As important as genes are, they do not act in a vacuum. Everyday metabolic activities—even breathing—expose cells to biochemical substances that can promote random DNAdamage and other cellular breakdowns. Of these factors, oxygen radicals and crosslinking of proteins have become focal points of scientific exploration. Gerontologists also are studying other important proteins—heat shock proteins, hormones, and growth factors—that may play a role in aging and longevity. In short, the biochemistry of aging is a rich territory with an expanding frontier.
Oxygen Radicals
Oxygen sustains us. Every cell in the body needs it to survive. Yet, paradoxically, oxygen also wreaks havoc in the body and may be a primary catalyst for much of the damage we associate with aging. This damage occurs as a direct result of how cells metabolize it.
Oxygen is processed within a cell by tiny oganelles called mitochondria. Mitochondria convert oxygen and food into adenosine triphosphate (ATP), an energy-releasing molecule that powers most cellular processes. In essence, mitochondria are furnaces, and like all furnaces, they produce potentially harmful by–products. In cells, these by–products are called oxygen free radicals, also known as reactive oxygen species.
A free radical can be produced from almost any molecule when it loses an electron from one or more of its atoms. In cells, they are commonly created when mitochondria combine oxygen with hydrogen to form water. This transformation releases energy into the cell, but it also can shred electrons from oxygen. When this happens it leaves the oxygen atom—now an unstable oxygen free radical—with one unpaired electron. Because electrons are most stable when they are paired, oxygen free radicals steal mates for their lone electrons from other molecules. These molecules, in turn, become unstable and combine readily with other molecules.
| Antioxidants and Aging Nematodes |
|
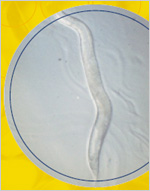 A worm barely the size of a comma printed on this page may change how investigators think about oxygen radicals, antioxidants, and aging. C. elegans nematodes immersed in a liquid containing a potent antioxidant drug lived 44 percent longer than worms not treated with this substance. It was the first time that any drug had extended the lifespan of any multi-cellular organism. The finding lends credence to the hypothesis that oxygen radicals have a significant role in the aging process. A worm barely the size of a comma printed on this page may change how investigators think about oxygen radicals, antioxidants, and aging. C. elegans nematodes immersed in a liquid containing a potent antioxidant drug lived 44 percent longer than worms not treated with this substance. It was the first time that any drug had extended the lifespan of any multi-cellular organism. The finding lends credence to the hypothesis that oxygen radicals have a significant role in the aging process.
In addition, the international team of investigators, led by Simon Melov, Ph.D., of the Buck Center for Research in Aging in California, restored a normal lifespan to mutant worms that had a mitochondrial defect, which caused increased oxygen radical production and accelerated aging. The worms showed no apparent ill effects from the treatment.
EUK-134, the drug used in the experiment, was a synthetic form of superoxide dismutase (SOD) and catalase, two enzymes that counteract the effects of oxidative stress. Like other antioxidants, such as vitamin E, these compounds convert oxygen radicals to water. But they are much more potent.
While it is only one study, and its results have not been confirmed in other species, this investigation supports the concept that antioxidant defenses may be critical during aging. It also suggests that one day it may be possible to use similar interventions to treat age-related conditions in humans. |
This process, called oxidation, can spark a chain reaction resulting in a series of products, some of which are actually beneficial. The immune system, for instance, uses free radicals to destroy bacteria and other pathogens. Another oxidizing molecule, called nitric oxide, helps nerve cells in the brain communicate with each other.
| |
Oxygen Free Radicals |
| |
|
| |
Oxygen free radicals, the bright yellow spots in this illustration, can attack and damage DNA, leading to mutations. |
Free radicals, however, also can be vandals that cause extensive damage to proteins, membranes, and DNA. Mitochondria are particularly prone to free radical damage. The major source of free radical production in the body, they are also one of its prime targets. As the damage mounts, mitochondria become less efficient, progressively generating less ATP and more free radicals. Over time, according to the free radical theory, oxidative damage accumulates in our cells and tissues, triggering many of the bodily changes that occur as we age. Free radicals have been implicated not only in aging but also in degenerative disorders, including cancer, atherosclerosis, cataracts, and neurodegeneration.
