g., genomics, proteomics, transgenic animals) will become available to determine useful endpoints for drug safety assessment, especially concerning such adverse effects as systemic hypersensitivity, autoimmunity, and photoallergy (Adkinson et al., 2002; Dean, 1997; Moser et al., 2001). Sponsors are encouraged to contact the appropriate CDER review division when signs of immunotoxicity in toxicology studies or clinical trials suggest follow-up studies. REFERENCESAder, R., and Cohen, N. (1993). “Psychoneuroimmunology: Conditioning and Stress.” Ann. Rev. Psychol., 44, 53-85. Adkinson, N.F., Jr. (1998). “Drug Allergy.” In Allergy: Principles & Practice, Vol. II (E. Middleton, Jr., E.F. Ellis, J.W. Yunginger, C.E. Reed, N.F. Adkinson, Jr., and W.W. Busse, Eds.) pp. 1212-1224, Mosby, St. Louis, Missouri. Adkinson, N.F., Jr., Essayan, D., Gruchalla, R., Haggerty, H., Kawabata, T., Sandler, J.D., Updyke, L., Shear, N.H., and Wierda, D. (2002). “Task Force Report: Future Research Needs for the Prevention and Management of Immune-Mediated Drug Hypersensitivity Reactions.” J. Allergy Clin. Immunol., 109, S461-S478. Albers, R., Van der Pijl, A., Bol, M., Bleumink, R., Seinen, W., and Pieters, R. (1999). “Distinct Immunomodulation by Autoimmunogenic Xenobiotics in Susceptible and Resistant Mice.” Toxicol. Appl. Pharmacol., 160, 156-162. Anderson, J.M., and Langone, J.J. (1999). “Issues and Perspectives on the Biocompatibility and Immunotoxicity Evaluation of Implanted Controlled Release Systems.” J. Controlled Release, 57, 107-113. Andrews, A.G., Dysko, R.C., Spilman, S.C., Kunkel, R.G., Brammer, D.W., and Johnson, K.J. (1994). “Immune Complex Vasculitis With Secondary Ulcerative Dermatitis in Aged C57BL/6NNia Mice.” Vet. Pathol., 31, 293-300. Arts, J.H.E., Kuper, C.F., Spoor, S.M., and Bloksma, N. (1998). “Airway Morphology and Function of Rats Following Dermal Sensitization and Respiratory Challenge With Low Molecular Weight Chemicals.” Toxicol. Appl. Pharmacol., 152, 66-76. Bagenstose, L.M., Salgame, P., and Monestier, M. (1999). “Cytokine Regulation of a Rodent Model of Mercuric Chloride-Induced Autoimmunity.” Environ. Health Perspect., 107, 807-810. Basketter, D.A., Bremmer, J.N., Buckley, P., Kammuller, M.E., Kawabata, T., Kimber, I., Loveless, S.E., Magda, S., Stringer, D.A., and Vohr, H.-W. (1995). “Pathology Considerations for, and Subsequent Risk Assessment of, Chemicals Identified as Immunosuppressive in Routine Toxicology.” Food Chem. Toxicol., 33, 239-243. Basketter, D.A., and Scholes, E.W. (1992). “Comparison of the Local Lymph Node Assay With the Guinea-Pig Maximization Test for the Detection of a Range of Contact Allergens.” Food Chem. Toxicol., 30, 65-69. Basketter, D.A., Scholes, E.W., Kimber, I., Botham, P.A., Hilton, J., Miller, K., Robbins, M.C., Harrison, P.T.C., and Waite, S.J. (1991). “Interlaboratory Evaluation of the Local Lymph Node Assay With 25 Chemicals and Comparison With Guinea Pig Test Data.” Toxicol. Methods, 1, 30-43. Basketter, D.A., Selbie, E., Scholes, E.W., Lees, D., Kimber, I., and Botham, P.A. (1993). “Results With OECD Recommended Positive Control Sensitizers in the Maximization, Buehler and Local Lymph Node Assays.” Food Chem. Toxicol., 31, 63-67. Baxter, A.B., Lazarus, S.C., and Brasch, R.C. (1993). “In Vitro Histamine Release Induced by Magnetic Resonance Imaging and Iodinated Contrast Media.” Invest. Radiol., 28, 308-312. Biagini, R.E. (1998). “Epidemiology Studies in Immunotoxicity Evaluations.” Toxicology, 129, 37-54. Blaikie, L., Morrow, T., Wilson, A.P., Hext, P., Hartrop, P.J., Rattray, N.J., Woodcock, D., and Botham, P.A. (1995). “A Two-Centre Study for the Evaluation and Validation of an Animal Model for the Assessment of the Potential of Small Molecular Weight Chemicals to Cause Respiratory Allergy.” Toxicology, 96, 37-50. Bloksma, N., Kubicka-Muranyi, M., Schuppe, H.C., Gleichmann, E., and Gleichmann, H. (1995). “Predictive Immunotoxicological Test Systems: Suitability of the Politeal Lymph Node Assay in Mice and Rats.” Crit. Rev. Toxicol., 25, 3693-3696. Bloom, J.C., and Brandt, J.T. (2001). “Toxic Responses of The Blood.” In Casarett & Doull’s Toxicology (C.D. Klaassen, Ed.), pp. 389-417, McGraw-Hill, New York. Boorman, G.A., Luster, M.I., Dean, J.H., and Campbell, M.L. (1982). “Assessment of Myelotoxicity Caused by Environmental Chemicals.” Environ. Health Perspect., 43, 129-135. Botham, P.A., Basketter, D.A., Maurer, T., Mueller, D., Potokar, M., and Bontinck, W.J. (1991). “Skin Sensitization -- A Critical Review of Predictive Test Methods in Animals and Man.” Food Chem. Toxicol., 29, 275-286. Briatico-Vangosa, G., Braun, C.L.J., Cookman, G., Hofmann, T., Kimber, I., Loveless, S.E., Morrow, T., Pauluhn, J., Sorensen, T., and