Stratospheric Ozone Layer (Antarctic, Arctic, and Global)
In the stratosphere, the region of the atmosphere between about 10 and 50 kilometers (6-30 miles) above the Earth's surface, ozone (O3) plays a vital role by absorbing harmful ultraviolet radiation from the sun. Stratospheric ozone is threatened by some of the human-made gases that have been released into the atmosphere, including those known as chlorofluorocarbons (CFCs).
Once widely used as propellants in spray cans, refrigerants, electronics cleaning agents, and in foam and insulating products, the CFCs had been hailed as the "wonder chemicals." But the very properties that make them useful - chemical inertness, non-toxicity, insolubility in water - also make them resistant to removal in the lower atmosphere.
CFCs are mixed worldwide by the large-scale motions of the atmosphere and survive until, after 1-2 years, they reach the stratosphere and are broken down by ultraviolet radiation. The chlorine atoms within them are released and directly attack ozone. In the process of destroying ozone, the chlorine atoms are regenerated and begin to attack other ozone molecules... and so on, for thousands of cycles before the chlorine atoms are removed from the stratosphere by other processes.
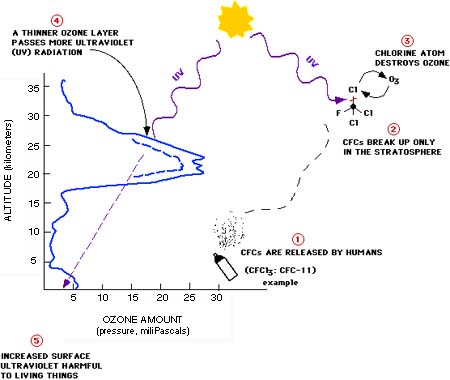 The Ozone Layer. This profile shows how the amount of ozone (O3) varies with height in the atmosphere. Note that most of the ozone is in the lower stratosphere, at an altitude of about 20-25 kilometers (12-15 miles) above sea level. This is the so-called "ozone layer." It acts as a shield by absorbing biologically active ultraviolet light (called UV-B) from the sun. If the ozone layer is depleted, more of this UV-B radiation reaches the surface of the earth. Increased exposure to UV-B has harmful effects on plants and animals, including humans. The chlorine and bromine in human-produced chemicals such as the ones known as chlorofluorocarbons (CFCs) and halons are depleting ozone in the stratosphere. The figure shows a simplified cycle of reactions in which chlorine (Cl) destroys ozone (O3).
The Ozone Layer. This profile shows how the amount of ozone (O3) varies with height in the atmosphere. Note that most of the ozone is in the lower stratosphere, at an altitude of about 20-25 kilometers (12-15 miles) above sea level. This is the so-called "ozone layer." It acts as a shield by absorbing biologically active ultraviolet light (called UV-B) from the sun. If the ozone layer is depleted, more of this UV-B radiation reaches the surface of the earth. Increased exposure to UV-B has harmful effects on plants and animals, including humans. The chlorine and bromine in human-produced chemicals such as the ones known as chlorofluorocarbons (CFCs) and halons are depleting ozone in the stratosphere. The figure shows a simplified cycle of reactions in which chlorine (Cl) destroys ozone (O3).
At CSD, researchers are conducting laboratory and field experiments and designing computer models to study this issue. CSD researchers have traveled to Antarctica to study the ozone "hole" that has been occurring there since the late 1970s each Southern-Hemisphere spring. Their measurements confirmed their theory that the ozone hole is caused as a result of the interaction of CFCs, sunlight, and the ice clouds that form in that special region of the Earth.
CSD has played leading roles in the key international field campaigns that have led to this and other findings related to the ozone hole and, more generally, the ozone layer. These missions were organized by NASA and involved the collaboration of several scientists from institutions around the world.
The National Ozone Expedition (see photo below) was the first of these, occurring just one year after the 1985 publication of the discovery of the ozone hole by the British Antarctic Survey. First-time measurements of key chlorine-containing and nitrogen-containing gases were made by members of the CSD's Chemistry and Climate Processes program (formerly Middle Atmosphere and Atmospheric Chemical Kinetics).
 NOZE I Research Team Arrives in Antarctica. Researchers arrive in Antarctica at noon, 23 August 1986, as part of the first National Ozone Expedition (NOZE I) to study the phenomenon known as the ozone hole. CSD scientist Dr. Susan Solomon led a team of 16 scientists who made measurements of trace gases and physical properties of the atmosphere. The data, along with additional findings from the NOZE II mission the next year, showed conclusively that human-produced trace gases that contain chlorine and bromine were causing the ozone hole. Scientists from the National Aeronautics and Space Administration (NASA), NOAA, and other organizations made the key measurements and analyses that showed the CFC/ozone/polar stratospheric cloud theories of the ozone hole, and not other theories, were consistent with the observations.
NOZE I Research Team Arrives in Antarctica. Researchers arrive in Antarctica at noon, 23 August 1986, as part of the first National Ozone Expedition (NOZE I) to study the phenomenon known as the ozone hole. CSD scientist Dr. Susan Solomon led a team of 16 scientists who made measurements of trace gases and physical properties of the atmosphere. The data, along with additional findings from the NOZE II mission the next year, showed conclusively that human-produced trace gases that contain chlorine and bromine were causing the ozone hole. Scientists from the National Aeronautics and Space Administration (NASA), NOAA, and other organizations made the key measurements and analyses that showed the CFC/ozone/polar stratospheric cloud theories of the ozone hole, and not other theories, were consistent with the observations.
