|
Regulatory Programs |
Farm-to-Table Initiatives For Safer Domestic, Imported Food
 |
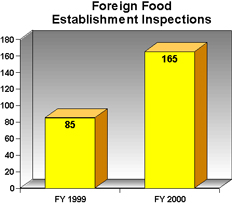 |
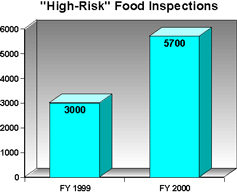
With the help of dedicated food safety funding, FDA -- particularly, its Office of Regulatory Affairs -- has been able to step up its efforts to ensure that FDA-regulated products comply with the consumer protection laws and regulations enforced by the agency. For example, in FY2000, FDA conducted about 5,700 food inspections for products at "high risk" of microbiological contamination, a 90 percent increase from its 3,000 such inspections for FY 1999. With respect to on-site inspections of foreign food establishments to complement inspections of incoming food at the U.S. border, FDA undertook 165 in FY 2000, nearly double the number in the previous fiscal year.
Sprouts and eggs saw considerable progress in safety measures in FY 2000, as did juice and dairy products and other foods, whether produced in the United States or in other countries. Samples of standout accomplishments follow:
- Steps for Safer Eggs
Recognizing egg safety as a public health issue warranting immediate federal
interagency action, FDA, in partnership with USDA
and
CDC, developed an "Egg Safety Action
Plan," which was announced in December 1999.
The joint FDA/USDA action plan identified
practices to be implemented to achieve a reduction
and ultimately an elimination of eggs as a source
of human Salmonella Enteritidis (SE) illness.
An interim goal of the egg safety action plan is a
50 percent reduction in egg-associated SE
illnesses by 2005.
"It's an important foodborne illness to address," says John Sheehan, Director of CFSAN's Division of Dairy and Egg Safety. "Reducing the frequency of these illnesses will have a tremendously positive impact on society. That many more people will be at work, productive and healthy."
After holding two public meetings and considering stakeholder input, in July 2000, FDA and the USDA's Food Safety Inspection Service (FSIS) made available the agencies' "Current Thinking Papers" on national standards for egg safety and met with representatives from industry, academia and consumer groups to discuss whether the documents represented the most effective approach to carrying out the Egg Safety Action Plan.
The first rule to come out of these FY2000 steps, a rule that requires shell eggs to carry safe handling statements and to be refrigerated under specific conditions at retail establishments such as grocery stores, nursing homes and restaurants, was published in December 2000. Specifically, the safe handling instructions state, "To prevent illness from bacteria: keep eggs refrigerated, cook eggs until yolks are firm, and cook foods containing eggs thoroughly." The refrigeration portion of the rule requires that eggs be placed promptly under refrigeration at 45 degrees Fahrenheit or lower upon delivery at retail establishments.
"You can get a pretty bad case of illness from a relatively low dose of the organism, so refrigeration is needed to preclude the rapid growth of Salmonella," Sheehan explains. "It's a worthwhile effort that is supported by the egg industry and marks another important step toward eliminating egg-associated Salmonella Enteritidis."
- Steps for Safer Sprouts

Significant scientific strides were made in FY 2000 toward improving the safety of sprouts, including development of an early-warning method for identifying contaminated sprouts by testing the irrigation water used to grow them. By testing the irrigation water early in the three- or four-day sprouting process, the testing is complete by the time the sprouts mature.
The significance of this advance: "Without a system of testing sprouts prior to selling them on the market, there was no guarantee that there weren't pathogens in the sprouts," says Charles Sizer, Ph.D., Director of the National Center for Food Safety and Technology (NCFST). An FDA guidance document based on the scientific development, titled "Guidance for Industry: Sampling and Microbial Testing of Spent Irrigation Water During Sprout Production," became available in FY 2000, as did a more general guidance document on "Reducing Microbial Food Safety Hazards for Sprouted Seeds."
FDA followed up on the guidance documents with inspections to survey the production practices of 150 sprout growers to see whether the agency's guidance was being followed. Preliminary estimates indicate that about half of producers were following the guidance and testing irrigation water to ensure that the sprouts were not contaminated prior to being marketed.
