- Radiative Forcing of Climate by non-CO2 Atmospheric Gases
- What is Radiative Forcing of Climate by Trace Gases?
- What are the non-CO2 greenhouse gases?
- Why are the non-CO2 atmospheric gases important?
- What do we know?
- What don't we know?
- What is NOAA's role?
- What will we need to know in the future?
- How does society benefit from this knowledge?
- Programmatic Information
Radiative Forcing of Climate by non-CO2 Atmospheric Gases
What is Radiative Forcing of Climate by Trace Gases?
Radiative forcing of climate by trace gases is commonly referred to as the "greenhouse effect." Solar radiation that passes through clouds and that is not reflected back to space strikes the Earth's surface. The longer wavelength (infrared) radiation created there is reflected upwards, and then is absorbed by clouds and the greenhouse gases (GHGs include carbon dioxide (CO2), methane (CH4), nitrous oxide (N2O), etc.). Those constituents reradiate upwards and downwards, thereby heating the Earth's surface. The Earth's surface temperature is 35 K warmer than its effective blackbody temperature, because of the presence of clouds and GHGs or called the natural greenhouse effect. Increasing concentrations of GHGs can cause more warming (+ or positive values), and vice-versa, decreasing means less warming or even cooling (- or negative values). For the entire group of climate forcing agents identified by the Intergovernmental Panel for Climate Change (IPCC), the climate forcing for the well-mixed GHGs is the most certain (left-most bar, Figure 1). The total effective climate forcing for all GHGs including CO2 and ozone (O3) from the beginning of the industrial revolution in 1750 to the year 2000 is 2.63 watts per square meter. As a good comparison, the natural greenhouse effect that warms the Earth's surface by 35 K is, on average, ~150 watts per square meter.
What are the non-CO2 greenhouse gases?
Like carbon dioxide, many non-CO2 atmospheric gases absorb in the infrared and contribute to climate forcing. The major non-CO2 GHGs or classes of gases listed by the Kyoto Protocol of the United Nations Framework Convention on Climate Change are CH4, N2O, hydrofluorocarbons (HFCs), sulfur hexafluoride (SF6), and perfluorocarbons (PFCs, includes CF4 and C2F6, etc.). Other classes of GHGs are included in the Montreal Protocol for Substances that Deplete the Ozone Layer and its subsequent amendments and are the chlorofluorocarbons (CFCs), halons, and chlorine- and bromine- containing (halogenated) solvents (methyl chloroform (CH3CCl3), carbon tetrachloride (CCl4), bromochloromethane (CH2BrCl), etc.). Emissions of some of the non-CO2 GHGs may be more cost-effective or easier to limit or reduce than those of CO2. Water vapor is a major greenhouse gas, but it is considered as a feedback in the climate system related to the human activities on other greenhouse gases and by changes in land use activity.
Why are the non-CO2 atmospheric gases important?
Radiatively speaking, the most important non-CO2 GHGs are CH4, N2O, CFCs, and ozone (O3) (Figure 1). The non-CO2 GHGs have added 1.17 watts per square meter (44.5% of total from all GHGs) of climate forcing from 1750 to 2000. Regardless of our future national energy strategy (fossil fuels (oil, coal) versus renewable energy (solar, wind, biofuels, tidal, etc.)), there will still exist the need to feed the ever-growing population (N2O released thru fertilizer use), refrigerate food for storage (leakage and release of the refrigerant, HFCs), and distribute electrical power (dielectric gases used like SF6). Many of the halogen-containing, non-CO2 atmospheric gases are also stratospheric ozone-depleting gases including the CFCs, halons, chlorinated solvents, and other halocarbons. The stratospheric ozone depletion resulting from these ozone- depleting gases has amounted to a -0.16 watt per square meter (medium understanding compared to the well-mixed GHGs). Tropospheric ozone, which is produced by man-made emissions of O3-forming gases, has increased 35% from 1750 to 2000, and adds 0.35 watt per square meter with also a medium degree of uncertainty.
