Justification - National Institute on Aging
Authorizing Legislation: Section 301 and Title IV of the Public Health Service Act, as amended. Reauthorizing legislation will be submitted.
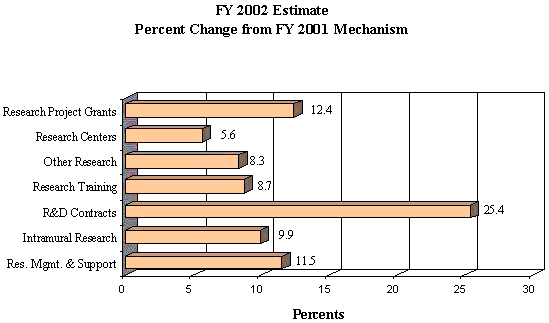
Budget Authority
FY 2000 Actual | FY 2001 Estimate | FY 2002 Estimate | Increase or Decrease |
| FTEs | BA | FTEs | BA | FTEs | BA | FTEs | BA |
| 384 | $687,969,000 | 457 | $786,452,000 | 457 | $879,961,000 | 0 | $93,509,000 |
This document provides justification for the FY 2002 activities of the National Institute on Aging (NIA), including HIV/AIDS activities. A more detailed description of NIH-wide Fiscal Year 2002 HIV/AIDS activities can be found in the NIH section entitled "Office of AIDS Research (OAR)."
Introduction
Older Americans are generally better off—healthier and wealthier—than ever before.(1) Average life expectancy in the United States has at least doubled over the past century, and a baby born today is expected to live almost 30 years longer than one born in the year 1900. These improvements in life expectancy, from an average of 49 years in 1900 to age 76 at the turn of the 21st century, plus the advent of such programs as Medicare and Social Security have helped to improve both the physical and fiscal well-being of the Nation's older population. The added years of life have allowed the vast majority of older Americans to enjoy a healthy and active retirement. A majority of people 65 and older rate their health as good or excellent.
But good health is far from a universal reality for older Americans. The latest national surveys indicate that about one-fifth of people age 65 and older, more than 7 million people, report some disability.(2)
Chronic disease, memory impairment, and depressive symptoms affect large numbers of older people and the risk of such problems significantly rises with age. Nearly half of those age 85 and older suffer from Alzheimer's disease.(3) These millions of less fortunate older people struggle with daily activities as simple as bathing and dressing, with families and friends taking on the difficult and often costly role of caregiver.
Understanding the difference between advanced years that are active and independent and those that are characterized by frailty and dependence is at the heart of the NIA's research program. Since the Institute's founding in 1974, research has shed considerable light on aging and health. It is now known that aging itself is not the cause of disease, disability, and frailty associated with advancing age. Indeed, the converse is true: It is disease and disabling processes, influenced by age-related changes in the body and by unhealthy choices and sedentary lifestyles, that are the most important factors in compromising the quality of life for older people. This fundamental shift in thinking was reinforced most recently with insights from the National Long Term Care Survey (NLTCS) and other such longitudinal analyses. According to the NLTCS, the rate of disability among older Americans dramatically declined from the 1980s through the mid 1990s, even among people age 85 and older, who are most vulnerable to disabling conditions. These findings, along with evidence from a number of clinical trials and studies testing specific interventions, suggest more strongly than ever that disease and disability can be addressed and are not inevitable consequences of aging.
The challenge now is to maintain and even accelerate the trend in declining disability and to reduce rates of disease amid a steep rise in the number and proportion of older people. The task is urgent. Demographic projections show that the U.S. population is beginning to age at a rapid pace, with the first baby boomers turning 65 in 2011. Between now and the year 2030, the number of individuals age 65 and older likely will double, reaching 70.3 million and comprising a larger proportion of the entire population, up from 13% today to 20% in 2030.(4) Of great interest is the explosive growth anticipated among those most at risk of disease and disability, people aged 85 and older. Their ranks are expected to grow from 4.3 million (1.6%) in 2000, to at least 19.4 million (4.8%) in 2050.3 The racial and ethnic makeup of the older population will change dramatically as well, bringing with it possibly even greater racial and ethnic disparities in health among a more diverse population of older Americans. These demographic factors threaten to combine to increase the burden of age-related diseases and conditions on individuals, families, and society. Unless new understandings and interventions are developed and implemented to reduce disease and disability before the population ages so intensively, the costs, in both human and financial terms, could be extraordinary.
In the 20th century, health research and public health practices did much to extend life and improve health. At the start of this new millennium, the NIA's research portfolio is aimed primarily at increasing "healthspan," or years of healthy active life expectancy. Aging research is at the threshold of discovery, poised to build upon the work of recent years to make a difference in the lives of older Americans and their families. Toward that end, NIA's overall program is wide-ranging and includes research on: the biochemical, genetic, and physiological mechanisms of aging in humans and animal models; the structure and function of the aging nervous system; social and behavioral aspects of aging processes and the place of older people in society; and the pathophysiology, diagnosis, treatment, and prevention of age-related diseases, degenerative conditions, and disabilities. The NIA is the lead federal agency for Alzheimer's disease research.
In close collaboration with the National Advisory Council on Aging and other public and private organizations, the NIA has developed a strategic plan for aging research, to identify goals for the next 5 years. These goals address scientific areas that hold the greatest promise for advancing knowledge, many outlined in this narrative. The NIA also recently completed a strategic plan on disparities in health status of older Americans of different racial and ethnic backgrounds. In this narrative, the Institute focuses on recent progress and future directions for research in four key areas: Section I.) Alzheimer's disease and the neuroscience of aging; Section II.) reducing chronic disease and disability; Section III.) the biology of aging; and Section IV.) the behavioral and social aspects of growing older. In each of these efforts and more broadly, the Institute is paying special attention to reducing health disparities among different groups of Americans (Section V.). Interspersed within the narrative, in two sections, are "Stories of Discovery," which follow the history and the drama of unfolding scientific knowledge in a specific area of interest.
Alzheimer's Disease and the Neuroscience of Aging
Alzheimer's disease (AD) is a progressive, currently irreversible brain disorder. People with AD gradually suffer memory loss and a decline in thinking abilities, as well as major personality change. These losses in cognitive function are accompanied by pathologic changes in the brain, including the build up of amyloid plaques and tau-containing neurofibrillary tangles, which result in death of brain cells and breakdown of the connections between them. AD advances by stages, from early, mild forgetfulness to a severe loss of mental function called dementia. Eventually, all reasoning ability is lost and people with AD become dependent on others for every aspect of their care. The risk of developing AD increases exponentially with age, but it is not a part of normal aging.
The most common cause of dementia among people age 65 and older, AD is a major health issue for the United States because of its enormous impact on individuals, families, the health care system, and society as a whole. Scientists estimate that as many as 4 million people currently suffer with the disease, (5) and annual costs associated with AD are estimated to exceed $100 billion. (6)
As the population ages, it is projected that the numbers of people with AD and costs associated with increased prevalence could rise significantly.
The following section on Alzheimer's disease and neuroscience describes recent research advances in 5 areas of AD research—improvements in early diagnosis, defining normal age-related cognitive change, the role of environmental factors in development of AD, pre-clinical research on animal models to better characterize what may be happening in the brain during the course of AD, and clinical trials to test new therapies that may delay or even prevent development of the disease. The discussion on AD begins with a Story of Discovery on exciting developments leading to potential new therapies to attack the formation of amyloid plaques in the brain, including a possible vaccine. It concludes with a look at future directions for research, outlining the NIA's AD Prevention Initiative and studies seeking to find how to maintain the health of the brain with age.
Story of Discovery: Alzheimer's Disease Amyloid—Discovery of Molecular Processes Leads to New Therapeutic Approaches
Alois Alzheimer first described the plaques and tangles found in the brains of dementia patients in 1906. He had a patient who became demented in her 50s and died at age 55. Upon her death, Dr. Alzheimer performed an autopsy and found plaques and tangles in her brain tissue. For the next 60 years, scientists and clinicians thought that this form of early-onset dementia was very different from the dementia of old age. Indeed, dementia in older people was considered an almost inevitable part of aging. Then, in the 1970s, scientists discovered that older people with dementia often had exactly the same plaques and tangles in their brains as those described so clearly by Dr. Alzheimer.