But free radicals, which also can be produced by tobacco smoke, sun exposure, and other environmental factors, do not go unchecked. Cells utilize substances called antioxidants to counteract them. These substances including nutrients—the familiar vitamins C and E—as well as enzymes produced in the cell, such as superoxide dismutase (SOD), catalase, and glutathione peroxidase, prevent most oxidative damage. Nonetheless, some free radicals manage to circumvent these defenses and do harm. As a result, cellular repair mechanisms eventually falter and some internal breakdowns are inevitable. These breakdowns can lead to cellular senescence, and eventually may trigger apoptosis, a form of programmed cell death.
Support for the free radical theory, which was first proposed in 1956 by chemist Denham Harman, M.D., comes from studies of antioxidants, particularly SOD. SOD converts an oxygen radical known as superoxide anion into hydrogen peroxide, which can be degraded by an enzyme, called catalase, into oxygen and water. Studies have shown that inserting extra copies of the SOD gene into fruit flies extends their average lifespan by as much as 30 percent.
Other experimental evidence lends support to the free radical hypothesis. For example, higher levels of SOD and catalase have been found in long-lived nematodes. In one compelling study, giving nematodes synthetic forms of these antioxidants significantly extended their normal lifespan. (See Antioxidants and Aging Nematodes).
The discovery of antioxidants raised hopes that people could retard aging simply by adding them to the diet. So far, studies of antioxidant-laden foods and supplements in humans have yielded little support for this premise. Further research, including largescale epidemiological studies, might clarify whether dietary antioxidants can help people live longer, healthier lives. For now, however, the effectiveness of dietary antioxidant supplementation remains controversial. In the meantime, gerontologists are investigating other intriguing biochemical processes affected by free radicals, including protein crosslinking.
| Research on Sunlight May Help Explain What Happens to Skin as We Age |
|
As anyone who reads beauty magazines knows, sunlight damages skin in ways that seem similar to aging. It’s well-established that long-term, sunlight-induced damage causes wrinkles. And in both normal aging and photoaging—the process initiated by sunlight—the skin becomes drier and loses elasticity. Although gerontologists think that the normal or intrinsic aging process is probably not the same as photoaging, there are enough similarities to make this a tantalizing field of study.
The process of photoaging may hold clues to normal aging because many of the same cells are affected. Photoaging, for example, damages collagen and elastin, the two proteins that give skin its elasticity. These proteins decline as we age, along with the fibroblast cells that manufacture them. In addition, the enzymes that break down collagen and elastin increase.
Other changes occur in keratinocytes, upper-layer skin cells that are shed and renewed regularly. In the normal aging process the turnover of keratinocytes slows down and in photoaging they are damaged. Still other skin cells, called melanocytes, are also affected by both processes: they decline with normal aging and are killed in photoaging. (Stopped in their tracks by sunlight, these normally migratory cells show up as freckles in light skin.)
What we don’t know yet is exactly how photoaging damages cells. Ultraviolet light can damage DNA and could be the culprit. Free radicals could be involved in some way. Researchers continue to explore these and other factors in the effort to understand photoaging. |
Protein Crosslinking
Blood sugar—glucose—is another suspect in cellular deterioration. In a process called non-enzymatic glycosylation or glycation, glucose molecules attach themselves to proteins, setting in motion a chain of chemical reactions that ends in the proteins binding together or crosslinking, thus altering their biological and structural roles. The process is slow and complex, but crosslinked proteins accumulate with time and eventually disrupt cellular function.
Investigators suspect that glycation and oxidation are interdependent processes since free radicals and crosslinks seem to accelerate the formation of one another.
Crosslinks, also known as advanced glycation end products (AGEs), seem to “stiffen” tissues and may cause some of the deterioration associated with aging. Collagen, for instance, the most common protein molecule in our bodies, forms the connective tissue that provides structure and support for organs and joints. When glucose binds with collagen—as it tends to do as we age—this normally supple protein loses much of its flexibility. As a result, lungs, arteries, tendons, and other tissues stiffen and become less efficient. In the circulatory system, AGEs may help trap LDL (the so-called “bad”) cholesterol in artery walls, and thus contribute to the development of atherosclerosis. They also have been linked to clouded lenses (cataracts), reduced kidney function (nephropathy), and age-related neurological disorders including Alzheimer’s disease.