Niessen, H.J. (1994). “Respiratory Allergy: Hazard Identification and Risk Assessment.” Fund. Appl. Toxicol., 23, 145-158. Buhles, W.C. (1998). “Application Of Immunologic Methods In Clinical Trials.” Toxicology 129, 73-89. Burchiel, S.W., Lauer, F.T., Gurulé, D., Mounho, B.J., and Salas, V.M. (1999). “Uses and Future Applications of Flow Cytometry in Immunotoxicity Testing.” Methods, 19, 28-35. Burleson, G.R., Dean, J.H., and Munson, A.E. (Eds.) (1995a). Methods in Immunotoxicology, Vol. 1, Wiley-Liss, New York. Burleson, G.R., Dean, J.H., and Munson, A.E. (Eds.) (1995b). Methods in Immunotoxicology, Vol. 2, Wiley-Liss, New York. Burns-Naas, L.A., Meade, B.J., and Munson, A.E. (2001). “Toxic Responses of the Immune System.” In Casarett & Doull’s Toxicology (C.D. Klaassen, Ed.), pp. 419-470, McGraw-Hill, New York. Chabner, B.A., Allegra, C.J., Curt, G.A., and Calabresi, P. (1996). “Antineoplastic Agents.” In Goodman and Gilman’s The Pharmacological Basis of Therapeutics, Ninth Edition (J.G. Hardman, L.E. Limbird, P.B. Molinoff, R.W. Ruddon, and A.G. Gilman, Eds.), pp. 1233-1287, McGraw-Hill, New York. Chazal, I., Verdier, F., Virat, M., and Descotes, J. (1994). “Prediction of Drug-Induced Immediate Hypersensitivity in Guinea Pigs.” Toxicol. In Vitro, 8, 1045-1047. Choquet-Kastylevsky, G., and Descotes, J. (1998). “Value of Animal Models for Predicting Hypersensitivity Reactions to Medicinal Products.” Toxicology, 129, 27-35. Clarke, J.B., Thomas, C., Chen, M., Hastings, K.L., and Gandolfi, A.J. (1995). “Halogenated Anesthetics Form Liver Adducts and Antigens That Cross-React With Halothane-Induced Antibodies.” Int. Arch. Allergy Immunol., 108, 24-32. Cohen, M.D., Schook, L.B., Oppenheim, J.J., Freed, B.M., and Rodgers, K.E. (1999). “Symposium Overview: Alterations in Cytokine Receptors by Xenobiotics.” Toxicol. Sci., 48, 163-169. Colville-Nash, P.R., and Gilroy, D.W. (2001). “Potential Adverse Effects of Cyclooxygenase-2 Inhibition: Evidence From Animal Models of Inflammation” BioDrugs, 15, 1-9. Coombs, R.R.A., and Gell, P.G.H. (1975). “Classification of Allergic Reactions Responsible for Clinical Hypersensitivity and Disease.” In Clinical Aspects of Immunology (P.G.H. Gell, R.R.A. Coombs, and P.J. Lachman, Eds.), pp. 761-778, Lippincott, Philadelphia. Cornacoff, J.B., Graham, C.S., and LaBrie, T.K. (1995). “Phenotypic Identification of Peripheral Blood Mononuclear Leukocytes by Flow Cytometry as an Adjunct to Immunotoxicity Evaluation.” In Methods in Immunotoxicology, Vol. 1 (G.R. Burleson, J.H. Dean, and A.E. Munson, Eds.), pp. 211-226, Wiley-Liss, New York. Dansette, P.M., Bonierbale, E., Minoletti, C., Beaune, P.H., Pessayre, D., and Mansuy, D. (1998). “Drug-Induced Immunotoxicity.” Eur. J. Drug Metab. Pharmacokin., 23, 443-451. Dean, J.H. (1997). “Issues With Introducing New Immunotoxicology Methods Into the Safety Assessment of Pharmaceuticals.” Toxicology, 119, 95-101. Dean, J.H., Hincks, J.R., and Remandet, B. (1998). “Immunotoxicology Assessment in the Pharmaceutical Industry.” Toxicol. Letts., 102-103, 247-255. Dean, J.H., Luster, M.I., Boorman, G.A., Luebke, R.W., and Lauer, L.D. (1982). “Application of Tumor, Bacterial and Parasite Susceptibility Assays to Study Immune Alterations Induced by Environmental Chemicals.” Environ. Health Perspect., 43, 81-88. Dean, J.H., Luster, M.I., Boorman, G.A., Padarathsingh, M.L., and Luebke, R.W. (1981). “Host Resistance Models as Endpoints for Assessing Immune Alterations Following Chemical Exposures: Studies with Diethylstilbestrol, Cyclophosphamide and 2,3,7,8-Tetrachlorodibenzo-p-dioxin. In The Biological Relevance of Immunosuppression Induced by Therapeutic and Environmental Agents (J.H. Dean, and M.L. Padarathsingh, Eds.), pp. 233-255, Van Norstrand Reinhold, New York. Dean, J.H., House, R.V., and Luster, M.I. (2001a). “Immunotoxicology: Effects of, and Responses to, Drugs and Chemicals.” In Principles and Methods of Toxicology: Fourth Edition (A.W. Hayes, Ed.), pp. 1415-1450, Taylor & Francis, Philadelphia, Pennsylvania. Dean, J.H., Twerdok, L.E., Tice, R.R., Sailstad, D.M., Hattan, D.G., and Stokes, W.S. (2001b). “ICCVAM Evaluation of the Murine Local Lymph Node Assay. II. Conclusions and Recommendations of an Independent Scientific Peer Review Panel.” Reg. Toxicol. Pharmacol., 34, 258-273. Dearman, R.J., Basketter, D.A., and Kimber, I. (1995). “Differential Cytokine Production Following Chronic Exposure of Mice to Chemical Respiratory and Contact Allergens.” Immunology, 86, 545-550. Dearman, R.J., Basketter, D.A., and Kimber, I. (1996). “Characterization of Chemical Allergens as a Function of Divergent Cytokine Secretion Profiles Induced in Mice.” Toxicol. Appl. Pharmacol., 138, 308-316. DeGeorge, J.J., Ahn, C.