Two abnormal structures in the brain are hallmarks of AD: amyloid plaques and neurofibrillary tangles. Plaques are dense deposits of protein and cellular material outside and around the brain's nerve cells. Tangles are twisted fibers that build up inside the nerve cells. Though scientists have known about plaques and tangles since Dr. Alzheimer's time, the intensive research efforts of the last two decades have revealed much about their composition, how they form, and their possible roles in the development of AD. The deposition of amyloid in the form of plaques is thought by many to trigger the cascade of events leading to AD pathology. Amyloid now is believed to be a critical target for eventual treatment. In both the public and the private sector, important efforts are underway to expand understanding of the amyloid deposition process while, at the same time, developing potential therapies based on current knowledge.
The unfolding story of amyloid, from hypothesis to potential breakthroughs in treatment, illustrates the dynamics of AD research and the synergy of public and private efforts to challenge this dreaded disease. In the 1980s, amyloid from the brain tissue of an AD patient was found to consist of a short protein fragment (a peptide). The likely DNA sequence coding for this amyloid peptide was predicted from its amino acid sequence and was then used as a molecular probe to search the 100,000 or so genes present in human DNA for the specific gene that produces amyloid. Several research groups found the gene, naming it the amyloid precursor protein (APP) gene. By the mid 1990s, three mutated genes causing major forms of inherited early-onset AD were identified: the APP gene itself, as well as genes named presenilin 1 and presenilin 2.
These discoveries initiated the modern era of AD research. Building on this basic research, primarily funded by NIH, scientists from all sectors zeroed in on the proteins made by these genes and their metabolic pathways, uncovering major biological clues to the sequence of events in the development of AD pathology. Understanding these pathways and gene products now allows the design of treatments targeted to the early events that underlie the pathology of AD. By interfering with the disease early on, this approach aims to arrest disease before it affects brain function and causes clinical symptoms.
A major advance was developing the first animal models of AD by inserting mutated human APP genes into mouse eggs and observing formation of amyloid plaques and other AD-like pathologies in the brains of these "transgenic" mice as they age. Since then, numerous transgenic models have been developed, allowing scientists to understand better how a complex array of intercellular pathways can interact to affect the production of AD plaques. These transgenic animal models are also beginning to provide a means of testing the efficacy of different treatments on reducing build up of plaques and on cognitive function.
Much research has focused on the possibility that amyloid may play a causative role in AD. Interfering with amyloid production or its aggregation into plaques may represent an approach to treating or preventing disease. Using both transgenic animal models and experiments in tissue culture, scientists now understand more about how amyloid is snipped out of APP, how amyloid aggregates into plaques, and how plaques might lead to the brain destruction of later stages of AD. Importantly, these discoveries are also facilitating discovery of ways in which plaque production may be slowed. Many leads are being pursued, including development of compounds to halt amyloid deposition at various steps along the pathway, or to prevent downstream harmful effects of amyloid deposits.
During the past year, NIH- and industry-funded scientists provided evidence that one of the proteins (enzymes) that snip amyloid out of APP may actually be identical to one of the genes, presenilin 1, whose mutations can cause inherited early onset AD. Scientists from several pharmaceutical companies also identified the other snipping enzyme. Now, more effective drugs to inhibit production of amyloid by these enzymes can be developed. Prototypes are being developed and tested for safety in industry-sponsored trials.
Recent papers describing a potential vaccine for preventing plaque formation have excited AD researchers. Testing this unconventional approach in transgenic mice that make human amyloid in brain tissue, one industry scientist found that injecting an amyloid solution into the mice provoked an immune reaction to the injected amyloid. Further, this study indicated, if mice were immunized repeatedly for many months, plaque development was all but halted. This breakthrough has been successfully replicated in different strains of transgenic mice by a number of NIH-funded laboratories. One recent report shows that nasal inhalation of the amyloid can also retard plaque production, in a route of delivery that may be better tolerated than repetitive injections. A report at the recent Alzheimer's Disease World Congress went even further, demonstrating that vaccination prevented cognitive decline in a transgenic mouse model.
Though very preliminary safety testing of the injected vaccine in humans has shown promise for its tolerability so far, it is much too soon to know if such a vaccine could work in humans as it has in mice. Both technical and theoretical hurdles need to be overcome. Would there be harmful side effects? Preliminary industry-sponsored human safety trials have shown no harmful effects of one vaccine injection and trials looking at effects of multiple injections are ongoing. Will an intervention that prevents plaque formation indeed have an effect on the neuronal death and symptoms of AD? Ultimately, clinical trials in humans will provide answers to these questions.
Early Diagnosis of AD
The earlier an accurate diagnosis of AD is made, the better. This holds true for everyone involved, from individuals and their families to clinicians and researchers. For patients and their families, a definitive diagnosis early on provides an opportunity to plan and to pursue options for treatment and care while the patient can still take an active role in decision making. Clinicians increasingly will need effective tools for identifying people in the early stages of the disease, as new interventions are developed to stop or slow progression of symptoms. In research, earlier and more accurate diagnosis will simplify and improve recruitment for clinical trials to test new, preventive drugs.
Research suggests that earliest AD pathology may begin to develop in the brain 10 to 20 years before clinical symptoms yield a diagnosis. Scientists have been actively looking for ways to diagnose AD in its presymptomatic or preclinical stages, and enormous progress has been made in this area. Described below are recent advances in imaging and clinical assessment that will help to identify patients in very early stages of AD. Eventually, combinations of specific imaging strategies with genetic, clinical, and neuropsychological assessments may become the key to identifying people at very high risk of developing AD.
Use of Positron Emission Tomography (PET) Imaging To Identify Presymptomatic Decline in Brain Function. The gene APOE-ε4 has been associated with increased risk of AD. Scientists have been increasingly interested in whether the brain and brain function of people who carry one or more copies of APOE-ε4 are different from those of individuals who do not carry the gene to ultimately see whether AD-like symptoms can be identified before the disease is diagnosed clinically. PET imaging can provide information on metabolic function of specific brain regions. Recent studies using PET show that, despite similarities in age, gender, education, family history of dementia, and baseline performance on memory and other cognitive tasks, individuals with the APOE-ε4 gene(s) have reduced cerebral glucose metabolism in several areas of the brain compared to people who have none. The differences in metabolism were even greater two years after initial evaluation. Lower baseline metabolism at the start of the study predicted a greater cognitive decline in subjects at genetic risk for AD. Though longer follow-up studies are needed to determine how many of the APOE-ε4 carriers actually develop AD, these findings suggest that a combination of cerebral metabolic rate and genetic risk factors may be one way to help detect AD preclinically. actually develop AD, these findings suggest that a combination of cerebral metabolic rate and genetic risk factors may be one way to help detect AD preclinically.
Use of Magnetic Resonance Imaging (MRI) To Predict Development of AD. A recent study used a much more common technique than PET imaging, MRI, to determine whether persons in a very early phase of developing AD could be identified prior to a clinical diagnosis. Participants received MRI scans at the start of the study and then were followed for three years to determine who subsequently developed changes that met clinical criteria for AD. The researchers found that they could identify people who would develop AD over time with high accuracy, based on significantly smaller baseline volume measurements for specific brain regions, likely reflecting loss of brain cells in these areas. This study implicates specific brain areas in the underlying early pathology of AD and suggests that, by focusing on these areas, it may be possible to use existing imaging techniques to better identify people at greatest risk for AD. This promising MRI technique will need further research, refinement, and validation before it can become a part of standard clinical practice
In Vivo Detection of Amyloid Plaques. Scientists have been searching for a marker to be used in living patients (in vivo) to identify amyloid plaques that may be present in brain long before clinical diagnosis of the disease. A new molecular probe has recently been developed that sensitively labels plaques in post mortem AD brain sections. This probe now has been shown as well to label plaques throughout the brain after intracerebral injection in living transgenic mice. This probe is a prototype for molecules that could be used for radiological imaging of plaques in the brains of living people, permitting monitoring of the development and progression of AD as well as the clearance of plaques in response to antiamyloid therapies.