These conditions appear at younger ages in people with diabetes, who have high glucose levels (hyperglycemia). Glycosylated hemoglobin in red blood cells, for instance, is an important marker doctors use to measure hyperglycemia. While the physiological effects of glycosylated hemoglobin are unclear, the disease it helps doctors detect—diabetes—is sometimes considered an accelerated model of aging. Not only do the complications of diabetes mimic the physiologic changes that can accompany old age, but people with this condition have shorter-than-average life expectancies. As a result, much research on crosslinking has focused on its relationship to diabetes as well as aging.
| |
Macrophages |
| |
|
| |
Macrophages, such as the one shown here, recognize foreign bodies such as bacteria, or altered molecules such as crosslinked proteins, and remove them from circulation. |
Just as the body has antioxidants to fight freeradical damage, it has other guardians, immune cells called macrophages, which combat glycation. Macrophages with special receptors for AGEs seek out and engulf them. Once AGEs are broken down, they are ejected into the blood stream where they are filtered out by the kidneys and eliminated in urine.
The only apparent drawback to this defense system is that it is not complete and levels of AGEs increase steadily with age. One reason is that kidney function tends to decline with advancing age. Another is that macrophages, like certain other components of the immune system, become less active.
Why this happens is not known, but immunologists are beginning to learn more about how the immune system affects, and is affected by aging (See The Immune System). And in the meantime, diabetes researchers are investigating drugs that could supplement the body’s natural defenses by blocking AGEs formation.
Crosslinking interests gerontologists for several reasons. It is associated with disorders that are common among older people, such as diabetes and heart disease; it progresses with age; and AGEs are potential targets for drugs. In addition, cross-linking may play a role in damage to DNA, which is another important focus for research on aging.
DNA Repair and Synthesis
In the normal wear and tear of cellular life, DNA undergoes continual damage. Attacked by oxygen radicals, ultraviolet light, and other toxic agents, it suffers damage in the form of deletions, or deleted sections, and mutations, or changes in the sequence of DNA bases that make up the genetic code. In addition, sometimes the DNA replication machinery makes an error.
Biologists theorize that this DNA damage, which gradually accumulates, leads to malfunctioning genes, proteins, cells, and, as the years go by, deteriorating tissues and organs.
Not surprisingly, numerous enzyme systems in the cell have evolved to detect and repair damaged DNA. For repair, transcription, and replication to occur, the double-helical structure that makes up DNA must be partially unwound. Enzymes called helicases do the unwinding. Investigators have found that people who have Werner’s syndrome (WS), a rare disease with several features of premature aging, have a defect in one of their helicases. George Martin, M.D., of the University of Washington and other investigators are exploring the mechanisms involved in DNA repair in WS and similar disorders, collectively known as progeroid syndromes. This research could help explain why DNA repair becomes less efficient during normal human aging.
The repair process interests gerontologists for many reasons. It is known that an animal’s ability to repair certain types of DNA damage is directly related to the lifespan of its species. Humans repair DNA, for example, more quickly and efficiently than mice or other animals with shorter lifespans. This suggests that DNA damage and repair are in some way part of the aging puzzle.
In addition, researchers have found defects in DNA repair in people with a genetic or familial susceptibility to cancer. If DNA repair processes decline with age while damage accumulates as scientists hypothesize, it could help explain why cancer is more common among older people.
