

The Assistant Secretary for Health and Surgeon General chaired the third and final review of progress in achieving Healthy People 2000 objectives for Food and Drug Safety. The review was organized by the HHS Food and Drug Administration (FDA) and the USDA Food Safety Inspection Service (FSIS). Through a satellite broadcast, participants were linked with viewers at remote sites, who were able to submit questions by telephone and fax. Of the eight objectives in this priority area, one has met the year 2000 target, six have made progress, and one has regressed. The progress review drew attention to the following objectives:
12.1 Infections Caused by Key Foodborne Pathogens. The year 2000 target of no more than 16 cases of infection per 100,000 caused by Salmonella species was achieved every year since 1990. In 1997, the incidence of infection was 14. The incidence of infection per 100,000 caused by Campylobacter jejuni decreased from 50 in 1987 to 25 in 1997, meeting its year 2000 target. Incidence of Escherichia coli O157:H7 infection decreased from eight in 1987 to two in 1997, and surpassed its year 2000 target of four. The incidence of Listeria monocytogenes decreased from 0.7 in 1987 to 0.5 in 1996, also meeting the year 2000 target.
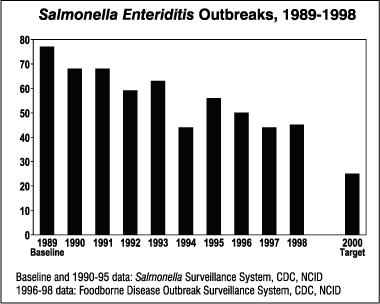
12.2 Outbreaks of Salmonella Enteritidis. The number of outbreaks of infections due to Salmonella Enteritidis decreased from 77 outbreaks in 1989 to 45 in 1998. Year to year surveillance of Salmonella Enteritidis outbreaks from 1989 to 1998 reveal a steadily declining total number of outbreaks. The year 2000 target is 25 outbreaks.
12.3 Refrigeration and Cutting Board Practices. The proportion of households in which the principal food preparers routinely refrigerate perishable foods in less than 2 hours increased from the 1988 baseline of 70 percent to 72 percent in 1993. The proportion of households in which the principal food preparer routinely washed the cutting board with soap increased from the 1988 baseline of 66 percent to 71 percent in 1998. The year 2000 target is 75 percent for all food preparation practices.
12.4 States’ and Territories’ Adoption of the Model Food Code. The proportion of states, the District of Columbia, and U.S. Commonwealth of Puerto Rico that adopted the FDA’s Food Code increased from the 1994 baseline of 2 percent to 31 percent in 1999. The year 2000 target is 70 percent.
12.5 Linked Pharmacy Systems. There was an increase from 95 percent in 1993 to 98 percent in 1995, in the proportion of pharmacies and other dispensers of prescription medications that use computer systems for individual patients. Unfortunately, while the computer capabilities may be in place in individual pharmacies, specific data to assess the extent to which linked systems are in place to inform pharmacists and protect patients are not available. The year 2000 target is 75 percent.
12.6 Medication Review for Patients Aged 65 and Older. In 1992, internists already had met their targets but for other health providers only about 50 to 60 percent were conducting this important medication review. Since that time, new data show an increase from 55 percent in 1992 to 68 percent in 1997-1998 in the proportion of nurse practitioners who routinely review with their patients 65 years and older all prescribed and over-the-counter medicines each time a new medication is prescribed. The proportion of nurse practitioners who routinely maintain current medication lists for patients aged 65 and older increased from 63 percent in 1992 to 71 percent in 1997. The year 2000 target is 75 percent. Assessment of the proportions of physicians reviewing medication was not possible due to low response rates of the 1997-1998 Prevention in Primary Care Study.
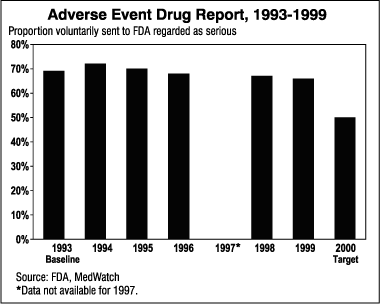
12.7 Voluntary Reports of Adverse Drug Events. The proportion of adverse drug event reports that are sent voluntarily to the FDA and that are regarded as serious decreased from its high of 72 percent in 1994 to 66 percent in 1999. Data collected from 1993 to 1999 show a decline in the proportions of adverse drug event reports voluntarily sent to the FDA that are regarded as serious. The year 2000 target is 75 percent.
12.8 Written and Verbal Information about New Prescriptions.
Since
1992, there has been a significant increase in the sharing of written
information for prescribed medicines. The proportion of patients receiving
written information for new prescriptions from dispensers increased from 32 percent in
1992 to 59 percent in 1994. The proportion of patients who received verbal information
from prescribers increased from 61 percent in 1992 to 69 percent in 1998; from pharmacists, verbal
information increased from 37 percent in 1992 to 43 percent in 1998. The year 2000 target is
75 percent for both prescribers and dispensers.
DEVELOPMENTS
Annually, an estimated 5,000 people die as the result of microorganisms
in food. The annual cost of foodborne illness in the United States is estimated
to be between $7.7 and $23 billion.
Only 11 percent of health care organizations have fully functional
computer-based patient records, according to a 1999 survey. In 1998, only 27 percent
of pharmacy computer systems in hospital settings allowed for the transfer of
information between inpatient and ambulatory care settings. Since 1996, the Foodborne Diseases Active Surveillance Network (FoodNet)
has produced national estimates of the burden and sources of specific diseases
in the United States through active surveillance and other studies.
Since October 1997, the Fight BAC!™ Campaign has used the BAC!™
character to put a “face” on microscopic foodborne bacteria and focus national
attention on the four basic food safety messages: Clean, Separate, Cook, and
Chill.
Since 1993, PulseNet computer networking, enabling rapid comparison of
bacterial pathogens at the CDC, has expanded to include FDA, FSIS, and public health
labs in all but 16 states.
DEVELOPMENTS
(Cont.)
Since 1993, MedWatch, an FDA Medical Reporting and Safety Information
Program, has instructed health professionals about monitoring suspected adverse
drug and device reactions and has facilitated systematic reporting by these
health professionals to the FDA and the manufacturer. For Healthy People 2010, the USDA’s FSIS joins the FDA as a co-lead for
the Food Safety focus area. Drug Safety will be included in a separate focus
area entitled Medical Product Safety. FOLLOW-UP Improve the exchange of information at the point of prescribing and at
the point of dispensing medications.
Collect specific data to assess the extent to which linked computer
systems are in place to inform pharmacists and protect patients from adverse
drug events. Develop food safety messages appropriate for food retailers, food
transporters, and food producers. Expand FoodNet’s active surveillance among additional selected States
and FDA and pilot electronic reporting for outbreaks. Develop methods for predicting the risk associated with foodborne
pathogens. PARTICIPANTS
American Medical Association Progress
Review Page | Healthy
People 2000 Home Page | ODPHP | NHIC
American Nurses Association
American Society for Health System Pharmacists
Centers for Disease Control and Prevention
Conference for Food Protection
Council of State and Territorial Epidemiologists
Food and Drug Administration
Food Safety and Inspection Service, U.S. Department of Agriculture
Food Safety Training and Educational Alliance
Hadassah
National Association of County and City Health Officials
National Consumers League
National Council on Patient Information and Education
National Restaurant Association Educational Foundation
Office of Disease Prevention and Health Promotion
Office of Public Health and Science
Rhode Island Department of Health