|
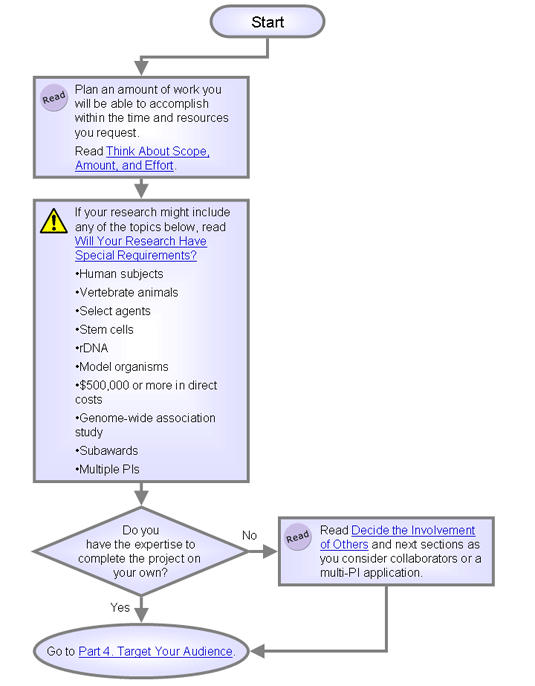
Table of Contents
Are You Ready for This Part?
Part 3. Define Your Project tells you how to scale the research scope and design your project, including resources, effort, special requirements, and collaborators.
Before reading this page, be sure that you . . .
- Know how to use this tutorial and where to find tools. Read How to Use the NIH Grant Cycle in Start Here.
Find helpful Tools:
application samples, checklists, timelines, and contacts.
- Know if you qualify for an NIH grant and NIH's and your institution's expectations -- see Part 1. Qualifying for a Grant.
- Have talked to NIH program officers to gauge where your application might belong and help choose a topic, award type, and approach -- see next bullets.
- Have picked a topic, hypothesis, and award type. Read Part 2. Game Plan to learn how to create a strategy for your research, including timing and choosing a topic and an award type.
- Know whether your application will be investigator-initiated, or you will respond to a request for applications; understand funding opportunity announcements. Read Part 2. Game Plan.
- Understand NIH peer review. You'll need this knowledge to write your application. See Part 8. Assignment and Review.
Think About Scope, Amount, and Effort
| Consider overestimating the amount of time and underestimating the amount of work. |
|
At this point, you have started planning your project and are ready to design the project in more detail.
 Our Advice: Less Is More Our Advice: Less Is More
It is key that you propose an amount of work you will be able to accomplish within the time and resources you request.
Scope
Remember the axiom "less is more." Keep the scope of the research narrow. New applicants often propose too much work, compromising their credibility with reviewers.
Think about overestimating time and underestimating work, for example, requesting five years even if you think the work will take three. This strategy can impress reviewers when you renew your grant by potentially letting you accomplish at least as much or -- even better -- more than you proposed!
Tempering expectations also gives you a healthy reality check: people generally find that their research takes longer than expected.
Amount
Here's another reason for keeping the scope narrow: to ensure that your budget stays within a reasonable target.
Make that target in sync with your experience and the needs of the research. If you are a new investigator, you should probably stay within the $250,000 limit of a modular budget. Remember that if you're from a foreign institution, you must include a detailed budget for all competing applications.
At this point, you are simply defining a budget level to aim for. As you design your experiments, you will continually revise and keep track of the size of your budget to arrive at the final figure, as we describe later in Strategy for Planning a Budget.
Ensure that your budget stays within a reasonable target. |
|
To help you gauge the amount to request, here are the levels your peers requested in FY 2007:
- Around 80 percent of all NIAID grantees had a modular grant; most requested either $225,000 or $250,000.
- The average competing R01 application received $253,012 in direct costs and $381,866 in total costs (includes institution's overhead, called facilities and administrative costs). See the table.
NIAID FY 2007 R01s -- Average Annual Cost
| Type |
Direct Cost |
Total Cost |
| Competing |
$253,012 |
$381,866 |
| Non-Competing |
$239,871 |
$360,051 |
Effort
For research grants such as the R01, NIH does not set a minimum effort requirement. However, peer reviewers expect to see a level appropriate to the work proposed, i.e., enough time to effectively manage and oversee the project.
For new investigators, we recommend that you list at least a 25 percent level of effort on each application. The level of effort you request can limit the number of applications you can submit at the same time.
Be careful not to spread yourself too thin.
- Keep in mind your teaching load as well as availability of your grad students, postdocs, and others.
- Your level of effort can't exceed 100 percent for your grants and any applications whose project period would overlap if funded.
Find more information online:
Will Your Research Have Special Requirements?
Special requirements will make your application more complex. |
|
Do you plan to use human embryonic stem cells or select agents? Will you involve animals or identifiable human products?
 Our Advice: Try to Avoid Five Complicated Areas Our Advice: Try to Avoid Five Complicated Areas
If possible, design your research to accomplish your goals without falling into any of the five areas listed below, which involve additional application and reporting requirements. For human subjects, for example, you could bypass requirements by using unidentified human data or specimens.