Gerontologists who study DNA damage and repair have begun to uncover numerous complexities. Even within a single organism, repair rates can vary among cells, with the most efficient repair going on in germ (sperm and egg) cells. Moreover, certain genes are repaired more quickly than others, including those that regulate cell proliferation.
| |
DNA |
| |
|
| |
DNA, the double helix cellular molecule that contains thousands of genes necessary for life, is constantly being damaged and repaired. Gerontologists suspect DNA repair mechanisms become less efficient with age. Accumulating DNA damage, including breaks in its structure or changes in its nucleotide sequences, can lead to some of the physical changes we associate with aging. |
Especially intriguing is repair to a kind of DNA that resides not in the cell’s nucleus but in its mitochondria. These small organelles are the principal sites of metabolism and energy production, and cells have hundreds of them. Investigators suspect mitochondrial DNA is injured at a much greater rate than nuclear DNA, possibly because the mitochondria produce a stream of damaging oxygen radicals during metabolism. Adding to its vulnerability, mitochondrial DNA is unprotected by the protein coat that helps shield DNA in the nucleus from damage.
Research has shown that mitochondrial DNA damage increases exponentially with age, and as a result, energy production in cells diminishes over time. These changes may cause declines in physiological performance, and may play a role in the development of age-related diseases. Investigators are examining how much mitochondrial DNA damage occurs in specific parts of the body such as the brain, what causes the damage, and whether it can be prevented.
Heat Shock Proteins
In the early 1960s, investigators noticed fruit flies did something unusual. When these insects were exposed to a burst of heat, they produced proteins that helped their cells survive the temperature change. Intrigued, researchers looked for these proteins in other animals, and found them in virtually every living thing including plants, bacteria, worms, mice, and yes, humans. Today, the role of these substances, known as heat shock proteins, in the aging process is under scrutiny.
Despite their name, heat shock proteins (HSPs) are produced when cells are exposed to various stresses, not only heat. Their expression can be triggered by exposure to toxic substances such as heavy metals and chemicals and even by behavioral and psychological stress.
What attracts aging researchers to HSPs is the finding that the levels at which they are produced depend on age. Old animals placed under stress—short term, physical restraint, for example—have lower levels of a heat shock protein designated HSP-70 than young animals under similar stress. Moreover, in laboratory cultures of cells, researchers have found a striking decline in HSP-70 production as cells approach senescence.
Exactly what role HSPs play in the aging process is not yet clear. They are known to help cells dismantle and dispose of damaged proteins. They also facilitate the making and transport of new proteins. But what proteins are involved and how they relate to aging is still the subject of speculation and study.
While at the NIA, Nikki Holbrook, Ph.D., and other researchers investigated the action of HSP-70 in specific sites, such as the adrenal cortex (the outer layer of the adrenal gland). In this gland as well as in blood vessels and possibly other sites, the expression of HSP-70 appears closely related to hormones released in response to stress, such as the glucocorticoids and catecholamines. Eventually, answers to the puzzle of HSPs may throw light on some parts of the neuroendocrine system, whose hormones and growth factors might have an important influence on the aging process.
Hormones
Hormones are powerful chemicals that help keep our bodies working normally. Made by specialized groups of cells called glands, hormones stimulate, regulate, and control the function of various tissues and organs. They are involved in virtually every biological process including sexual reproduction, growth, metabolism, and immune function. These glands, including the pituitary, thyroid, adrenal, ovaries, and testes, release various hormones into the body as needed.
As we age, production of certain hormones, such as testosterone and estrogen, tends to decrease. Hormones with less familiar names, like melatonin and dehydroepiandrosterone (DHEA) are also not as abundant in older people as in younger adults. But what influence, if any, these natural hormonal declines have on the aging process is unclear.
| |
Hormones |
| |
|
| |
In this colored transmission electron micrograph, growth hormone granules (brown) are visible in the cytoplasm (yellow) of a growth-hormone secreting endocrine cell from the pituitary gland. Visible cell organelles include mitochondria (round, green) and the nucleus (purple, center). |
Hormone Replacement
In the late 1980s, at Veterans Administration hospitals in Milwaukee and Chicago, 12 men age 60 and older began receiving injections three times a week that dramatically reversed some signs of aging. The injections increased their lean body (and presumably muscle) mass, reduced excess fat, and thickened skin. When the injections stopped, these changes reversed, and the signs of aging returned. What the men were taking was recombinant human growth hormone (hGH), a synthetic version of the hormone that is produced in the pituitary gland and plays a critical part in normal childhood growth and development. At the same time, evidence was accumulating that menopausal hormone therapy with estrogen (alone or in combination with a progestin in women with a uterus) could benefit postmenopausal women by reducing cardiovascular disease, colon cancer, and other diseases of aging. Further studies have indicated that, although estrogen remains an effective way to control hot flashes, long-term use of these hormones may increase risk for several major age-related diseases in some women, especially then treatment is started years after menopause. The finding that levels of testosterone in men decreased with aging raised the question of whether they too might benefit from sex hormone treatment.