Standardized Clinical Information Can Predict Conversion to AD. Researchers have identified components of a standardized clinical assessment instrument that also appear to predict which individuals with very mild impairment (symptoms) or "questionable" AD have a high likelihood of converting to AD over time. The assessment instrument was the Clinical Dementia Rating (CDR), a clinical interview which stages AD from normal to severe based on six functional categories. After receiving a CDR rating of normal or questionable, participants were followed for three years to determine who converted to probable AD. Likelihood of progression to AD during follow-up was related to the sum of the scores in the six CDR categories. This score, combined with selected clinical interview questions, identified 89% of those questionable individuals who subsequently converted to AD in the study. These findings provide guidelines for using a clinical assessment to identify patients most likely to convert from questionable AD to AD, improving the possibility of earlier diagnosis and earlier implementation of available interventions.
Normal Age-Related Cognitive Change
Improved characterization of normal cognitive function and underlying brain changes over the life course will help in distinguishing and understanding normal from abnormal changes in memory, learning, and attention with age. Such understanding will help either confirm or, hopefully, alleviate the anxiety of many older Americans and their families, who may observe modest but perceptible changes in cognitive function in themselves or in a loved one and fear that such changes are the harbingers of a decline into AD or dementia.
Imaging Studies of Age Differences in Performing Memory Tasks. A recent study has shown that older adults show activation of more brain regions when performing a memory task than young adults. While both age groups were similarly accurate in performing the memory task, the older group was slower than the younger. Using PET imaging to measure cerebral blood flow, investigators reported activation of both frontal lobes of the brain among older people performing the memory task whereas young adults showed activation of only one of the frontal lobes. These results imply that the older brain is either changing or is compensating in some fashion in order to maintain appropriate cognitive function. Discovering more about why the older brain may perform differently will enable scientists to better determine how to maintain the ability to perform cognitive tasks. To date, intervention trials of cognitive training or aerobic exercise show selective but beneficial effects on cognitive function among older study participants. Understanding the relationship between these environmental modifications and brain function will permit even greater understanding of normal cognitive capacity in the elderly and offer clues for maintaining or improving cognitive function.
Early Life Environmental Factors and AD
Early Life Childhood and Adolescent Environment is Associated with the Risk of AD. Early-life environment has been implicated as a risk factor for many adult chronic diseases. A recent study looked at the association of AD risk with factors including mother's age at patient's birth, birth order, number of siblings, and area of residence prior to age 18. Results indicated that an increased number of siblings was associated with increased risk of AD and growing up in the suburbs was associated with a decreased risk. These associations were not explained by patients' educational level or APOE status. Such results are consistent with possible linkage of socioeconomic or environmental variables with altered brain growth and development, which in turn may affect the risk of developing AD later in life.
Preclinical Research
None of the treatments presently approved for AD alter the progressive underlying pathology of the disease. Early pathologic changes in the brain, including amyloid deposits and formation of neurofibrillary tangles, may play a causative role in AD. Interfering with these processes may be one way to treat or prevent the disease. Two promising approaches were reported this year; one involves blocking the activity of enzymes involved in the formation of amyloid and the other focuses on stopping the development of amyloid plaques by immunization. A new fruit fly model of Parkinson's disease (PD), another neurodegenerative disease, has been developed and could provide information about the etiology of these types of disorders.
Identification of the Amyloid-ß Forming Enzymes Offers New Targets for Drug Development. Amyloid is a small peptide fragment produced as a result of snipping (cleavage) of the much larger amyloid precursor protein (APP) by two enzymes known as beta (ß) and gamma (γ) secretases. For years, scientists knew that something was snipping the APP into fragments and they even went so far as to name the suspect secretases. But no one had been able to physically and precisely identify the enzymes that did the actual clipping of APP until the past year, when the identities of the ß and γ secretases at last were revealed.
The identity of ß secretase was discovered simultaneously by several drug companies. However, γ secretase has proven more elusive. Its activity was known to be affected by mutations in one of the genes (presenilin 1 or PS1) that cause AD in early onset families. PS1 was identified several years ago and structural evidence suggested it might actually be the γ secretase. To test this possibility, scientists identified a radioactive molecule that binds tightly to the active site of the enzyme, thus labeling the enzyme molecules. They found that PS1 was the labeled protein, strongly suggesting that it itself is the γ secretase. It is believed this line of research could lead to the discovery of drugs that inhibit the production of amyloid without inhibiting other essential functions these secretase enzymes might have. Ultimately, clinical trials on such secretase-inhibiting drugs will show whether this approach will work.
Immunization Against Amyloid-ß Can Reduce Brain Amyloid-ß Deposition. Recent studies in animal models have been important in understanding the etiology of AD and in testing potential new therapies. In transgenic mouse models showing extensive plaque formation with advancing age, researchers are now evaluating plaque-reducing drugs. The results of this research have been promising. In one breakthrough, pharmaceutical company scientists showed that repeated long-term injections of an amyloid vaccine can cause an immune response in test mice, nearly eliminating amyloid plaques and associated neuropathology, with no obvious toxicity. A number of NIH-funded scientists have confirmed and extended these observations. In a novel approach, one group administered the vaccine to mice nasally, and also induced an immune response. In that study, when young transgenic mice were repeatedly given the human amyloid-ß via the nasal route, the mice had a much lower amyloid burden at middle age than animals not receiving the vaccine. Interest in the vaccine approach heightened upon recent preliminary reports that amyloid vaccination prevents cognitive decline in another transgenic mouse model of AD, suggesting that a vaccine might indeed make a difference in the clinical symptoms of AD. Human trials are only now beginning to test both the safety and the efficacy of these vaccines as a possible therapy for people with AD.
A New Model of Parkinson's Disease (PD). There are many similarities among neurodegenerative diseases such as AD, PD, and other dementias, and research on one can provide valuable clues about the others. PD is a common age-related and progressive neurodegenerative disorder characterized by death of neurons that make the neurotransmitter dopamine. Loss of these neurons results in rigidity, tremor, slowed movement, and impaired gait. Another hallmark of PD is the formation of fibrous protein deposits, called Lewy bodies, in neurons. Mutations in the α-synuclein gene have been linked to some forms of inherited PD and insoluble α-synuclein accumulates in Lewy bodies, as well as in plaques in AD. A new α-synuclein transgenic model has been developed, using the fruit fly Drosophila, that exhibits many essential features of human PD including age-dependent onset, progressive loss of dopamine neurons and motor function, and development of Lewy body-like pathology. This model will be useful in identifying underlying mechanisms mediating α-synuclein toxicity and in identifying genes that modify the α-synuclein mediated neurodegeneration, and which may play a role in the pathogenesis of PD. These transgenic flies may also be valuable in screening potential drugs affecting the onset and progression of PD.
Clinical Trials
Today, an estimated 50 to 60 compounds are presently or will soon be tested in human AD clinical trials. These studies are sponsored by a number of sources, including the NIA, other NIH institutes and the private sector, primarily pharmaceutical companies. Compounds now under scrutiny focus on three major areas of treatment: short-term maintenance of cognitive function; slowing the progress of the disease, delaying AD's onset, or preventing the disease altogether; and managing behavioral problems associated with AD.
Currently available FDA-approved drugs maintain cognitive function in a subset of AD patients, but only for a limited time. NIH-funded clinical trials are, for the first time, targeting prevention of disease. Current clinical trials are examining a number of compounds to determine what works—and what may not—to slow the onset of AD or retard its development. Interest is now focusing on compounds which directly target disease-related pathologies. These include estrogen, anti-inflammatory agents, and antioxidants. Recently completed studies have moved our knowledge forward, and it is hoped that a great deal more will be learned from newly initiated efforts. Interestingly, some important new findings, in patients who already have AD, have been negative, showing no relationship between treatment with certain drugs and an effect on progression of the disease.