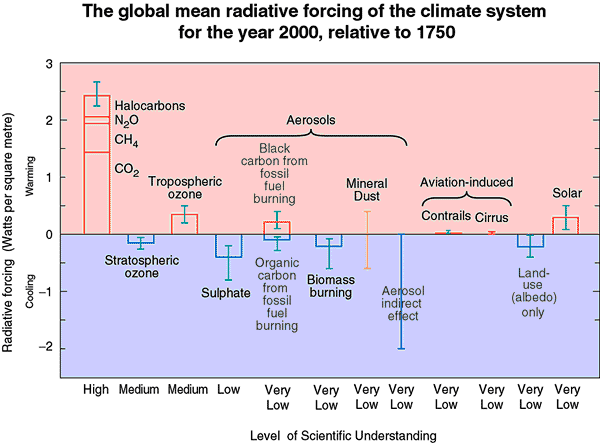
Figure 1. Major climate forcing agents and their forcing from the beginning of the industrial revolution to 2000.
What do we know?
- The physics of radiative forcing is well known, and the level of understanding in the climate forcing from the well-mixed, longer lifetime GHGs is high.
- The atmospheric lifetime of a GHG in the atmosphere is important to the length of time that the gas affects climate forcing (time horizons used in climate forcing calculations). Through chemical and inventory studies, the atmospheric lifetimes for the majority of the GHGs are well known.
- Concentrations of methane in the global atmosphere are leveling off (Figure 2).
- Nitrous oxide and sulfur hexafluoride are increasing at a nearly constant rate because of Earth's population's increased requirements for fertilizer and electrical power distribution (Figures 2 and 3).
- The Montreal Protocol and its subsequent amendments have decreased the concentrations of many CFCs and chlorinated solvents where total equivalent chlorine peaked in 1993-94 and has decreased by 8% by the end of 2005 (Figure 2).
- The growth rate for atmospheric concentration of the HCFC-14Xb series, CFC replacement compounds, are slowing down as a result of reduced production in developed countries since 2004, due to the Montreal Protocol and its subsequent amendments (Figure 3).
- The atmospheric growth of two of the key CFC replacements, HFC-134a and - 152a are increasing in the atmosphere. It is predicted that the HFC-134a radiative forcing will be as great or greater as all of the CFCs were in the early 1990s (Figure 3)
- Three long-term Northern Hemispheric station trends of O3 mixing ratios from ozonesondes in the lower troposphere since 1970 show increases that were significant (5-25% increase) in the first 10-15 years, however the most recent 15 years show a flat trend. Recent records of surface O3 from U.S. and global background stations over the past 33 years from ESRL show a mixed picture with some locations with increases and others with little change. Cape Point, South Africa since 1990 (10% increase) and European stations since 1978 (20-30%) have showed significant increases over time in tropospheric ozone.
- The global total column of O3 has decreased 3% from before the 1980s to 1997-2001 period, mostly in the mid-latitudes. The Antarctic Ozone Hole in the Austral spring is still present. However, there are indications from satellite observations that mid-latitude stratospheric O3 may have started to recover at some altitudes and during some of the seasons. Once the Montreal Protocol is under full compliance with halocarbon regulations (developing nations as well as developed, and total elimination of the production of HCFCs), the positive climate forcing from the ozone-depleting gases and the negative climate forcing from decreased O3 concentrations should both be reduced while the ozone layer recovers over the 21st century.
- Boulder, Colorado balloon-borne observations have shown that stratospheric H2O has increased, on average, 1% per year, from 1980 to 2006. The recent Boulder balloon record (1992-2006) however shows no statistically significant change, which is consistent with the slowdown of the growth rate of source gas, methane (Figure 2).
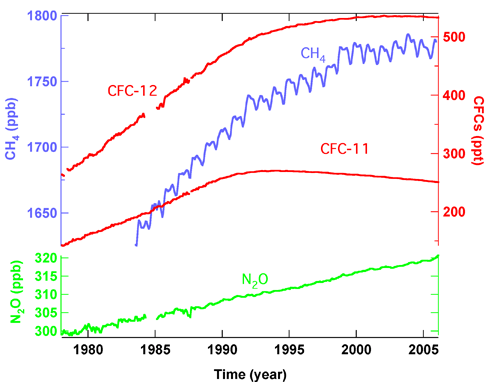
Figure 2. Global trends of the mixing ratios of the well-mixed greenhouse gases versus time from NOAA observations.
