DURAGESIC® (fentanyl transdermal system
CII) Recall Notice
Product Photos
Janssen Pharmaceutica Products, L.P., recalled one lot of DURAGESIC® (fentanyl
transdermal system CII) 75 mcg per hour patches, control
number 0327192. No other dosage strengths and lot numbers
were affected. A control number is listed on every DURAGESIC® 75
mcg per hour on every patch carton and patch pouch. See the photos
below for the location of the control number and dosage strength.
Photo of DURAGESIC 75 mcg/hr product carton
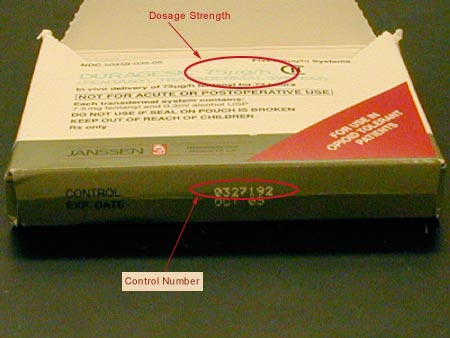
Only DURAGESIC 75 mcg/hr with Control number 0327192 are being
recalled
DURAGESIC 75 mcg/hr product carton

Photo of DURAGESIC 75 mcg/hr product patch pouch

DURAGESIC 75 mcg/hour patch

Return to 2004
Safety Summary