As a result of these preliminary observational findings, the NIA launched a series of research initiatives to clarify what influence hormone replacement therapy might have on the aging process. So far, most of these studies have been inconclusive, but have led many investigators to question whether the risks of hormone replacement may outweigh any benefit. Supplements of hGH, for instance, can promote diabetes, joint pain, carpal tunnel syndrome, and pooling of fluid in the skin and other tissues, which may lead to high blood pressure and heart failure. Studies in mice have raised other concerns about the hormone. Investigators have found that mice deficient in growth hormone production live substantially longer than normal mice, while mice overproducing growth hormone live shorter than average lives. This finding suggests that even if hGH replacement therapy is initially beneficial, ultimately it may be harmful and actually might curtail longevity.
Similarly, there is scant evidence that testosterone supplementation has any positive impact in healthy older men. In fact, some studies suggest supplementation might trigger excessive red blood cell production in some men. This side effect can increase a man’s risk of stroke.
Estrogen is perhaps the most well studied of all hormones. Yet results from the Women’s Health Initiative (WHI), the first major placebo-controlled, randomized clinical trial of estrogen therapy with or without progestin to prevent some chronic diseases of aging, surprised the medical community. There were more cases of stroke, blood clots, heart disease, and breast cancer in postmenopausal women using estrogen and progestin in the study, and more cases of possible dementia in women over age 65, than in those using the placebo. But, there were also fewer bone fractures and cases of colon cancer. In postmenopausal women using estrogen alone, there were more cases of stroke and fewer bone fractures than in those women on placebo. Other studies indicate that menopausal hormone therapy is effective in controlling moderate-to-severe menopausal symptoms, so research is ongoing to evaluate benefits and risks in menopausal and younger postmenopausal women.
As research continues, the pros and cons of hormone replacement may become more precisely defined. These hormonal supplements appear to increase risk and provide few clear-cut benefits for healthy individuals and do not seem to slow the aging process.
| Hormones and Research on Aging |
|
Produced by glands, organs, and tissues, hormones are the body’s chemical messengers, flowing through the blood stream and searching out cells fitted with special receptors. Each receptor, like a lock, can be opened by the specific hormone that fits it and also, to a lesser extent, by closely related hormones. Here are some of the hormones and other growth factors of special interest to gerontologists.
 ESTROGEN > Although it is primarily associated with women, men also produce small amounts of this sex hormone. Among its many roles, estrogen slows the bone thinning that accompanies aging. In premenopausal women the ovaries are the main manufacturers of estrogen (see image). After menopause, fat tissue is the major source of smaller amounts and weaker forms of estrogen than that produced by the ovaries. While many women with menopausal symptoms are helped by hormone therapy during and after menopause, some are placed at higher risk for certain diseases if they take it. The results of the WHI are prompting further studies about the usefulness and safety of this therapy when used by younger menopausal and postmenopausal women to control symptoms, such as hot flashes, and to prevent chronic diseases. ESTROGEN > Although it is primarily associated with women, men also produce small amounts of this sex hormone. Among its many roles, estrogen slows the bone thinning that accompanies aging. In premenopausal women the ovaries are the main manufacturers of estrogen (see image). After menopause, fat tissue is the major source of smaller amounts and weaker forms of estrogen than that produced by the ovaries. While many women with menopausal symptoms are helped by hormone therapy during and after menopause, some are placed at higher risk for certain diseases if they take it. The results of the WHI are prompting further studies about the usefulness and safety of this therapy when used by younger menopausal and postmenopausal women to control symptoms, such as hot flashes, and to prevent chronic diseases.