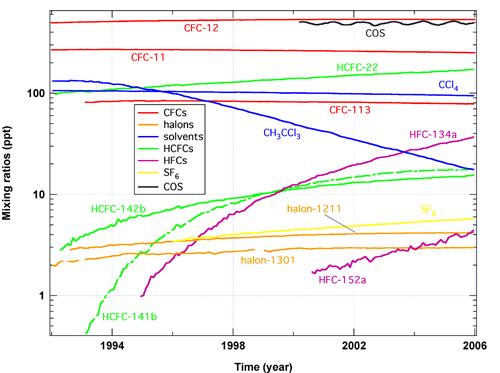
Figure 3. Logarithmic (base 10) mixing ratios of radiatively-active halocarbon and sulfur species versus time from NOAA observations.
What don't we know?
- Why are the global atmospheric concentrations of methane leveling off? Many theories exist to explain this occurrence but there is no definitive answer or smoking gun.
- Is the background level of the atmospheric cleanser, hydroxide radical, OH, changing? It could have major consequences for many GHGs, including methane, HCFCs, and HFCs.
- Our current understanding of the atmospheric emissions for the feedstock of the CFCs, CCl4, does not allow us to predict its concentration in the atmosphere.
- What is the fate of the short-lived halogen gases in the atmosphere, particularly their bromine organic species? Are they changing the total bromine in the stratosphere via tropospheric-stratospheric exchange and natural and man-made emissions?
- What are the uptake and loss strengths, biological and chemical, of important non-CO2 greenhouse and ozone-depleting gases into and out of the ocean (methyl halides, N2O, carbonyl sulfide (COS), etc.)?
- There are no perfect replacements for either the fire extinguishing agents (halons) or fumigants (methyl bromide). When new replacement compounds are considered economically feasible, then the chemistry and atmospheric lifetime will need to be established for each replacement compound.
- Some of the mid-latitude increase of stratospheric water vapor (1% per year) over the period of 1980-2006 can be explained by the increase of atmospheric methane, but not all. Is this an early indication of the feedback of water vapor in climate change, transport, or unknown chemistry?
What is NOAA's role?
Climate is one of the four major goals of NOAA. NOAA's Earth System Research Laboratory provides the expertise to conduct monitoring of the GHGs worldwide, comparison of actual emissions with industrial inventories and/or modeled natural emissions, run numerical models for radiative forcing and gas-phase chemistry, conduct laboratory studies, and airborne and ground based field campaigns.
What will we need to know in the future?
We will need to better understand the feedback mechanisms involved in the most important greenhouse gas, H2O, in the Earth's atmosphere. Increased cloud cover will block solar radiation from striking the ground, and cool the Earth's surface, while increasing the water content of the atmosphere will increase warming. Future climate models will be required that include an encompassing system approach (chemistry, transport, economical and societal factors) in order to adequately predict climate change. Air quality, through changes in the tropospheric ozone concentration, reactive chemistry, and the amount of the atmospheric cleanser, hydroxide radical, OH, may effect the strength and sign of climate forcing. Finally, how well are international agreements like the Montreal and Kyoto Protocols working to control future emissions of these GHGs?
How does society benefit from this knowledge?
The research accomplished here is available to the public and scientists alike through the web, peer-reviewed scientific literature, and "state of the art" assessments of climate and ozone depletion. NOAA provides the data and scientific analysis for public policymakers to make informed decisions on how to deal with these environmental problems facing the nation.
References
IPCC, Climate Change 2001: The Scientific Basis, Contribution of Working Group I to the Third Assessment Report of the Intergovernmental Panel on Climate Change, edited by J. T. Houghton, Y. Ding, D.J. Griggs, M. Noguer, P.J. van der Linden, X. Dai, K. Maskell, C.A. Johnson, pp. 881, Cambridge University Press, Cambridge, U.K., 2001.