The Meteorological Chemistry Program and Chemistry & Climate Processes Program of CSD have a long history of conducting field and theoretical research that makes use of data gathered by instruments aboard the NASA ER-2 stratospheric research aircraft. In 1987, the Airborne Antarctic Ozone Experiment (AAOE) gave the first clear evidence of exactly how the chemistry of the Antarctic stratosphere is perturbed by the ice particles (it was found that the ice particles remove nitrogen-containing gases from the atmosphere which, in turn, allows chlorine to stay in its active ozone-destroying forms longer). Instrumentation developed at CSD for measuring nitrogen-containing gases, ozone, and water vapor provided critical data that led to those findings. More recently, CSD scientist Dr. Adrian Tuck served as Project Scientist and several other CSD scientists were investigators in the 1994 Airborne Southern Hemisphere Ozone Expedition (ASHOE), which furthered the understanding of chemistry that depletes ozone over Antarctic and other latitudes of the Southern Hemisphere.
 Preparation of the NASA ER-2 for stratospheric studies. CSD researchers install chemical instrumentation on the ER-2 aircraft. The ER-2 carries only a pilot and the automated, remotely operated instrument payloads for making meteorological and chemical measurements. Several agencies and universities provide the instrumentation; CSD instruments measure reactive nitrogen compounds, ozone, and water vapor. Those are located in the instrument bay beneath the cockpit, where the investigators in the photo are shown working. Other payloads are carried in the nose, areas of the wings, and rear areas of the aircraft. The ER-2 has been used in several key international field campaigns concerned with stratospheric ozone, including AAOE, AASE, and ASHOE (see text for descriptions).
Preparation of the NASA ER-2 for stratospheric studies. CSD researchers install chemical instrumentation on the ER-2 aircraft. The ER-2 carries only a pilot and the automated, remotely operated instrument payloads for making meteorological and chemical measurements. Several agencies and universities provide the instrumentation; CSD instruments measure reactive nitrogen compounds, ozone, and water vapor. Those are located in the instrument bay beneath the cockpit, where the investigators in the photo are shown working. Other payloads are carried in the nose, areas of the wings, and rear areas of the aircraft. The ER-2 has been used in several key international field campaigns concerned with stratospheric ozone, including AAOE, AASE, and ASHOE (see text for descriptions).
It is clear that the decreases in Arctic ozone are far less dramatic than those of the Antarctic. CSD's field, theoretical, and laboratory research has demonstrated some of the key processes that contribute to this observed difference. Data from the 1988/89 and 1991 Airborne Arctic Stratospheric Expeditions (AASE-1 and AASE-2), combined with theoretical and laboratory work at CSD, revealed that the nitrogen-removing processes are far less effective in the north. Several CSD scientists were investigators on those missions, and Dr. Adrian Tuck served as Mission Scientist.
Other stratospheric research is concerned with the downward trends in global ozone that have recently been measured over populated regions in the midlatitudes. A special interest is the role of volcanic eruptions (in particular, the atmospheric particles that result from them) in the chemistry of ozone depletion. Here, CSD scientists are making contributions from all corners (field measurements, theoretical modeling, and laboratory studies) that are furthering our understanding of heterogeneous (in this case, gas-particle) chemistry - truly one of the "frontier areas" of atmospheric science.
Major Collaborative Efforts: Some Examples
Like the expeditions mentioned above, the stratospheric work of CSD is often carried out in close collaboration with colleagues from other institutions in the U.S. and abroad. As examples we describe two major collaborative efforts:
- The STRAT program (Stratospheric Tracers of Atmospheric Transport) investigated how gases and particles are transported in the stratosphere. This major field campaign especially clarifies how gases and particles from the exhaust of potential future fleets of supersonic airplanes would be distributed around the globe. The findings are expected to increase the scientific understanding of how exhaust products might influence the stratospheric ozone layer. During 1996, the NASA ER-2 research plane carried several instruments for measuring gases, winds, and temperature into the upper troposphere and lower stratosphere. Flights began in 1995 and were repeated on a regular basis through 1997 in a broad region from the tropics to the middle latitudes of the Northern Hemisphere, allowing scientists to study both the latitudinal and seasonal characteristics of the stratosphere. Additional measurements were obtained from satellite instruments and from instruments that were carried aloft by balloons in the summer of 1996. The project involved an international team of researchers from NASA, NOAA ESRL, and several universities and other organizations. STRAT is co-sponsored by NASA and NOAA.
- Fieldwork of the Photochemistry of Ozone Loss in the Arctic Region in Summer (POLARIS) mission was completed in 1997. This experiment was designed to study ozone photochemistry in the summertime lower stratosphere at middle and high latitudes, with the aim of understanding the processes that cause ozone column amounts to decline during the summer/early fall period at northern high latitudes. Chemical and meteorological data were obtained from instruments aboard the NASA ER-2 high-altitude aircraft, other aircraft, balloons, and satellites. The project involved an international team of researchers from NASA, NOAA ESRL, and several universities and other organizations.
The STRAT and POLARIS missions have scientific objectives that are consistent with the goals of SPARC (Stratospheric Processes and their Role in Climate), an international program to facilitate stratospheric research efforts on climate-related issues.