"FDA believes that its guidance will have a significant effect on sprout safety, as more and more sprouters adopt it," says Nega Beru, Director of CFSAN's Division of Plant Product Safety.
To help sprout producers (as well as retailers, regulators and others) better understand sprout production and current recommendations for best production practices, FDA, in cooperation with the California Department of Health, also developed an educational video for sprout producers in FY 2000.
The sprout video was the first in a series of food safety training programs jointly planned by FDA and the California Department of Health. So far, the video "seems to be a big hit" with industry, FDA personnel and other regulators, according to FDA's Beru.
- Sampling Produce for Pathogens: Import Survey Completed, Domestic Begun

A "1,000-sample survey" of imported produce, under which FDA investigators collected and analyzed samples of broccoli, cantaloupe, celery, cilantro, culantro, loose-leaf lettuce, parsley, scallions, strawberries and tomatoes for Salmonella and E. coli 0157:H7 (and in most cases for Shigella), was completed in FY 2000.
Of 1,003 samples, 96 percent were not contaminated with any of the three pathogens. Of the 44 contaminated samples (4 percent), 35 were contaminated with Salmonella and nine were contaminated with Shigella. None was contaminated with E. coli 0157:H7.
"Results varied greatly depending on the commodities being tested," Beru says. "This kind of objective information provides invaluable science-based support for how we target our surveillance of imported produce."
Based on violative samples, 21 firms were placed on restricted importation, called detention without physical examination (DWPE). (Some of those firms were later removed from DWPE based on evidence that the problematic conditions had been resolved.)
In FY 2000, FDA followed up on the imported survey by initiating in May a complementary 1000-sample assignment for domestic produce. Like the imported-produce assignment, the goals of the domestic project are to collect baseline information on pathogens in produce and to conduct regulatory follow-up, including at the grower level, when positive samples occur. The assignment calls for the collection of 125 samples each of the following products: cantaloupes, celery, cilantro, loose-leaf lettuce, parsley, scallions, strawberries and tomatoes. All of the samples will be analyzed for E. coli 0157:H7 and Salmonella, and most products will also be analyzed for Shigella.
The survey results will help the agency appropriately focus surveillance, research and educational efforts to lessen the risk of foodborne disease. Because these products are not grown in a sterile environment, the goal is to minimize microbial contamination to the greatest extent possible.
Also in FY 2000, FDA assisted USDA's National Agricultural Statistical Service (NASS) in conducting a fruit and vegetable agricultural practices survey that will establish a baseline of grower and packer practices. Survey results will be available in FY 2001. Followup surveys will provide a measure of the degree of adoption of FDA's "Guide to Minimize Microbial Food Safety Hazards for Fresh Fruits and Vegetables."
- Seafood HACCP Program

FY 2000 saw continuing progress in ensuring the safety of U.S. seafood. In its third year of seafood Hazard Analysis and Critical Control Point (HACCP) inspections, FDA sent 148 seafood HACCP warning letters, and in June 2000 brought the first injunctive action for noncompliance under the seafood HACCP regulations. The injunction was pursued against the owners of a Seattle-based company that was processing smoked fish without an acceptable HACCP plan and was not adequately controlling the hazard Clostridium botulinum. The firm entered into a consent decree and agreed to stop processing until the hazard could be controlled.
The seafood HACCP program has made dramatic progress toward improving the safety of U.S. seafood and reducing seafood-related illnesses to the lowest possible levels.
For example, before the landmark program was initiated in 1997, FDA conducted inspections of seafood processors only once every four years, on average. After the program's implementation, the frequency of inspections increased to an average of nearly once every year.
A recent evaluation of the seafood HACCP program documented that the program is being implemented by about 3,600 U.S. seafood processors (most of them small businesses), which collectively process more than 350 species of fish. The evaluation showed that a significant majority of processors are doing well on most of the individual elements of the program, but also identified areas where significant gaps remain.