The research focus now is turning to prevention trials, and a number are underway to test the effectiveness of therapies in people without symptoms or who have only slight memory problems. Under scrutiny in these studies are further examination of estrogen and studies of various classes of anti-inflammatory drugs and antioxidants. Recruitment is complete for the first NIH AD prevention trial, to take place at more than 70 sites across the U.S. This trial compares the effects of vitamin E and donepezil (brand name Aricept) in preventing the development of AD in people diagnosed with mild cognitive impairment, a population at high risk for developing AD. Ongoing trials are also examining the effectiveness of naproxen and celecoxib (anti-inflammatory drugs) in reducing the risk of AD in persons with a family history of dementia, the effect of estrogen replacement therapy in preventing AD in women with a family history of the disease, and whether treatment with a variety of agents, such as aspirin, vitamin E, antioxidants, or combined folate/B6/B12 supplementation can prevent older women from developing age-related memory impairment or AD. As scientists test these currently available medications, the next generation of drugs is being developed, targeting specific abnormal cellular pathways uncovered by recent discoveries, including plaque and tangle formation and death of brain cells. Prevention trials are among the most costly of research projects, but, if successful, the payoff in terms of reduced disease and disability will be significant.
Caregiving of AD Patients
Ongoing Research Highlights Importance of Testing Interventions. REACH (Resources for Enhancing Alzheimer's Caregiver Health) is a multisite intervention trial, at six sites and a coordinating center, to conduct social and behavioral research on interventions designed to help caregivers of patients with AD and related disorders. REACH projects are testing such interventions as educational support groups, behavioral skills training programs, family-based interventions, environmental modifications, and computer-based information and communication services. Some 1,222 caregivers and care recipients have participated in the study, which includes large numbers of African Americans, Cuban Americans, and Mexican Americans. Data from the REACH study are just being analyzed, but very preliminary general findings suggest that testing of interventions to determine effectiveness in different groups is important, and research in this area continues.
Selected Future Research Directions in AD and the Neuroscience of Aging
Preventing Alzheimer's Disease: The AD Prevention Initiative. The NIA AD Prevention Initiative is an intensive, coordinated effort to accelerate basic research and the movement of basic research findings into the development of novel compounds to delay or slow the progress of AD or to prevent the disease entirely. Potentially promising strategies are being identified, based on new information about the initial stages and events in the brain that lead to AD, as well as data from studies of genetic and environmental risk factors. To follow up on these leads, NIH is implementing a five-year research initiative to speed the development of vaccine and other novel approaches for preventing AD. Another research initiative will examine changes in immune function with age, including response to different vaccination protocols. Along with the prevention initiative, other studies will continue to look at the many similarities in the biological mechanisms underlying neurodegenerative diseases such as AD, PD, and other dementias and will help to characterize age-related change in the normal, healthy brain.
As new leads are identified and developed in the test tube and in laboratory animals, findings are being translated into clinical interventions. The translation process will involve testing of drugs that target crucial pathways as well as incorporation of efficient processes for channeling drugs of interest into appropriately designed clinical trials. Plans for these clinical trials will increasingly emphasize AD prevention, including trials recruiting people with normal cognition and those with cognitive impairment. Continuing development of tools for early diagnosis will be pursued to help both clinicians and researchers in the treatment and the study of AD. Intervention studies aimed at people caring for AD patients will also be launched to develop and test additional ways of managing the daily activities and stresses of caregiving and to reduce caregiver burden. Investigations into long-term care issues involving AD will look at how to prevent hospitalizations and delay nursing home admissions.
Maintaining a Healthy Brain. Much is known and publicized about maintaining a healthy heart, but relatively little emphasis has been given to maintaining a healthy brain. Like the cardiovascular system, the brain and brain function have been shown to change over time. As people age, there can be positive changes in cognitive function such as greater wisdom or integrative prowess. Researchers have also measured performance deficits with age in the cognitive behaviors of attention, language, learning, decision making, and memory, as well as in sensory and motor systems, all of which can produce frustration and concern for older people. The molecular and cellular bases for these age-related deficits are being defined, and other possible risk factors are being assessed. Different life experiences and cultural factors, for example, are increasingly recognized as playing an important role in modulating cognitive content and performance throughout the lifespan. Scientists want to know, for example, how early life factors, education, social interactions and self-concept may influence brain health and behavior in later life through immune, endocrine, or other pathways.
Research to date has provided some basis for understanding the risk factors associated with compromised brain function. But while we are beginning to sort out the biological, environmental, and social factors that may be involved in brain health, much remains to be discovered. In an effort to accelerate the pace of scientific advances in the fields of cognition and emotion, a trans-NIH Healthy Brain Research Initiative is planned. This activity will combine the efforts of the National Institute of Neurological Disorders and Stroke, the National Institute of Mental Health, and the NIA in these areas. To start, an analysis of trans-NIH research in these areas will identify what is known, pinpoint gaps in knowledge, and map future directions for research.
As these areas are further investigated, additional research should be conducted to describe the "normal" course of cognitive change at very advanced ages. Studies of individuals age 85 and older, the fastest growing segment of the population, will provide information on healthy cognitive aging, onset of mild cognitive impairment, or dementia in this age group. Given findings suggesting that racial and ethnic minorities may be at greater risk of developing AD, there will also be a particular focus on potential differences in healthy brain aging for older racial and ethnic minorities, including effects of education, health and other life course variables.
Reducing Chronic Disease and Disability
Besides AD, chronic disease and disability can compromise the quality of life for older people. Some 79 percent of people age 70 and older have at least one of seven potentially disabling chronic conditions (arthritis, hypertension, heart disease, diabetes, respiratory diseases, stroke, and cancer).(7) The burden of such chronic conditions and disability poses a challenge not only to individuals, but to families, employers, and to the health care system as well. Research to improve understanding of the risk and protective factors for chronic disease and disability can offer effective prevention strategies. Described in this section are recent research advances on the benefits of exercise, treatment of various diseases, and the molecular underpinnings of disease. New directions for research are also outlined.
Benefits of Exercise
Regular exercise is essential to maintain health and function at any age. In older adults, it has been shown that exercise in four key areas—endurance, strength, balance, and flexibility—can improve function and reduce disease. Endurance activity is important for function of the heart, lungs, and circulatory system and may help prevent such diseases as diabetes, colon cancer, heart disease, and stroke. Even in very old adults, strength exercises build muscles, increase metabolism, and help to keep weight and blood sugar in check. Strength exercises have been shown to help prevent osteoporosis. Balance and flexibility exercises help prevent falls and other injuries. As research continues to measure such benefits, investigators also are delving deeper into how exercise does what it does. Better understanding in this area, for instance, in how exercise affects metabolic processes within the older body, may lead to new and innovative interventions.
Fitness Affects Mortality Risk Regardless of Body Fat. Both obesity and being unfit increase risk for chronic disease and death. However, the interrelationship between fitness, body fat, and mortality has not been clear. Recent research suggests that it is fitness, not fat, that may count most. In one study, investigators followed men 30–83 years of age for an average of eight years, classifying participants according to body fat as well as relative fitness based on exercise testing. Not surprisingly, the study showed that the higher the level of fat, the lower the level of fitness. But what intrigued researchers most were data showing that, within each category of body fat, "fit" men were at lower risk of death. Most strikingly, among those more fit, obesity was not significantly related to risk of death. In another study, low fitness increased mortality risk in men approximately fivefold for cardiovascular disease, and threefold for all-cause mortality. These findings suggest that, beyond interventions focusing on weight-loss to prevent and treat obesity-associated conditions, there may also be important benefits for the obese from improved fitness.