-H., Andrews, P.A., Brower, M.E., Choi, Y.S., Chun, M.Y., Du, T., Lee-Ham, D.Y., McGuinn, W.D., Pei, L., Sancilio, L.F., Schmidt, W., Sheevers, H.V., Sun, C.J., Tripathi, S., Vogel, W.M., Whitehurst, V., Williams, S., and Taylor, A.S. (1997). “Considerations for Toxicology Studies of Respiratory Drug Products” Reg. Toxicol. Pharmacol., 25, 189-193. De Jong, W.H., Kroese, E.D., Vos, J.G., and Van Loveren, H. (1999). “Detection of Immunotoxicity of Benzo[A]Pyrene in a Subacute Toxicity Study After Oral Exposure in Rats.” Toxicol. Sci., 50, 214-220. Deldar, A., House, R.V., and Wierda, D. (1995). “Bone Marrow Colony-Forming Assays.” In Methods in Immunotoxicology, Vol. 1 (G.R. Burleson, J.H. Dean, and A.E. Munson, Eds.), pp. 227-250, Wiley-Liss, New York. Del Giudice, G., Podda, A., and Rappuoli, R. (2002). “What Are the Limits of Adjuvanticity?” Vaccine, 20, S38-S41. Descotes, J. (1990). “Drug-Induced Immune Diseases.” In Drug-Induced Disorders, Vol. 4 (M.N.G. Dukes, Ed.), Elsevier, New York. Descotes, J. (1986). “Pseudo-Allergic Drug Reactions.” Clin. Res. Practices & Drug Reg. Affairs, 4, 75-84. Descotes, J., Choquet-Kastylevsky, G., Van Ganse, E., and Vial, T. (2000). “Responses of the Immune System to Injury.” Toxicol. Pathol., 28, 479-481. Descotes, J., and Verdier, F. (1995). “Popliteal Lymph Node Assay.” In Methods in Immunotoxicology, Vol. 1 (G.R. Burleson, J.H. Dean, and A.E. Munson, Eds.), pp. 189-196, Wiley-Liss, New York. De Waal, E.J., Timmerman, H.H., Dortant, P.M., Kranjc, M.A.M., and Van Loveren, H. (1995). “Investigation of a Screening Battery for Immunotoxicity of Pharmaceuticals Within a 28-Day Oral Toxicity Study Using Azathioprine and Cyclosporin A as Model Compounds.” Reg. Toxicol. Pharmacol., 21, 327-338. Dewdney, J.M., and Edwards, R.G. (1992). “Hypersensitivity: Adverse Drug Reactions.” In Principles and Practice of Immunotoxicology (K. Miller, J.L. Turk, and S. Nicklin, Eds.), pp. 265-278, Blackwell Scientific Publications, Oxford. De Weck, A.L. (1974). “Low Molecular Weight Antigens.” In The Antigens, Vol. 2 (M. Sela, Ed.), pp. 141-248, Academic Press, New York. Djeu, J.Y. (1995). “Natural Killer Activity.” In Methods in Immunotoxicology, Vol. 1 (G.R. Burleson, J.H. Dean, and A.E. Munson, Eds.), pp. 437-449. Duncan, J.R., Prasse, K.W., and Mahaffey, E.A. (1994). Veterinary Laboratory Medicine (3rd edition), Iowa State Press, Ames, Iowa. European Centre for Ecotoxicology and Toxicology of Chemicals. (1993). “Respiratory Allergy.” Monograph No. 19, ECETOC, Brussels, Belgium. Edwards, D.A., Soranno, T.M., Amoruso, M.A., House, R.V., Tummey, A.C., Trimmer, G.W., Thomas, P.T., and Ribeiro, P.L. (1994). “Screening Petrochemicals for Contact Hypersensitivity Potential: A Comparison of the Murine Local Lymph Node Assay With Guinea Pig and Human Test Data.” Fund. Appl. Toxicol., 23, 179-187. Exon, J.H., and Talcott, P.A. (1995). “Enzyme-Linked Immunosorbent Assay (ELISA) for Detection of Specific IgG Antibody in Rats.” In Methods in Immunotoxicology, Vol. 1 (G.R. Burleson, J.H. Dean, and A.E. Munson, Eds.), pp. 109-124, Wiley-Liss, New York. Furst, S.M., Chen, M, and Gandolfi, A.J. (1996). “Use of Halothane as a Model for Investigating Chemical-Induced Autoimmmune Hepatotoxicity.” Drug Info. J., 30, 301-307. Gad, S.C., Dunn, B.J., Dobbs, D.W., Reilly, C., and Walsh, R.D. (1986). “Development and Validation of an Alternative Dermal Sensitization Test: The Mouse Ear Swelling Test (MEST).” Toxicol. Appl. Pharmacol., 84, 93-114. Gad, S.C., Dobbs, B.W., Dunn, B.J., Reilly, C., Walsh, R.D., Auletta, C.S., Hile, R.A., Reagan, E., and Yenser, B. (1987). “Interlaboratory Validation Test (MEST).” In In Vitro Toxicology: Approaches to Validation (A.M. Goldberg, Ed.), pp. 275-292, Mary Ann Leibert, New York. Gerberick, G.F., and Ryan, C.A. (1989). “Contact Photoallergy Testing Of Sunscreens in Guinea Pigs.” Contact Dermatitis, 20, 251-259. Goebel, C., Griem, P., Sachs, B., Bloksma, N., and Gleichmann, E. (1996). “The Popliteal Lymph Node Assay in Mice: Screening of Drugs and Other Chemicals for Immunotoxic Hazard.” Inflamm. Res., 45, S85-S90. Gossett, K.A., Narayanan, P.K., Williams, D.M., Gore, E.R., Herzyk, D.J., Hart, T.K., and Sellers, T.S. (1999). “Flow Cytometry in the Preclinical Development of Biopharmaceuticals” Toxicol. Pathol., 27, 32-37. Graziano, F.M., Gunderson, L., Larson, L., and Askenase, P.W. (1983). “IgE Antibody-Mediated Cutaneous Basophil Hypersensitivity Reactions in Guinea Pigs.” J. Immunol., 131, 2675-2681. Gutting, B.W., Schomaker, S.J., Kaplan, A.H., and Amacher, D.E. (1999). “A Comparison of the Direct and Reporter