Looking ahead, FDA is intensifying its focus on seafood processors whose products present the highest risk to consumers. Specifically, FDA plans more frequent inspections of noncompliant firms, more extensive laboratory testing for pathogens and histamines, and followup enforcement action where appropriate. FDA is also working on additional guidance, training and certification programs to help industry prevent contamination of its product, and the agency will increase its emphasis on compliance by foreign processors and on surveillance of imports.
- Pilot Training for Dairy HACCP
In cooperation with the National Conference on Interstate Milk Shipments, a HACCP pilot program was implemented by FDA in November 1999 to examine HACCP as a possible alternative to the traditional system of Inspection/Rating/Check Rating under the Pasteurized Milk Ordinance. The one-year pilot took place at six plants from different states, chosen to represent a variety of plant sizes and product mixes.
"HACCP is a modern way of looking at the safety of foods," says Les Bluhm, Director of CFSAN's Division of HACCP Programs. "HACCP methods of monitoring and documentation allow a continuous tracking of a food from the start of manufacturing through distribution," Bluhm explains, whereas "with conventional inspections, you get just a snapshot on that day."
For consumers' part, HACCP can provide a greater degree of assurance that a food is safe, says Bluhm, because it is a preventive system that "prevents bad things from happening and greatly reduces the potential for microbial contamination and foodborne illness."
According to interim results reported by evaluators, "the assurance of food safety appears to be enhanced" in the HACCP pilot plants.
- Juice HACCP Nears Finalization

FDA took substantial steps in FY 2000 toward improving the safety of fresh and processed fruit and vegetable juices by requiring domestic and foreign processors of packaged juices to equip their plants for prevention of microbiological, chemical and physical contamination of their products. Specifically, under a rule developed in FY 2000 (published in January 2001), juice processors must use Hazard Analysis and Critical Control Point, or HACCP, principles to increase protection of consumers from illness-causing microbes and other hazards in juices. HACCP systems are already federally required for seafood, meat and poultry processors.
It is estimated that each year, the rule will help prevent at least 6,000 juice-related foodborne illnesses, which have been on the rise in recent years. A 1996 E. coli 0157:H7 outbreak associated with apple juice products sickened 70 people, including a child who died as a result. A Salmonella Enteritidis outbreak in 2000 caused by unpasteurized orange juice sickened 88. And a Salmonella Muenchen outbreak in 1999 caused by unpasteurized orange juice caused 423 illnesses and one death.
In FY 2000 FDA also initiated development of a juice hazard guide to support juice manufacturers in identifying potential hazards. Also, the agency held two workshops with the juice industry about HACCP and producing a safe juice product generally. FDA started work, as well, on a juice safety video with the California Department of Health Services.
- Expedited Review Deadlines Met
FDA completed the safety evaluation in less than 360 days for all food additive petitions that qualified for expedited review in FY 2000 under CFSAN's policy of giving priority review to additives expected to have a significant impact in reducing microbial contamination.
Ionizing radiation for shell eggs was one technology that received approval under the expedited system. To substantially decrease the potential for unprocessed eggs to be contaminated with Salmonella, FDA issued a final rule in July 2000 amending the food additive regulations to provide for the safe use of this technology in shell eggs. While the process can't ensure the elimination of every organism, it can achieve a considerable reduction.
"This technology offers another alternative for eggs with reduced risk of Salmonella Enteritidis, which can be particularly dangerous for people with compromised immune systems and other vulnerable populations," says CFSAN's Associate Senior Science Advisor, Patricia Hansen.
Three other food additive petitions received approval in FY 2000 under CFSAN's expedited review process: acidified sodium chlorite solutions for meat products, sodium chlorite for poultry processing water, and chlorine dioxide to disinfect poultry and processed fruits and vegetables. Also, FDA announced in October 2000 that it has approved the use of ionizing radiation on seeds to be used for producing sprouts to reduce the amount of pathogens in and on the seeds.
- Juice Irradiation Final Rule
After data submitted to FDA demonstrated that ultraviolet (UV) radiation was followed by significant reductions in human pathogens in four juices tested (orange, apple, carrot and garden vegetable), the agency concluded that this proposed use of UV irradiation, while not eliminating microorganisms in juice, can reduce them without introducing other hazards. The final rule on juice irradiation was published in November 2000.