Stress Testing May Not Be Needed for Starting an Exercise Program. The role of exercise stress testing and safety monitoring for older people who want to start an exercise program is unclear. Current guidelines for routine exercise stress testing may deter older people from beginning an exercise program, either because of the cost of testing or because it may lead people to believe that exercise poses higher risks than it actually does. The latest research suggests that, in the absence of cardiovascular contraindications, the benefits of exercise for the elderly, balanced against a somewhat minor increase in risk, may be sufficient for starting an exercise program without prior exercise stress testing. Identifying Gene Variants That Influence the Interaction Between Exercise and Cholesterol Levels. The increase in body fat and loss of muscle mass that occur with age raise risk for disease, including diabetes and cardiovascular disease. Proper diet and/or exercise can be effective in helping to prevent or improve these conditions. However, there can be large variation in response to these interventions, in part because of genetic influences. Detection of specific genes affecting obesity, muscle mass, and blood cholesterol levels may provide a way to identify individuals likely to respond favorably to a particular intervention. In overweight, postmenopausal women, one genetic variation of an enzyme, lipoprotein lipase (LPL), was found to be associated with lower levels of both total cholesterol and LDL-cholesterol, risk factors for heart disease. The same LPL gene variant was found in older men with greater exercise-induced increases in HDL-cholesterol, a protective factor against heart disease. In another study, variants of the gene for apolipoprotein E (APOE), previously shown to influence blood cholesterol levels, were related to exercise-induced increases in HDL-cholesterol in middle-aged and older, overweight men. One form (APOE2) was associated with greater exercise-induced increases in HDL, compared with other variants of the APOE gene. Knowledge of how such specific gene variants interact with each other and with exercise and diet will lead to the development of more targeted and individualized prevention and treatment strategies.
Treatment and Prevention of Disease
Treatment of disease in older people can be complicated by the presence of other diseases and disorders and by the use of multiple medications to treat various conditions. Potential interactions of multiple medications, including those of prescribed drugs with over-the-counter drugs and dietary supplements, represent additional concerns. Moreover, compliance with treatment regimens can be difficult, as older patients often must maintain a complex schedule for taking several different medications. Research is ongoing to determine the best treatment approaches for older patients, particularly those with multiple comorbidities, and to identify strategies for improving compliance and minimizing potentially harmful effects of multiple medications.
Inadequate Treatment of Hypertension and Atrial Fibrillation (AF) in the Elderly. According to national surveys, 60–70% of older Americans aged 60 years and older have high blood pressure.(8) Despite the considerable amount of scientific evidence that hypertension is an important risk factor for cardiovascular disease in all age groups, survey data suggest that in hypertensives over age 70, only 25% of African Americans and only 18% of white Americans have achieved the blood pressure goals (140/90 mmHg) recommended by the Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure.(9)
Uncontrolled or inadequately controlled high blood pressure can lead to heart attack, stroke, heart failure, kidney disease, dementia or blindness. Another common cardiovascular problem is AF, a heart rhythm abnormality that can lead to circulating blood clots. Warfarin, a drug used to inhibit blood clotting, dramatically reduces the risk of stroke in patients with AF. Two recent studies suggest that older patients may be under-treated for both hypertension and AF. One found that developing new strategies to improve blood pressure control in older hypertensive patients might prevent an estimated 15% of heart attacks in this population. The other found that many patients with AF and at least one additional stroke risk factor, especially high blood pressure, did not receive appropriate warfarin therapy when such treatment may have been appropriate. These findings suggest the need for new strategies to enhance the appropriate use of currently available warfarin and antihypertensive treatments in standard clinical care.
Commonly Prescribed Diuretic Protects Against Osteoporosis. The lifetime risk of osteoporotic fracture in the U.S. is 40% in women and 13% in men. Because age-related bone loss increases susceptibility to fracture, strategies aimed at preserving bone mass are important. Large observational studies have consistently shown that the use of thiazide diuretics, usually prescribed to treat high blood pressure, is associated with higher bone density and about a 30% lower risk of hip fracture. Investigators recently completed a clinical trial to directly test the effect of taking thiazides on bone density in older men and women with normal blood pressure. Among healthy older adults, low-dose hydrochlorothiazide did preserve bone density at the hip and spine. The modest effects observed over three years, if accumulated over 10–20 years, may explain the 30% reduction in hip fracture risk associated with thiazides in the earlier observational studies. The results of this trial suggest that low-dose thiazide therapy may have a role in strategies to prevent osteoporosis.
Molecular Understanding of Disease Processes: Diabetes, Atherosclerosis
Diabetes is one of the leading causes of death and disability in the U.S. Type 2 diabetes, the most common form, usually develops in adults over 40 and is most common in adults 55 and older. Diabetes accelerates the narrowing of blood vessels by atherosclerosis and is a major risk factor for peripheral arterial disease. Atherosclerosis, which impairs blood circulation by narrowing arteries, is the most common form of occlusive vascular disease, a factor in both heart attacks and strokes. Most individuals with peripheral arterial disease have atherosclerosis, and symptoms can include painful cramping in leg muscles during physical activity. Studies reported here include: a new molecular approach for treating type 2 diabetes; gene therapy advances in possible treatment for peripheral artery disease; and advances in understanding and preventing the atherosclerotic process and how to reverse it.
Exendin-4 as a Treatment for Type 2 Diabetes. Type 2 diabetes mellitus (DM) is caused by an inability of the beta cells of the pancreas to compensate for increasing insulin demands; consequently, blood glucose levels rise. Scientists are searching for compounds that act on the pancreatic beta cells to prevent this progressive rise in blood glucose. GLP-1, a gut peptide, can stimulate beta cells to produce more insulin even in type 2 DM; however, its biologic half-life is short and its effects quickly wear off. Exendin-4, a newly studied peptide analog of GLP-1, is long-lived and more potent than GLP-1, and has been shown to reduce blood glucose levels in rodents. A recent study with small numbers of diabetic and nondiabetic humans demonstrated Exendin-4's efficacy in inducing insulin and normalizing blood sugar, even in diabetics. In the near future, an exendin-like drug possibly may become an effective treatment for type 2 DM.
Gene Transfer in Animal Model Stimulates Growth of New Vessels for Treatment of Peripheral Muscle Ischemia. Patients with blocked arteries in limbs are at risk of disabling symptoms in the part of the limb to which blood flow has been interrupted (ischemia). Restoring blood flow involves treatment with factors that promote artery regrowth (angiogenic factors). Until now, these angiogenic factors have been successfully tested only in animals whose blood supply is permanently blocked, and clinical trials in humans with similar permanent blockages are underway. Whether angiogenic treatment could also be used to treat people with intermittent ischemia, a condition in which the symptoms associated with blockage can recur periodically, is not known. Research is suggesting that intervention may be possible. In one recent study, scientists delivered an angiogenic factor by gene transfer into normal rat leg muscle several weeks before inducing ischemia by interrupting blood flow to the same leg. In that study, the angiogenic factor markedly increased formation of new blood vessels to the normal muscle, and these new blood vessels played a role in restoring blood flow and normal metabolism more quickly to the limb following ischemia. Successful development of this technique in humans might permit early intervention in patients with intermittent ischemia.
Regulation of TGF-ß Type II Receptor and Atherosclerosis. Atherosclerosis or narrowing of the arteries is the major risk factor for both heart disease and stroke and is a major complication after arteries have been surgically enlarged by balloon angioplasty. Throughout life, artery wall cells successfully repair injuries related to smoking, high blood pressure or cholesterol, making new cells to replace damaged ones. But constant exposure to such stresses eventually causes the artery wall cells to lose control of their replication. The growing mass of cells forms plaque, which eventually clogs the vessels and causes reduced blood flow. New research is helping to identify the complex series of cellular events causing cells to lose control of their division. In normal circumstances, a protein called TGF-ß1 prevents excessive cell division. It acts on the cells through binding to a protein receptor on the cell surface, the TGF-ß1 receptor, causing intracellular changes that stop cells dividing. In atherosclerotic lesions, it has been shown, unrestricted growth in some cells is caused by mutations in this receptor, inactivating it. Another way of preventing normal receptor function is to make too little TGF-ß1 receptor to be effective. One protein that inhibits the production of TGF-ß1 receptor is called Egr-1. This protein is found at very high levels in plaques, perhaps being induced by artery injury. Finding drugs to repress the activity of Egr-1 may be one way of keeping the key TGF-ß1 receptor functioning effectively to stop excessive cell division and prevent atherosclerosis.