Antigen Popliteal Lymph Node Assay for the Detection of Immunomodulation by Low Molecular Weight Compounds.” Toxicol. Sci., 51, 71-79. Hall, R.L. (2001). “Principles Of Clinical Pathology for Toxicology Studies.” In Principles and Methods of Toxicology, Fourth Edition (A.W. Hayes, Ed.), pp. 1001-1038, Taylor & Francis, Philadelphia, Pennsylvania. Haneke, K.E., Tice, R.R., Carson, B.L., Margolin, B.H., and Stokes, W.S. (2001). “ICCVAM Evaluation of the Murine Local Lymph Node Assay. III. Data Analysis Completed by the National Toxicology Program Interagency Center for the Evaluation of Alternative Toxicological Methods.” Reg. Toxicol. Pharmacol., 34, 274-286. Hannet, I., Erkeller-Yuksel, F., Lydyard, P., Deneys, V., and DeBruyere, M. (1992). “Developmental and Maturational Changes in Human Blood Lymphocyte Subpopulations.” Immunol. Today, 13, 215-218. Hilton, J., Dearman, R.J., Basketter, D.A., and Kimber, I. (1995). “Identification of Chemical Respiratory Allergens: Dose Response Relationships in the Mouse IgE Test.” Toxicol. Methods, 5, 51-60. Holsapple, M.P. (1995). “The Plaque-Forming Cell (PFC) Response in Immunotoxicology: An Approach to Monitoring the Primary Effector Function of B-Lymphocytes.” In Methods in Immunotoxicology, Vol. 1 (G.R. Burleson, J.H. Dean, and A.E. Munson, Eds.), pp. 71-108, Wiley-Liss, New York. Holsapple, M.P., Page, D.G., Shopp, G.M., and Bick, P.H. (1984). “Characterization of the Delayed Hypersensitivity Response to a Protein Antigen in the Mouse.” Int. J. Immunopharmacol., 6, 399-405. House, R.V. (1995). “Cytokine Bioassays for Assessment of Immunomodulation: Applications, Procedures, and Practical Considerations.” In Methods in Immunotoxicology, Vol. 1 (G.R. Burleson, J.H. Dean, and A.E. Munson, Eds.), pp. 251-276, Wiley-Liss, New York. House, R.V. (1999). “Theory and Practice of Cytokine Assessment in Immunotoxicology.” Methods, 19, 17-27. House, R.V., and Thomas, P.T. (1995). “In VitroInduction of Cytotoxic T Lymphocytes.” In Methods in Immunotoxicology, Vol. 1 (G.R. Burleson, J.H. Dean, and A.E. Munson, Eds.), pp. 159-171. Hubbard, A.K. (1999). “Effects of Xenobiotics on Macrophage Function: Evaluation In Vitro.” Methods, 19, 8-16. Hubbard, A.K., Roth, T.P., Gandolfi, A.J., Brown, B.R., Jr., Webster, N.R., and Nunn, J.F. (1988). “Halothane Hepatitis Patients Generate an Antibody Response Toward a Covalently Bound Metabolite of Halothane.” Anesthesiology, 68, 791-796. ICH (1994). ICH-S5A guidance for industry on Detection of Toxicity to Reproduction for Medicinal Products (http://www.fda.gov/cder/guidance/index.htm). Immunotoxicology Technical Committee, International Life Sciences Institute Health and Environmental Sciences Institute. (2001). “Application of Flow Cytometry to Immunotoxicity Testing: Summary of a Workshop.” Toxicology, 163, 39-48. Immunotoxicology Technical Committee, International Life Sciences Institute Health and Environmental Sciences Institute. (1995). “Immunotoxicity Testing and Risk Assessment: Summary of a 1994 Workshop.” Food Chem. Toxicol., 33, 887-894. International Collaborative Immunotoxicity Study (1998). “Report of Validation Study of Assessment of Direct Immunotoxicity in the Rat.” Toxicology, 125, 183-201. Johansen, K.S. (1983). “Nitroblue Tetrazolium Slide Test.” Use of the phorbol-myristate-acetate-stimulated NBT-reduction slide test for routine and prenatal detection of chronic granulomatous disease and diagnosis of heterozygous carriers.” Acta Pathol. Microbiol. Immunol. Scand. C, 91, 349-354. Johnson, C.W., Williams, W.C., Copeland, C.B., DeVito, M.J., and Smialowicz, R.J. (2000). “Sensitivity of the SRBC PFC Assay Versus ELISA for Detection of Immunosuppression by TCDD and TCDD-Like Congeners.” Toxicology, 156, 1-11. Jones, R.D., Offutt, D.M., and Longmoor, B.A. (2000). “Capture ELISA and Flow Cytometry Methods for Toxicologic Assessment Following Immunization and Cyclophosphamide Challenges in Beagles.” Toxicol. Letts., 115, 33-44. Kammüller, M.E., Bloksma, N., and Seinen, W. (1989). “Autoimmunity and Toxicology. Immune Disregulation Induced by Drugs and Chemicals.” In Autoimmunity and Toxicology (M.E. Kammüller, N. Bloksma, and W. Seinen, Eds.), pp. 3-34, Elsevier, Amsterdam, The Netherlands. Karol, M.H. (1995). “Assays to Evaluate Pulmonary Hypersensitivity.” In Methods in Immunotoxicology, Vol. 2 (G.R. Burleson, J.H. Dean, and A.E. Munson, Eds.), pp. 401-409, Wiley-Liss, New York. Karol, M.H., Stadler, J., and Magreni, C.M. (1985). “Immunotoxicologic Evaluation of the Respiratory System: Animal Models for Immediate and Delayed-Onset Pulmonary Hypersensitivity.” Fund. Appl. Toxicol., 5, 459-472. Kawabata, T.T. (1995a). “Enumeration of Antigen-Specific