GROWTH HORMONE > This product of the pituitary gland appears to play a role in body composition and muscle and bone strength. It is released through the action of another trophic factor called growth hormone releasing hormone, which is produced in the brain. It works, in part, by stimulating the production of insulin-like growth factor, which comes mainly from the liver. All three hormones are being studied for their potential to strengthen muscle and bones and prevent frailty among older people. For now, however, there is no convincing evidence that taking growth hormone will improve the health of those who do not suffer a profound deficiency of this hormone.
MELATONIN > Contrary to some claims, secretion of this hormone, made by the pineal gland, does not necessarily diminish with age. Instead, a number of factors, including light, can affect production of this hormone, which seems to regulate various seasonal changes in the body. Current research does indicate that melatonin in low dosages may help some older individuals with their sleep. However, it is recommended that a physician knowledgeable in sleep medicine be consulted before self-medication. Claims that melatonin can slow or reverse aging are far from proven.
 TESTOSTERONE > In men, testosterone (see image) is produced in the testes (women also produce small amounts of this hormone). Production peaks in early adulthood. However, the range of normal testosterone production is vast. So while there are some declines in testosterone production with age, most older men stay well within normal limits. The NIA is investigating the role of testosterone supplementation in delaying or preventing frailty. Preliminary results have been inconclusive, and it remains unclear if supplementation of this hormone can sharpen memory or help men maintain stout muscles, sturdy bones, and robust sexual activity. Investigators are also looking at its side effects, which may include an increased risk of certain cancers, particularly prostate cancer. A small percentage of men with profound deficiencies may be helped by prescription testosterone supplements. TESTOSTERONE > In men, testosterone (see image) is produced in the testes (women also produce small amounts of this hormone). Production peaks in early adulthood. However, the range of normal testosterone production is vast. So while there are some declines in testosterone production with age, most older men stay well within normal limits. The NIA is investigating the role of testosterone supplementation in delaying or preventing frailty. Preliminary results have been inconclusive, and it remains unclear if supplementation of this hormone can sharpen memory or help men maintain stout muscles, sturdy bones, and robust sexual activity. Investigators are also looking at its side effects, which may include an increased risk of certain cancers, particularly prostate cancer. A small percentage of men with profound deficiencies may be helped by prescription testosterone supplements.
DHEA > Short for dehydroepiandrosterone, DHEA is produced in the adrenal glands. It is a precursor to some other hormones, including testosterone and estrogen. Production peaks in the mid-20s, and gradually declines with age. What this drop means or how it affects the aging process, if at all, is unclear. Investigators are working to find more definite answers about DHEA’s effects on aging, muscles, and the immune system. DHEA supplements, even when taken briefly, may cause liver damage and have other detrimental effects on the body. |
Growth Factors
Some types of hormones can be referred to as growth or trophic factors. These factors include substances such as insulin-like growth factor (IGF-I), which mediates many of the actions of hGH. Another trophic factor of interest to gerontologists is growth hormone releasing hormone, which stimulates the release of hGH. Growth factors might have an important role in longevity determination. In nematodes, for instance, mutations in at least two genes in the IGF-I pathway result in extended lifespan.
The mechanisms—how hormones and growth factors produce their effects—are still a matter of intense speculation and study. Scientists know that these chemical messengers selectively stimulate cell activities, which in turn affect critical events, such as the size and functioning of skeletal muscle. However, the pathway from hormone to muscle is complex and still unclear.
Consider growth hormone. It begins by stimulating production of IGF-I. Produced primarily in the liver, IGF-I enters and flows through the blood stream, seeking out special IGF-I receptors on the surface of various cells, including muscle cells. Through these receptors it signals the muscle cells to increase in size and number, perhaps by stimulating their genes to produce more of special, muscle-specific proteins. Also involved at some point in this process are one or more of the six known proteins that specifically bind with IGF-I; their regulatory roles are still a mystery.
As if the cellular complexities weren’t enough, the action of growth hormone also may be intertwined with a cluster of other factors—exercise, for example, which stimulates a certain amount of hGH secretion on its own, and obesity, which depresses production of hGH. Even the way fat is distributed in the body may make a difference; lower levels of hGH have been linked to excess abdominal fat but not to lower body fat.
<< Back | Next >>