Selected Future Research Directions To Reduce Disease and Disability
Cardiovascular Disease: Its Impact on Aging and Role in Dementia. Diseases of the heart and blood vessels are the leading cause of hospitalization and death in older Americans. The NIA is pursuing a broad program of basic and clinical cardiovascular research, often in collaboration with the National Heart, Lung, and Blood Institute (NHLBI). Characterization of age-associated changes in both the structure and function of the heart and blood vessels is vital to the development of newer, more effective prevention and treatment of cardiovascular disease. Research priorities include identifying genetic and environmental risk factors for hypertension, heart disease, and stroke as well as development of subclinical measures to predict development of cardiovascular disease. Studies are ongoing to determine the causes of age-associated increases in vascular stiffness, a potential risk factor for cardiovascular disease. Other research will focus on age-related changes in the structure and function of the heart's conduction system that can increase the risk of cardiac arrhythmias, especially atrial fibrillation that if uncorrected can lead to strokes. Additional priorities include determining the reasons for gender and racial differences in the aging cardiovascular system, delineating the relationship of cardiac enlargement to aging and disease development, reducing the progression of early atherosclerotic disease, and identification and testing of new therapeutic targets for congestive heart failure.
Further, the interrelationships among cardiovascular disease, cerebrovascular disease, and age-related cognitive declines, including dementia, need to be explored. Such research will help to better understand the range of factors that may contribute to mild cognitive deficits in elders and to develop improved methods for early identification of people at risk for dementia. Additional efforts in this area will look at new approaches for treatment and prevention, such as examining combined use of treatments targeted at cardiovascular disease, cerebrovascular disease, hypertension, and AD pathology. New technological developments in cardiac, vascular, and brain imaging and monitoring will be exploited to advance understanding of these disorders and their comorbidities, in animal models, including nonhuman primates, and in humans.
Treating and Reducing the Risk of Cancer. The second leading cause of death among the elderly is cancer. People age 65 and over account for 70 percent of cancer mortality in the U.S.(10) In collaboration with the National Cancer Institute (NCI), the NIA supports a research initiative to expand participation of older cancer patients in clinical trials and is expanding basic and clinical research on breast, prostate, and colon cancers. This research focuses on age-related changes that contribute to increased cancer incidence and mortality in older people, aggressive tumor behavior in the aged patient, the contributions of environmental versus hereditary factors to the risk of cancer, and the impact of previous or concurrent conditions and disabilities on the cancer experience of older patients. Specific research topics include: dose adjustment for antitumor agents and radiation therapy, diagnostic cancer imaging, the effect of coexisting diseases on cancer treatment and survival outcome, survival advantages or disadvantages of minority or ethnic populations, and underlying biological or environmental basis for cancers, such as prostate cancer, that disproportionately affect particular groups.
Type 2 Diabetes: An Age-Related Pathology. Unlike insulin-dependent diabetes, in which the pancreas makes no insulin, people with noninsulin-dependent diabetes (NIDDM) produce some insulin. However, not enough insulin is produced or the individual's cells are resistant to the insulin's action. NIDDM patients can often control their condition by weight loss through diet and exercise. Complications of diabetes are pervasive, including damage to the eyes, blood vessels, nervous system, and kidneys. A number of research lines within the NIA portfolio address issues relevant to diabetes. Work described earlier on a potential future drug treatment for type 2 diabetes, Exendin-4, is continuing. Increasing levels of physical activity and participation in exercise are often recommended for the prevention and treatment of common metabolic conditions such as obesity and insulin resistance/diabetes. NIA-funded scientists are investigating whether the metabolic benefits of exercise are mediated through body composition changes and/or occur independently of changes in body fat or leanness.
Potential Role of Caloric Restriction (CR) in Prevention of Multiple Diseases. In animal models, chronic CR has been shown to extend lifespan and delay the onset of age-related pathologies. A recent Request for Applications (RFA), has invited proposals for exploratory human studies on the effects of caloric restriction interventions on physiology, body composition, and risk factors for age-related pathologies, and encourages the use of such outcome measures as insulin sensitivity and glucose metabolism. Another current RFA is soliciting research proposals on the molecular and neural mechanisms underlying the beneficial effects of caloric restriction, and includes as a major objective investigation of the role played by reduction of blood glucose levels in the lifespan extension effects.
Enhancing Musculoskeletal Function. Osteoporosis, osteoarthritis, and age-related loss of muscle mass (or sarcopenia) contribute to frailty and injury among older people. The NIA supports several research initiatives to define the underlying mechanisms of aging in bone, muscle, and joints, and to design and evaluate effective prevention and intervention strategies for age-related musculoskeletal decline. In one area, scientists are exploring factors that may act to predispose older people to fractures. The role of exercise continues to be closely scrutinized. The physiological effects of exercise on muscle and bone are being examined, and the development of interventions to encourage older people to begin and maintain an exercise regimen is particularly important. Also, NIA participates in the NIH Federal Working Group on Bone Diseases, convened quarterly to provide opportunities for sharing information, identifying collaborative projects and carrying out specific joint activities such as conferences and research initiatives. In collaboration with the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), NIA will continue to evaluate future opportunities, currently encouraging researchers studying Paget's disease and osteogenesis imperfecta to focus on the effects of aging in patients with these conditions.
Biology of Aging
Aging is accompanied by gradual changes in most body systems. Research on the biology of aging focuses on understanding the cellular and molecular processes underlying these changes as well as those accompanying the onset of age-related diseases. As scientists learn more about these processes, experiments can be designed to better understand when and how pathological changes begin, providing important clues toward developing interventions to prevent or treat disease. A great deal has been learned about structural and functional changes that occur in different body systems, and progress is ongoing. Research has expanded our knowledge, too, of the biologic factors associated with extended longevity in humans and animal models. This section of the NIA's narrative discusses some recent advances in the biology of aging, on cloning and transplantation and on lifespan itself. Selected future research directions are described as well, including continuing efforts to find biologic interventions to promote healthy aging, to understand the genetic basis of aging, and to explore the potential of adult stem cells and cell replacement for reducing disease and improving function.
Cloning and Transplantation Strategies
There is enormous interest in the potential uses of cloning, gene therapy, and adult stem cell transplantation, as well as tissue transplantation, to combat diseases of aging. Cloning cells or animals could lead to new advances in medicine and agriculture, and each of these new techniques could lead to strategies to replace tissues and organs lost through disease.
Cloning Resets the Telomere Clock in Cattle. An important question in cloning research is whether cloned cells or organisms created from old or senescent cells will be biologically older than their normal counterparts. Telomeres are highly repetitive DNA sequences located at the end of chromosomes, and telomere length is associated with cell age. As cells divide, telomere length gets progressively shorter until eventually, proliferation stops entirely. Such cells, which have ceased dividing, are called senescent. In a recent study, nuclei from senescent bovine fibroblasts were transferred into egg cells from which the nucleus had been removed. The nuclei were reactivated and the egg cells were implanted into cows. Healthy calves were born, and were found to have telomere lengths that are more typical of young than old animals. Thus, telomere length was reset during gestation. Whether this will affect the lifespan of the cloned calves will not be known for many years; however it does appear from these data that cloned offspring in some, if not all, species will not be biologically older than normal offspring. Such information will be useful in developing cell replacement intervention strategies to restore cells damaged or lost through disease.
Cell Transplantation and Aging. An alternative to tissue or organ transplantation that appears to have great potential is formation of functional tissue from cell transplants. Recent research has shown that isolated cow or human adrenal gland cells inserted into immunodeficient mice formed functional adrenal tissue that resembles normal adrenal gland. This approach may potentially be used for any organ, either to study its functional regeneration in a living organism with age or to therapeutically regenerate lost function as in a case, for example, when defective genes might be replaced in cells isolated from a patient and then placed back into the same patient for tissue regeneration. This technique can also reduce the need for immunosuppressive therapies and offers an alternative to adult stem cell therapies.