Antibody-Forming Cells by the Enzyme-Linked Immunospot (ELISPOT) Assay.” In Methods in Immunotoxicology, Vol. 1 (G.R. Burleson, J.H. Dean, and A.E. Munson, Eds.), pp. 125-135, Wiley-Liss, New York. Kawabata, T.T., Babcock, L.S., and Horn, P.A. (1996). “Specific IgE And IgG1 Responses to Subtilisin Carlsberg (Alcalase) in Mice: Development of an Intratracheal Exposure Model.” Fund. Appl. Toxicol., 29, 238-243. Kawabata, T.T., Burleson, G.R., Ernst, P.B., and Ullrich, S.E. (1995b). “Immunotoxicology of Regional Lymphoid Tissue: The Respiratory and Gastrointestinal Tracts and Skin.” Fund. Appl. Toxicol., 26, 8-19. Kay, A.B. (2001a). “Allergy and Allergic Diseases.” New Engl. J. Med., 344, 30-37. Kay, A.B. (2001b). “Allergy and Allergic Diseases.” New Engl. J. Med., 344, 109-113. Kenna, J.G., Neuberger, J., and Williams, R. (1984). “An Enzyme-Linked Immunosorbent Assay For Detection Of Antibodies Against Halothane-Altered Hepatocyte Antigens.” J. Immunol. Methods, 75, 3-14. Keil, D., Luebke, R.W., Ensley, M., Gerard, P.D., and Pruett, S.B. (1999). “Evaluation of Multivariate Statistical Methods for Analysis and Modeling of Immunotoxicology Data.” Toxicol. Sci., 51, 245-258. Kimber, I. (2001). “The Local Lymph Node Assay and Potential Application to the Identification of Drug Allergens.” Toxicology, 158, 59-64. Kimber, I., Bernstein, I.L., Karol, M.H., Robinson, M.K., Sarlo, K., and Selgrade, M.K. (1996). “Identification of Respiratory Allergens.” Fundam. Appl. Toxicol., 33, 1-10. Kimber, I., Hilton, J., and Botham, P.A. (1990). “Identification of Contact Allergens Using the Murine Local Lymph Node Assay: Comparisons with the Buehler Occluded Patch Test in Guinea Pigs.” J. Appl. Toxicol., 10, 173-180. Kimber, I., Hilton, J., Botham, P.A., Basketter, D.A., Scholes, E.W., Miller, K., Robbins, M.C., Harrison, P.T.C., Gray, T.J.B., and Waite, S.J. (1991). “The Murine Local Lymph Node Assay: Results of an Inter-Laboratory Trial.” Toxicol. Lett., 55, 203-213. Kimber, I., Hilton, J., Dearman, R.J., Gerberick, G.F., Ryan, C.A., Basketter, D.A., Lea, L., House, R.V., Ladics, G.S., Loveless, S.E., and Hastings, K.L. (1998). “Assessment of the Skin Sensitization Potential of Topical Medicaments Using the Local Lymph Node Assay: An Interlaboratory Evaluation.” J. Toxicol. Environ. Health, 53, 563-579. Kimber, I., Hilton, J., Dearman, R.J., Gerberick, G.F., Ryan, C.A., Basketter, D.A., Scholes, E.W., Ladics, G.S., Loveless, S.E., House, R.V., and Guy, A. (1995). “An International Evaluation of the Murine Local Lymph Node Assay and Comparison of Modified Procedures.” Toxicology, 103, 63-73. Kimber, I., Kerkvliet, N.I., Taylor, S.L., Astwood, J.D., Sarlo, K., and Dearman, R.J. (1999). “Toxicology of Protein Allergenicity: Prediction and Characterization.” Toxicol. Sci., 48, 157-162. Klecak, G. (1996). “Test Methods for Allergic Contact Dermatitis in Animals.” In Dermatotoxicology, Fifth Edition (F.N. Marzulli and H.I. Maibach, Eds.), pp. 437-459, Taylor & Francis, Washington, DC. Kligman, A.M., and Basketter, D.A. (1995). “A Critical Commentary and Updating of the Guinea Pig Maximization Test.” Contact Dermatitis, 32, 129-134. Knowles, S.R., Uetrecht, J, and Shear, N.H. (2000). “ Idiosyncratic Drug Reactions: The Reactive Metabolite Syndromes.” Lancet, 356, 1587-1591. Kuper, C.F., Harleman, J.H., Richter-Reichelm, H.B., and Vos, J.G. (2000). “Histopathologic Approaches to Detect Changes Indicative of Immunotoxicity.” Toxicol. Pathol., 28, 454-466. Kuper, C.F., Schuurman, H.-J., and Vos, J.G. (1995). “Pathology in Immunotoxicology.” In Methods in Immunotoxicology, Vol. 1 (G.R. Burleson, J.H. Dean, and A.E. Munson, Eds.), pp. 397-436, Wiley-Liss, New York. Kwak, B., Mulhaupt, F., Myit, S., and Mach, F. (2000). “Statins as a Newly Recognized Type of Immunomodulator.” Nature Medicine, 6, 1399-1402. Ladics, G.S., Smith, C., Bunn, T.L., Dietert, R.R., Anderson, P.K., Wiescinski, C.M., and Holsapple, M.P. (2000). “Characterization of an Approach to Developmental Immunotoxicology Assessment in the Rat Using SRBC as the Antigen.” Toxicol. Methods, 10, 283-311. Ladics, G.S., Smith, C., Heaps, K., Elliott, G.S., Slone, T.W., and Loveless, S.E. (1995). “Possible Incorporation of An Immunotoxicological Functional Assay for Assessing Humoral Immunity for Hazard Identification Purposes in Rats on Standard Toxicology Study.” Toxicology, 96, 225-238. Lang, D.S., Meier, K.L., and Luster, M.I. (1993). “Comparative Effects of Immunotoxic Chemicals on In VitroProliferative Responses of Human and Rodent Lymphocytes.” Fund. Appl. Toxicol., 21, 535-545. Lebrec, H., Blot, C., Pequet, S., Roger, R., Bohuon, C., and Pallardy, M. (1994). “Immunotoxicological Investigation