Press Release 06-021
High-Tech Sieve Sifts for Hydrogen

New polymer use may yield cheaper way to separate hydrogen from impurities
February 2, 2006
Whether it's used in chemical laboratories or the fuel tanks of advanced automobiles, hydrogen is mostly produced from natural gas and other fossil fuels. However, to isolate the tiny hydrogen molecules, engineers must first remove impurities, and the currently available methods can require substantial equipment or toxic chemicals.
Now, in the Feb. 3 issue of the journal Science, engineers have announced the development of a simpler, safer material that can potentially assist, and in some places replace, existing processing methods. The rubbery, plastic film, similar to membranes already in use in biomedical devices, has applications for isolating not only hydrogen, but also natural gas itself.
"Our team originally set out to design membranes to purify hydrogen produced from coal," said co-author and National Science Foundation awardee Benny Freeman of The University of Texas. "We felt that a good improvement would be to design membranes more permeable to impurities than to hydrogen," he added. Until now, existing membranes had the opposite property--they were more permeable to hydrogen than to impurities.
Freeman collaborated in this research with colleagues at both The University of Texas at Austin and the Research Triangle Institute in Research Triangle Park, N.C.
Hydrogen is commonly generated from natural gas in a process called steam reforming, wherein treatments with hot steam convert methane into a gaseous mixture consisting of mainly carbon dioxide (CO2), carbon monoxide (CO) and hydrogen.
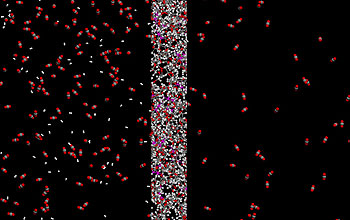
In a phenomenon that at first seems counterintuitive, larger gas molecules like CO2, and polar molecules, pass through the new film, while the much smaller hydrogen molecules stay behind.
The membrane works because the molecules in its structure have relatively "positive" parts that attract electrons and relatively "negative" parts that repel electrons. CO2 has some of these "polar" characteristics, so it is attracted to the membrane, dissolving into it as salt dissolves into a glass of water.
The molecules diffuse through the membrane at a rate that increases as more polar molecules become entrenched in the rubbery polymer, the researchers found. Even when the membrane is saturated with impurities, the polar properties continue to funnel the undesirable molecules along at a faster rate than for hydrogen, retaining most hydrogen molecules on the upstream side.
Unlike other methods, the new "reverse-selective" process can capture hydrogen at a pressure close to that of the incoming gas. This is a primary advantage for the membrane because high pressure is important for transport of the gas, and many applications, yet adds significant costs.
"The best you can do in terms of pressurization for any of these processes is make hydrogen at or near feed pressure," said Freeman. Conventional membranes, which would allow hydrogen to pass through while holding other gasses back, would decrease hydrogen pressure, he added.
While other hydrogen extraction methods still have advantages, the researchers believe there is great potential for future approaches to be hybrid processes that incorporate the new membrane within established systems.
-NSF-

Media Contacts
Joshua A. Chamot, NSF (703) 292-7730 jchamot@nsf.gov
Lisa Bistreich, RTI International (919) 316-3596 lbistreich@rti.org
Becky Rische, The University of Texas at Austin (512) 471-7272 brische@mail.utexas.edu
Program Contacts
Eric S. Peterson, NSF (703) 292-8371 epeterso@nsf.gov
Principal Investigators
Benny D. Freeman, The University of Texas at Austin (512) 232-2803 freeman@che.utexas.edu

The National Science Foundation (NSF) is an independent federal agency that supports fundamental research and education across all fields of science and engineering, with an annual budget of $6.06 billion. NSF funds reach all 50 states through grants to over 1,900 universities and institutions. Each year, NSF receives about 45,000 competitive requests for funding, and makes over 11,500 new funding awards. NSF also awards over $400 million in professional and service contracts yearly.
 Get News Updates by Email Get News Updates by Email
Useful NSF Web Sites:
NSF Home Page: http://www.nsf.gov
NSF News: http://www.nsf.gov/news/
For the News Media: http://www.nsf.gov/news/newsroom.jsp
Science and Engineering Statistics: http://www.nsf.gov/statistics/
Awards Searches: http://www.nsf.gov/awardsearch/
|