- Low Acid Canned Food Filing Improvements
To improve the efficiency of the system of receiving, reviewing and managing forms submitted under the Low Acid Canned Food (LACF) and Acidified Foods (AF) registration and process filing requirements, CFSAN took major strides in FY 2000 (which are continuing in FY 2001) to re-engineer the data management system for these types of information. The process is divisible into three parts:
Phase I entails moving computer data to the CFSAN Wide Area Network, allowing improved access to and management of data. Phase II involves development of an Internet component to permit filers to enter information directly into the system. This enhancement will speed the filing process and will include data quality checks during on-line filing. Phase III will allow for on-line analysis of process filings by CFSAN staff and for electronic notification of data errors or deficiencies, and will incorporate query and report generating functions.
"The data under this system is up-to-date and available instantaneously, which can expedite determinations of safety and avoid unnecessary hold-ups in the distribution of food," says FDA's Les Bluhm.
- Improvements in Imported Food Safety
The comprehensive "Imported Foods Action Plan," developed by the Department of Health and Human Services and the Department of Treasury and announced on Dec. 11, 2000, presents steps to better protect consumers from outbreaks of illnesses related to imported foods. The steps will penalize unscrupulous importers and help prevent the importation of food that jeopardizes the public health.
FDA has established a procedure to help prevent distribution of unsafe imported food by requiring that shipments for "bad actor" importers be held in a secure storage facility at the importers' expense until released by FDA. FDA also has established procedures to enhance interagency coordination and efficiently use the U.S. Customs Service's civil monetary penalties procedures against importers who attempt to bring food into the United States by means of a material false statement, act or omission.
The agency has published a proposed rule that would require marking of food shipments refused for entry into the United States for safety reasons to indicate that they were denied entry. The step will help eliminate the practice of "port shopping," in which importers whose cargo is denied entry at one port try to reintroduce it at another port without bringing the food into compliance with U.S. laws and regulations.
In another significant advance for the safety of imported foods, FDA is developing a proposed rule to establish standards for importers and other people who use sample collection services and/or private laboratories to demonstrate compliance with FDA requirements, including standards for sample collection and analysis.
- Evaluation of State Programs
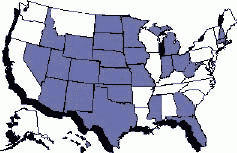 States in blue had adopted the Food Code as of April 2001 (Puerto Rico has also adopted) |
The agency in FY 2000 developed a comprehensive strategy to enhance FDA's evaluation of state inspection programs. As part of the plan, which is to be implemented over three years, FDA developed a "State Contracts Audit Course" to train FDA field personnel to audit establishment inspections conducted through the agency's state contract program.
Also in FY 2000, the number of states that have adopted the Food Code (a reference issued by FDA to guide retail outlets and institutions on how to prepare food to prevent foodborne illness) reached 20, exceeding FDA's goal to achieve adoption of the Code by 35 percent of states (18 states). The number of states that had adopted the Food Code continued to rise rapidly in early FY 2001.
"The food service, retail stores and institutional food service facilities where consumers eat and shop make up a critical sector in the farm-to-table food safety chain," says Jeannette Lyon, Consumer Safety Officer with FDA's Retail Food and Interstate Travel Team. "With each state that adopts the Food Code, we are another step closer to achieving an integrated, uniform system of national standards, which can help ensure that the food U.S. consumers eat is as safe as possible."
- Chemical Contaminants and Food Safety

In FY 2000, the food safety program focused not only on microbial pathogens themselves, but also on chemicals that may influence the growth of pathogenic bacteria. For example, FDA published two regulatory documents relating to such chemicals: (1) a draft guidance published in June 2000 titled "Guidance for Industry: Fumonisin Levels in Human Foods and Animal Feeds"; and (2) a draft compliance policy guide (CPG) published in June 2000 titled "Apple Juice, Apple Juice Concentrates, and Apple Juice Products _ Adulteration with Patulin," which advises FDA's field offices and the industry of enforcement actions that may be taken against apple juice products that contain patulin.