Using Pharmaceutical Drugs: In Vivo Evaluation of Immune Effects.” Fund. Appl. Toxicol., 23, 159-168. Lebrec, H., Kerdine, S., Gaspard, I., and Pallardy, M. (2001). “Th1/Th2 Responses to Drugs.” Toxicology, 158, 25-29. Lebrec, H., Roger, R., Blot, C., Burleson, G.R., Bohuon, C., and Pallardy, M. (1995). “Immunotoxicological Investigation Using Pharmaceutical Drugs: In Vitro Evaluation of Immune Effects Using Rodent or Human Immune Cells.” Toxicology, 96, 147-156. Lorenz, M., Evering, W.E., Provencher, A., Blue, J.T., Lewis, H.B., Hazelette, J.R., Rajagopalan, P., Meunier, P.C., and Car, B.D. (1999). “Atypical Antipsychotic-Induced Neutropenia in Dogs.” Toxicol. Appl. Pharmacol., 155, 227-236. Loveless, S.E., Ladics, G.S., Gerberick, G.F., Ryan, C.A., Basketter, D.A., Scholes, E.W., House, R.V., Hilton, J., Dearman, R.J., and Kimber, I. (1996). “Further Evaluation of the Local Lymph Node Assay in the Final Phase of an International Collaborative Trial.” Toxicology, 108, 141- 152. Luster, M.I., Munson, A.E., Thomas, P.T., Holsapple, M.P., Fenters, J.D., White, K.L., Jr., Lauer, L.D., Germolec, D.R., Rosenthal, G.J., and Dean, J.H. (1988). “Development of a Testing Battery to Assess Chemical-Induced Immunotoxicity: National Toxicology Program's Guidelines for Immunotoxicity Evaluation in Mice.” Fundam. Appl. Toxicol., 10, 2-19. Luster, M.I., Pait, D.G., Portier, C., Rosenthal, G.J., Germolec, D.R., Comment, C.E., Munson, A.E., White, K., and Pollock, P. (1992a). “Qualitative and Quantitative Experimental Models ao Aid in Risk Assessment for Immunotoxicology.” Toxicol. Lett., 64/65, 71-78. Luster, M.I., Portier, C., Pait, D.G., White, K.L., Jr., Gennings, C., Munson, A.E., and Rosenthal, G.J. (1992b). “Risk Assessment in Immunotoxicology I: Sensitivity and Predictability of Immune Tests.” Fundam. Appl. Toxicol., 18, 200-210. Luster, M I., Portier, C., Pait, D.G., Rosenthal, G.J., Germolec, D.R., Corsini, E., Blaylock, B.L., Pollock, P., Kouchi, Y., Craig, W., White, D.L., Munson, A.E., and Comment, C.E. (1993). “Risk Assessment in Immunotoxicology II: Relationships Between Immune and Host Resistance Tests.” Fundam. Appl. Toxicol., 21, 71-82. Luster, M.I., Simeonova, P., Germolec, D.R., Portier, C., and Munson, A.E. (1996). “Relationships Between Chemical-Induced Immunotoxicity and Carcinogenesis.” Drug Info. J., 30, 281-286. Manetz, T.S., and Meade, B.J. (1999). “Development of a Flow Cytometry Assay for the Identification and Differentiation of Chemicals With the Potential to Elicit Irritation, IgE-Mediated, or T Cell-Mediated Hypersensitivity Responses.” Toxicol. Sci., 48, 206-217. McCay, J.A. (1995). “Syngeneic Tumor Cell Models: B16F10 and PYB6.” In Methods in Immunotoxicology, Vol. 2 (G.R. Burleson, J.H. Dean, and A.E. Munson, Eds.), pp. 143-157, Wiley-Liss, New York. Mehling, A., Grabbe, S., Voskort, M., Schwarz, T., Luger, T.A., and Beissert, S. (2000). “Mycophenolate Mofetil Impairs the Maturation and Function of Murine Dendritic Cells.” J. Immunol., 165, 2374-2381. Mitsumori, K., Takegawa, K., Shimo, T., Onodera, H., Yasuhara, K., and Takahashi, M. (1996). “Morphometric and Immunohistochemical Studies on Atrophic Changes in Lympho-Hematopoietic Organs of Rats Treated With Piperonyl Butoxide or Subjected to Dietary Restriction.” Arch. Toxicol., 70, 809-814. Moser, R., Quesniaux, V., and Ryffel, B. (2001). “Use of Transgenic Animals to Investigate Drug Hypersensitivity.” Toxicology, 158, 75-83. Park, B.K., and Kitteringham, N.R. (1990). “Drug-Protein Conjugation and Its Immunological Consequences.” Drug Metab. Rev., 22, 87-144. Park, B.K., Pirmohamed, M., and Kitteringham, N.R. (1998). “Role of Drug Disposition in Drug Hypersensitivity: A Chemical, Molecular, and Clinical Perspective.” Chem. Res. Toxicol., 11, 969-988. Penn, I. (1998). “The Role of Immunosuppression in Lymphoma Formation.” Springer Semin. Immunopathol., 20, 343-355. Pieters, R. (2001). “The Popliteal Lymph Node Assay: A Tool for Predicting Drug Allergies.” Toxicology, 158, 65-69. Pieters, R., and Albers, R. (1999a). “Assessment of Autoimmunogenic Potential of Xenobiotics Using the Popliteal Lymph Node Assay.” Methods, 19, 71-77. Pieters, R., and Albers, R. (1999b). “Screening Tests for Autoimmune-Related Immunotoxicity.” Environ. Health Perspect., 107, Suppl. 5, 673-677. Pohl, L.R., Kenna, J.G., Satoh, H., Christ, D., and Martin, J.L. (1989). “Neoantigens Associated With Halothane Hepatitis.” Drug Metab. Rev., 20, 203-217. Pohl, L.R., Satoh, H., Christ, D.D., and Kenna, J.G. (1988). “The Immunologic and Metabolic Basis of Drug Hypersensitivities.” Ann. Rev. Pharmacol., 28, 367-387. Pruett, S.B., Collier, S., Wu, W.