Understanding and Extending the Lifespan
In order to understand the aging process, it is important to identify those factors that affect the overall lifespan of an organism. In mammals, there is a progressive physiologic decline with aging that is often accompanied by disease and disability. Understanding the responsible physiological mechanisms and, further, identifying ways to slow down age-related changes are important. Beyond any gains in lifespan, studies in this area are aimed more importantly at developing interventions to keep older people healthy and free of disease and/or disability as long as possible. Experiments in a number of animal models are providing valuable insights.
Extension of Average Lifespan of Nematodes by Pharmacological Intervention. It is widely accepted that oxidative stress is a factor in aging. To date, however, it has not been demonstrated convincingly that natural antioxidants such as vitamins C and E or ß-carotene extend lifespan in model experiments with mice, fruit flies, or nematodes (a kind of worm). Varied results have been obtained in genetically altered fruit flies over-expressing either superoxide dismutase (SOD) or SOD and catalase, enzymes that reduce oxidative damage. Now, an artificial compound, EUK-134, which mimics both SOD and catalase activity, has been shown to increase the average lifespan of nematodes by about 50%. EUK-134 also reversed premature aging in a nematode strain subject to elevated oxidative damage. These results strongly suggest that oxidative stress is a major factor in rate of aging in the nematode, and that this rate can be slowed by pharmacological intervention. It may be that similar compounds could lessen oxidative stress in humans and delay or reduce age-related pathology.
Genetically Mimicking Caloric Restriction (CR) Significantly Extends Yeast Lifespan. CR has been shown to significantly extend lifespan in a variety of organisms. In organisms studied to date (yeast, nematodes, fruit flies, mice and rats), CR increased both mean and maximum lifespan, as well as significantly reducing signs of disease. In all species examined, the extended longevity and health of the animals was accompanied by changes in the regulation of energy metabolism. Recent research has determined that genetic manipulation of glucose availability, metabolism, and signaling pathways can mimic the longevity-extending effects of CR in the yeast model. This discovery makes the yeast model of aging and longevity a powerful tool for uncovering the underlying cellular and molecular mechanisms responsible for increased longevity and health span, with a view to developing effective interventions.
CR Increases Neurotrophic Factor Production in the Brain and Protects Neurons. Beyond extending lifespan, CR also reduces development of age-related cancers, immune and neuroendocrine alterations, and motor dysfunction in rodents. Recent animal model studies of neurodegenerative disorders provide the first evidence that CR can also increase resistance of neurons to age-related and disease-specific stresses. One possible mechanism is that the mild metabolic stress associated with CR induces cells to produce proteins that increase cellular resistance to disease processes. Indeed, CR increases production of one such protein, a neuronal survival factor, BDNF. BDNF signaling in turn plays a central role in the neuroprotective effect of CR. This work suggests that CR may be an effective approach for reducing neuronal damage and neurodegenerative disorders in aging, providing insight into the design of approaches that might mimic CR's beneficial consequences.
Use of Gene Expression Microarrays in Aging Research. Aging is normally accompanied by changes in expression, or activity, of a large number of genes, but it is not clear which of these changes are critical in the aging process. Gene expression microarrays, which allow profiling the activity of many thousands of genes at once, provide an opportunity to obtain a more complete picture of what these changes are, and to design tests of whether these changes are causally associated with aging. In three recent studies, investigators looked at differences in gene expression patterns in young and old mouse skeletal muscle, liver, and brain tissue and also made several observations on changes brought about by caloric restriction. Though the data analyses are complex, some initial observations are: (1) aging results in lower levels of activity of metabolic and biosynthetic genes; (2) aging is accompanied by patterns of gene expression that are indicative of stress responses, including inflammatory and oxidative stress; (3) many, but not all, age-related changes in gene expression in mouse liver and skeletal muscle are slowed by caloric restriction; and (4) caloric restriction appears to increase expression of genes for repairing and/or preventing damage to cellular macromolecules. Microarray technology is proving to be an efficient approach to answering longstanding important questions about molecular mechanisms of aging and how these may be manipulated, for example, by calorie restriction. Profiling changes in gene activity may eventually provide useful biomarkers of the aging process itself, markers that might be important in assessing the effectiveness of strategies to retard aging-related processes.
Selected Future Research Directions in the Biology of Aging
Biological Interventions To Promote Healthy Aging. Counteracting the effects of aging by hormonal and dietary supplements, including estrogen, testosterone, human growth hormone, melatonin, and DHEA (dehydroepiandrosterone), is an area of active study. There are concerns that many middle-aged and older people may be taking such agents, before safety and efficacy of these substances for so-called "anti-aging" purposes have been adequately assessed. Although levels of some hormones may decline with age, maintaining levels that are normal at younger ages may not be needed, or even desirable, as a person grows older. Even if effective, supplementation may entail risks. More research is needed to determine how the biologic action of these hormones changes in older people and to assess whether replacement of these hormones will improve health.
CR is another biological intervention that may promote healthy aging. Some of CR's effects on longevity have been linked to changes in specific metabolic pathways. Studies are now planned to define the role of energy metabolism and metabolic regulation in mammalian aging, longevity and age-related disease, and uncover the cellular and molecular mechanisms that may be regulating aging processes, including those affected by CR. Most recently, researchers have identified changes in physiologic function in calorically restricted rhesus monkeys that suggest delays in aging-related decline. At this point, the effects of voluntary CR on lifespan and development of age-related diseases in humans are unknown. Preliminary human intervention studies are being designed to determine whether CR and physical activity differ in their long-term effects on obesity, body composition, prevention and susceptibility to age-related diseases.
Understanding the Genetic Basis of Aging, Longevity, Disease, and Behavior. Interactions between genetic and environmental factors are major determinants of aging and longevity in many species, including humans. NIA studies have begun to reveal the biologic factors associated with extended longevity in humans and animal models, implicating numerous genes in normal aging processes, age-related pathologies and diseases, and longevity. Some of these genes are associated with dramatic extension of lifespan. Using advanced technology, the NIA plans to accelerate its efforts to discover additional age- and longevity-related genes and to characterize their biological function. A new research initiative will extend studies of longevity-associated genes, changes in gene expression patterns, and the genetic epidemiology of human longevity. The ultimate goal of this effort is to develop interventions to reduce or delay age-related degenerative processes in humans. In addition, revolutionary advances in the fields of quantitative and molecular genetics hold great promise in the search for the genetic determinants of complex behaviors. Studies in humans can help identify the relative contributions of environment and heredity to dementia, cognitive abilities, physical functioning, well being, and social aging. New techniques can track the developmental course of genetic contributions to behavior, identify genetic heterogeneity, and explore genetic links between the normal and abnormal. Basic research will explore error accumulation in DNA with age and how the cell repairs such damage.
Exploring the Potential of Adult Stem Cells and Cell Replacement in Aging. Stem cells in adult human tissues retain the capacity for self-renewal and the potential to become many of the cell types in the human body. This capacity holds enormous potential for cell replacement or tissue repair therapy in many degenerative diseases of aging, including AD, PD, stroke, myocardial infarction, musculoskeletal disorders, immune system dysfunction, and diabetes. Emerging research findings suggest that it may be possible to harness the multipotential nature of adult stem cells to maintain tissue structure and function in aging. Much remains to be learned, however, about the basic biology of stem cells in animal models before effective cell therapy can be realized. The NIA is developing an research initiative on changes in stem cells and their environment with aging in animal models and in human nonfetal tissues. This research initiative will complement as well as encourage collaboration with other components of NIH.