Talk to your business office to find out if you have requisites in addition to NIH's listed here and in your funding
opportunity announcement.
- Human subjects -- Applications involving human subjects are complex.
- Vertebrate animals -- Applications involving animals in research also require extra documentation.
- Select agents -- If your research includes select agents, you have many regulations to comply with.
- Stem cells -- You need to conform to regulations on the NIH Stem Cell Information site.
- rDNA -- Get help. Rules are complex for research using Recombinant DNA.
Other Special Requirements
- Sharing resources.
- You must describe plans to share and distribute research resources or state reasons you cannot for the following areas:
- Model organisms. If you are using NIH funds to produce new, genetically modified variants of model organisms, include a plan for sharing and distributing these resources or provide reasons why sharing is restricted or not possible.
- Data sharing. Include a data sharing plan in the application if requesting $500,000 or more in direct costs in any year.
(You will also need to get NIAID approval before submitting any investigator-initiated application requesting that level of funding. For details, see the Big Grants SOP.)
- Data sharing for genome-wide association studies. Include a data sharing plan in the application for a study of variation across the entire human genome to identify genetic associations with observable traits or the presence or absence of a disease or condition. You'll need institutional
review board certification for this plan.
- Subaward budgets. If you are planning to pay collaborators a salary using grant money, you'll need to follow the Special Instructions for Preparing Applications with a Subaward/Consortium section of the Grant Application Guide.
- Multiple PIs. A multiple PI application needs a leadership plan and has other requirements. If you want to read more now, see Take Heed -- You Might Want to Avoid a Multiple PI Application below.
These items are a sample of additional information you may need to plan for. For more information, read the SF 424 R&R Application Guide. (See Section III Assurances to learn which assurances your institution must file before we can award a grant.)
Decide the Involvement of Others
It's a major decision: to what extent should you bring other people into your project? You can expand your reservoir of expertise by adding consultants or collaborators or participating in a multiple PI application.
 |
Do you have all the needed expertise to complete the project on your own?
- Yes. Skip ahead to Part 3. Define Your Project.
- No. Could consultants or collaborators fill any gaps? Consider submitting a multiple-PI application if the science demands it. Continue reading the next two sections for information and advice.
|
Using Consultants and Collaborators
Rely on consultants to help in areas where you're short
on expertise. |
|
 Our Advice: Find People to Lend Their Expertise Our Advice: Find People to Lend Their Expertise
To help fill gaps in your expertise, rely on consultants and collaborators.
Carefully
selecting consultants and collaborators can add credibility to your application and enhance its quality.
- They can fill gaps in your expertise and resources and will impress reviewers if well known and respected in the field.
- Reviewers may recognize the name of a well-known collaborator or consultant. That is particularly important if study section members have barely heard of you.
What is the difference between them? For a fee, consultants usually provide advice or services and may participate significantly in the research. Collaborators play an active role in the research, and often the grant pays part of their salary.
If you want to read more, see Consultants or Collaborators -- How They Differ.
Take Heed -- You Might Want to Avoid a Multiple PI Application
Use a multiple PI application only if it fits the science. |
|
When deciding whether to be part of a multiple PI application, the most important factor is whether the approach fits the science.
The multiple PI option is for collaborative, usually multidisciplinary, research where each scientist contributes critical expertise to complete at least one Specific Aim of the project.
 Our Advice: Proceed With Caution! Our Advice: Proceed With Caution!
Early evidence indicates that multiple PI applications have a harder time succeeding -- probably because they are more complex.
Think about using an alternative approach: divide the research into multiple applications where you each serve as a consultant for the others' applications.
Before you decide, consider the following:
- You can no longer apply as a new investigator once you apply for and receive a multiple PI award unless it is one of the awards listed on Are You "New"?
- A multiple PI application is usually appropriate only if you could not complete the research without the other person.
To succeed in peer review, your research must require extreme synergy.
- Early data show that the more investigators, the more likely an application is to be unscored.
- It is more difficult to write a multiple PI application.
- As is true for any application, the more complex, the more likely reviewers are to find problems.
- It's also hard to correlate the pieces so they are well integrated and coordinated.
- It's best that at least one applicant, preferably all, has already been a PI on an NIH grant.
NIH allows multiple PIs for most research project grant applications, such as the R01, Exploratory/Developmental Grant (R21), and Small Grant (R03). For initiatives, check the NIH Guide announcement to confirm.
If you decide to write a multiple PI application, keep the following in mind:
-
Contact the program
officer for your area of science:
|
|
Contact your program officer as soon as you can to discuss the appropriateness of the approach.
- All PIs must be registered as PIs in the eRA Commons and have a principal investigator signature assurance on file with their institutions.
- You can request more than one PI for a renewal or amended application even if the original application had only one PI. You cannot do so for a noncompeting application.
Find more information online:
Help us improve our outreach to you by emailing deaweb@niaid.nih.gov. |