-J., and Fan, R. (1999). “Quantitative Relationship Between the Suppression of Selected Immunological Parameters and the Area Under the Corticosterone Concentration Vs: Time Curve in B6C3F1 Mice Subjected to Exogenous Corticosterone or to Restraint Stress.” Toxicol. Sci., 49, 272-280. Pruett, S.B., Fan, R., Zheng, Q., Myers, L.P., and Hébert, P. (2000). “Modeling and Predicting Selected Immunological Effects of a Chemical Stressor (3,4-Dichloropropionanilide) Using the Area Under the Corticosterone Concentration Versus Time Curve.” Toxicol. Sci., 58, 77-87. Reilly, C.A., and Aust, S.A. (1999). “Red Blood Cell Lysis (Basic Protocol 4).” In Current Protocols in Toxicology, Vol. 1 (M.D. Maines, L.G. Costa, D.J. Reed, S. Sassa, I.G. Sipes, Eds.), p. 2.4.4, John Wiley & Sons, New York. Richter-Reichhelm, H-B., Dasenbrock, C.A., Descotes, G., Emmendorfer, A.C., Ernst, H.U., Harleman, J.H., Hildebrand, B., Kuttler, K., Ruhl-Fehlert, C.I., Schilling, K., Schulte, A.E., and Vohr, H.-W. (1995). “Validation of a Modified 28-Day Rat Study to Evidence Effects of Test Compounds on the Immune System.” Reg. Toxicol. Pharmacol., 22, 54-56. Richter-Reichhelm, H.-B., and Schulte, A.E. (1998). “Results of a Cyclosporin A Ringstudy.” Toxicology, 129, 91-94. Rose, N.R., and Bhatia, S. (1995). “Autoimmunity: Animal Models of Human Autoimmune Disease.” In Methods in Immunotoxicology, Vol. 2 (G.R. Burleson, J.H. Dean, and A.E. Munson, Eds.), pp. 427-445, Wiley-Liss, New York. Sailstad, D.M., Hattan, D., Hill, R.N., and Stokes, W.S. (2001). “ICCVAM Evaluation of the Murine Local Lymph Node Assay. I. The ICCVAM Review Process.” Reg. Toxicol. Pharmacol., 34, 249-257. Sarlo, K., and Clark, E.D. (1992). “A Tier Approach for Evaluating the Respiratory Allergenicity of Low Molecular Weight Chemicals.” Fund. Appl. Toxicol., 18, 107-114. Scholes, E.W., Basketter, D.A., Lovell, W.W., Sarll, A.E., and Pendlington, R.U. (1991). “The Identification of Photoallergic Potential in the Local Lymph Node Assay.” Photodermatol. Photoimmunol. Photomed., 8, 249-254. Scholes, E.W., Basketter, D.A., Sarll, A.E., Kimber, I., Evans, C.D., Miller, K., Robbins, M.C., Harrison, P.T.C., and Waite, S.J. (1992). “The Local Lymph Node Assay: Results of a Final Inter-Laboratory Validation Under Field Conditions.” J. Appl. Toxicol., 12, 217-222. Selgrade, M.K., Cooper, K.D., Devlin, R.B., Van Loveren, H., Biagini, R.E., and Luster, M.I. (1995). “Immunotoxicity -- Bridging the Gap Between Animal Research and Human Health Effects.” Fundam. Appl. Toxicol., 24, 13-21. Shinkai, K, Nakamura, K., Tsutsui, N., Kuninishi, Y., Iwaki, Y., Nishida, H., Suzuki, R., Vohr, H.-W., Takahashi, M., Takahashi, K., Kamimura, Y., and Maki, E. (1999). “Mouse Popliteal Lymph Node Assay for Assessment of Allergic and Autoimmunity-Inducing Potentials of Low-Molecular-Weight Drugs.” J. Toxicol. Sci., 24, 95-102. Smialowicz, R.J. (1995). “In VitroLymphocyte Proliferation Assays: The Mitogen-Stimulated Response and the Mixed-Lymphocyte Reaction in Immunotoxicity Testing.” In Methods in Immunotoxicology, Vol. 1 (G.R. Burleson, J.H. Dean, and A.E. Munson, Eds.), pp. 197-210, Wiley-Liss, New York. Szebeni, J. (2001). “Complement Activation-Related Pseudoallergy Caused by Liposomes, Micellar Carriers of Intravenous Drugs, and Radiocontrast Agents.” Crit. Rev. Therap. Drug Carrier Systems, 18, 567-606. Temple, L. Kawabata, T.T., Munson, A.E., and White, K.L., Jr. (1993). “Comparison of ELISA and Plaque-Forming Cell Assays for Measuring the Humoral Immune Response to SRBC in Rats and Mice Treated With Benzo[A]Pyrene or Cyclophosphamide.” Fundam. Appl. Toxicol., 21, 412- 419. Temple, L., Butterworth, L., Kawabata, T.T., Munson, A.E., and White, K.L., Jr. (1995). “ELISA to Measure SRBC Specific IgM: Method and Data Evaluation.” In Methods in Immunotoxicology Vol. 1 (G.R. Burleson, J.H. Dean, and A.E. Munson, Eds.), pp. 137-157, Wiley-Liss, New York. Thomas, P.T., and Sherwood, R.L. (1995). “Host Resistance Models in Immunotoxicology.” In Experimental Immunotoxicology (R.J. Smialowicz, and M.P. Holsapple, Eds.), CRC Press, Boca Raton, Florida. Toyoguchi, T., Ebihara, M., Ojima, F., Hosoya, J., Shoji, T., and Nakagawa, Y. (2000). “Histamine Release Induced by Antimicrobial Agents and Effects of Antimicrobial Agents on Vancomycin-Induced Histamine Release From Rat Peritoneal Mast Cells.” J. Pharm. Pharmacol., 52, 327-331. Trizio, D., Basketter, D.A., Botham, P.A., Graepel, P.H., Lambré, C., Magda, S.J., Pal, T.M., Riley, A.J., Ronneberger, H., Van Sittert, N.J., and Bontinck, W.J. (1988). “Identification of Immunotoxic Effects of Chemicals and Assessment of Their Relevance to Man.” Food Chem. Toxicol., 26, 527-539. Tryphonas, H., Arnold, D.L., Bryce, F., Huang, J., Hodgen, M., Ladouceur, D.T., Fernie, S., Lepage-Parenteau, M., and Hayward, S. (2001). “Effects Of Toxaphene On The Immune System Of Cynomolgus (Macaca Fascicularis) Monkeys.” Food Chem. Toxicol., 39, 947-958. Ulrich, P., Grenet, O., Bluemel, J., Vohr, H.-W., Wieman, C., Grundler, O., and Suter, W. (2001a). “Cytokine Expression Profiles During Murine Contact Allergy: T Helper 2 Cytokines Are Expressed Irrespective of the Type of Contact Allergen.” Arch. Toxicol., 75, 470-479. Ulrich, P., Homey, B., and Vohr, H.-W. (1998). “A Modified Murine Local Lymph Node Assay for the Differentiation of Contact Photoallergy From Phototoxicity by Analysis of Cytokine Expression in Skin-Draining Lymph Node Cells.” Toxicology, 125, 149-168. Ulrich, P., Streich, J., and Suter, W. (2001b). “Intralaboratory Validation of Alternative Endpoints in the Murine Local Lymph Node Assay for the Identification of Contact Allergic Potential: Primary Ear Skin Irritation and Ear-Draining Lymph Node Hyperplasia Induced by Topical Chemicals.” Arch. Toxicol., 74, 733-744. Vandebriel, R.J., De Jong, W.H., Spiekstra, S.W., Van Dijk, M., Fluitman, A., Garssen, J., and Van Loveren, H. (2000). “Assessment of Preferntial T-Helper 1 or T-Helper 2 Induction by Low Molecular Weight Compounds Using the Local Lymph Node Assay in Conjunction With RT-PCR and ELISA for Interferon-g and Interleukin-4.” Toxicol. Appl. Pharmacol., 162, 77-85. Vandebriel, R.J., Van Loveren, H., and Meredith, C. (1998). “Altered Cytokine (Receptor) mRNA Expression as a Tool in Immunotoxicology” Toxicology, 130, 43-67. Van Luyn, M.J.A., Plantinga, J.A., Brouwer, L.A., Khouw, I.M.S.L., De Leij, L.F.M.H., and Van Wachem, P.B. (2001). “Repetitive Subcutaneous Implantation of Different Types of (Biodegradable) Biomaterials Alters the Foreign Body Reaction.” Biomaterials, 22, 1385-1391. Verdier, F., Chazal, I., and Descotes, J. (1994). “Anaphylaxis Models in the Guinea-Pig.” Toxicology, 93, 55-61. Verdier, F., and Morgan, L. (2002). “Predictive Value of Pre-Clinical Work for Vaccine Safety Assessment.” Vaccine, 20, S21-S23. Verdier, F., Patriarca, C., and Descotes, J. (1997). “Autoantibodies in Conventional Toxicity Testing.” Toxicology, 119, 51-58. Vial, T. (1992). “Cancers in Immunocompromised Hosts.” J. Toxicol. Clin. Exper., 12, 385-393. Vial, T., Carleer, J., Legrain, B., Verdier, F., and Descotes, J. (1997). “The Popliteal Lymph Node Assay: Results of a Preliminary Interlaboratory Validation Study.” Toxicology, 122, 213-218. Vos, J.G., and Van Loveren, H. (1998). “Experimental Studies on Immunosuppression: How Do They Predict for Man?” Toxicology, 129, 13-26. Ward, J.M., Uno, H., and Frith, C.H. (1993). “Immunohistochemistry and Morphology of Reactive Lesions in Lymph Nodes and Spleen From Rats and Mice.” Toxicol. Pathol., 21, 199-205. Weingand, K., Brown, G., Hall, R., Davies, D., Gossett, K., Neptun, D., Waner, T., Matsuzawa, T., Salemink, P., Froelke, E., Provost, J.-P., Dal Negro, G., Batchelor, J., Nomura, M., Groetsch, H., Boink, A., Kimball, J., Woodman, D., York, M., Fabianson-Johnson, E., Lupart, M., and Melloni, E. (1996). “Harmonization of Animal Clinical Pathology Testing in Toxicity and Safety Studies.” Fund. Appl. Toxicol., 29, 198-201. Wierda, D. (2000). “Can Host Resistance Assays Be Used to Evaluate the Immunotoxicity of Pharmaceuticals?” Human Exptl. Toxicol., 19, 244-245. Wierda, D., Smith, H.W., and Zwickl, C.M. (2001). “Immunogenicity of Biopharmaceuticals in Laboratory Animals.” Toxicology, 158, 71-74. Wilson, S.D., Munson, A.E., and Meade, B.J. (1999). “Assessment of the Functional Integrity of the Humoral Immune Response: The Plaque-Forming Cell Assay and the Enzyme-Linked Immunosorbent Assay.” Methods, 19, 3-7. Winkelhake, J.L., and Gauny, S.S. (1990). “Human Recombinant Interleukin-2 as an Experimental Therapeutic.” Pharmacol. Rev., 42, 1-28. Winkler, U., Jensen, M., Manzke, O., Schulz, H., Diehl, V., and Engert, A. (1999). “Cytokine-Release Syndrome in Patients With B-Cell Chronic Lymphocytic Leukemia and High Lymphocyte Counts After Treatment With an Anti-CD20 Monoclonal Antibody (Rituximab, IDEC-C2B8).” Blood, 94, 2217-2224. Wood, S.C., Karras, J.G., and Holsapple, M.P. (1992). “Integration of the Human Lymphocyte Into Immunotoxicological Investigations.” Fund. Appl. Toxicol., 18, 450-459.
This guidance has been prepared by the Office of New Drugs in the Center for Drug Evaluation and Research (CDER) at the Food and Drug Administration (FDA). Sponsors of biological products should refer to the International Conference on Harmonisation (ICH) guidance S6 Preclinical Safety Evaluation of Biotechnology-Derived Pharamaceuticals (July 1997).
Date created: July 20, 2006 |