Behavioral and Social Aspects of Growing Older
Behavioral and lifestyle factors have a profound impact on health throughout the lifespan. Older adults can help to prevent disease and disability and improve their quality of life through healthy behaviors such as proper nutrition, exercise, use of preventive health care, and avoiding smoking and alcohol abuse. Several particularly encouraging studies have shown that disability rates are declining. NIA research is focusing on ways to sustain and even accelerate the decline in disability, including the use of behavioral interventions and optimizing use of the health care system by older people. In addition, important research efforts, such as the national Health and Retirement Study, continue to collect and analyze demographic data that inform public policy and planning for the health, economic, and social needs of a growing older population.
This section on behavioral and social research begins with a Story of Discovery, which follows the initially startling finding of a decline in the rate of disability among older Americans. The narrative then describes research advances in assessing reduced mortality rates in developed nations and in examining the influence of attitudes and emotions on health in older people. Selected future directions for research focus on the use of demographic research to assess and improve the health of the older population, social and behavioral influences on cognitive aging, and behavioral medicine and interventions.
Story of Discovery: The Declining Disability of Older Americans
Older Americans are healthier and they are living longer. As a result, they may be able to stay in the workforce longer and need less medical care as they advance in age. But they are also growing in number, as the baby boom ages and the proportion of the population over 65 increases dramatically. The changing demographics of the aging population are proving a challenge to policymakers, who need to account for these population trends in looking at the future of such key national programs as Social Security and Medicare. In the mid-1970s, for example, the Social Security Administration made life expectancy projections based on the assumption that mortality rates would stay constant, and that life expectancy would not improve over time. It soon became clear that such an approach was not accurate. In 1982, the National Commission on Social Security Reform was established to determine how to maintain Social Security solvency in the face of projected life expectancy increases at ages 65 and over. The commission recommended an increase in the normal retirement age for Social Security beneficiaries from 65 to 67 years in increments from 2000 to 2022. These modifications to the Social Security program were based on changes in life expectancy and did not address the health status of older individuals.
In 1993, a surprising new analysis suggested that the health status of older people might be changing radically as well, with equally important ramifications for policy and planning for an aging America. Researchers looking at trends from 1982 through 1989 in the National Long Term Care Survey (NLTCS) found that disability rates had declined significantly in the older population. In 1994, new NLTCS data confirmed that a decline was occurring in chronic disability and researchers and policymakers began looking even more closely at the data. At least 1.2 million fewer older Americans were disabled in 1994 than there would have been if disability rates had not improved since 1982. Today, a preliminary update from the same survey suggests a continuation of the decline and perhaps even a reduction in the absolute number of elderly disabled persons.
Additional studies, using different databases, have since confirmed the NLTCS findings. An analysis of data from the Survey of Income and Program Participation in 1998 showed a reduction in disability rates from 1984 to 1993 in every age group of elderly Americans 50 years and over. The 1991 to 1996 Medicare Current Beneficiary Survey also showed disability declines occurring at an even more rapid rate than in the NLTCS. These corroborating findings have helped establish that the observed disability decline may reflect real improvements in underlying physiological health of older people.
The implications of these studies are important. It is hoped that better functioning among growing numbers of older people could help hold down the demand for health care and, in turn, help to reduce health care costs, over and above investments in research, preventive health care, and treatment that might be needed to maintain or accelerate the decline in disability. The decline might also have an effect on the ratio of working age people to the disabled elderly. This is important because a continued decline in disability rates, with fewer disabled older people, might increase the ratio of working age people to disabled elderly, possibly reducing the burden of support that the younger population might have to bear for programs for an aging population. Research to understand these potential long-term impacts of the decline is underway, looking at economic consequences, the adoption of new medical technology, and changes in the burden of caregiving, including informal care, home health services, and institutional long-term care.
Research has also begun to focus on factors contributing to the decline in disability so that specific interventions and behavioral changes can be identified that might accelerate trends in improved function among older Americans. Some of these efforts are described in this section's discussion of selected future directions in behavioral and social research.
Mortality Continues To Decline in Industrialized Countries
During the twentieth century, mortality rates have shown steady and significant declines in the G7 countries of Canada, France, Italy, Germany, Japan, the United Kingdom, and the U.S. Mortality decline has occurred most significantly in older populations due to decreases in deaths from heart attack, stroke, and cancer. Examining mortality data of the G7 industrialized countries over the last five decades, researchers found that long-term patterns in mortality rates have continued to decline exponentially at a remarkably constant rate, without evidence of slowing. Therefore, official estimates of longevity in the G7 countries underestimate life expectancy and also understate the ratio of people 65 and older to working age people (20–64 year olds). By the year 2050, these ratios may be between 6 percent (UK) and 40 percent (Japan) higher than official projections. These findings have significant implications for public policy regarding future demands on health care, long-term care, retirement support, and other services.
Potential Impact of Attitudes on Health and Behavior
Emotional state has been associated with health and functional status in old age. Both positive and negative attitudes or emotions can influence health and physical and cognitive function.
Emotional Vitality Protects Against Mortality and Progression of Disability in Disabled Older Women. Using data from the Women's Health and Aging Study, a longitudinal study of community-dwelling disabled women aged 65 years and older, researchers examined whether emotional vitality protects against progression of disability and mortality. At the start of this study, a substantial proportion of even the most disabled older women were identified as emotionally vital. Three years later, results showed that these upbeat, positive women did better than women who were not emotionally vital in maintaining physical function over time. These results suggest that helping older people maintain a high level of emotional vitality might play an important role in slowing or preventing a downward spiral in health status. Further study may be warranted of why and when positive emotions protect against health decline in older people.
The Influence of Stereotypes on Cardiovascular Health and Cognitive Function. Recent research indicates that exposure to negative beliefs about aging can contribute to adverse health outcomes, even when an individual is not consciously aware of such exposure. In this study, exposure to negative stereotypes elicited heightened cardiovascular stress (increased blood pressure and heart rate in older adults) in response to mathematical and verbal challenges designed to elicit a stress response. Positive messages about aging protected participants from a stress response. The older adults exposed to positive stereotypes also exhibited more confidence in their ability to perform computations than those exposed to negative stereotypes, and then outperformed them as well. These preliminary findings suggest that further research is need to examine the potentially powerful influence of stereotypes not only on the physical well being of older adults but also on their performance in tasks known to become progressively more difficult with age. Perhaps positive age-related stereotypes could be used to reduce cardiovascular responses to stress and to improve cognitive performance and daily function.
Selected Future Directions in Behavioral and Social Research
Using Demographic Research To Assess and Improve Health, and Reduce Disability. As the world's older population grows, demographic research can be used to assess the impact of population aging on the global burden of chronic disease and disability. Health and economic trends can be identified, helping to target opportunities for research on their causes and impact. NIA will collaborate with other NIH institutes in studying the changes in health and functional status over time of disabled and chronically ill older people. Research is being developed to improve data on burdens and costs of diseases. One growing area of interest centers on the possible addition of biological measures of health in social surveys. Demographic research is also planned to track the dynamics underlying the increase in old-age life expectancy in the U.S. and to define the implications of changes in health, disability, and life expectancy for national policies on retirement and on programs for the elderly. A particular focus is being developed to provide the necessary data for understanding the large variations in health across racial and ethnic populations.
Special efforts are underway to identify more precisely factors that have specifically contributed to recent declines in disability, as discussed in the Story of Discovery above. Past demographic and health research has provided clues, noting the social, educational, public health, and biomedical variables that affect health and function. Further research will examine specific trends likely to extend the disability decline, such as improvements in health-related behaviors, increased education among older people, improved availability and effectiveness of assistive devices, disease prevention, and better treatment for conditions that lead to disability.
Another area of emphasis is work, retirement, and health. The NIA is the primary sponsor of the Health and Retirement Study (HRS), one of the largest and most innovative efforts done in the U.S. to understand the dynamics of health and retirement. Analyses of HRS data will focus on the relationships between health and wealth with age and the variability in savings at various income levels. Additional research in this area will examine the determinants of work and the influence that pension systems and incentives have on the decision to work or retire.
Social and Behavioral Influences on Cognitive Development. The cognitive health of older adults is influenced by a number of factors, and additional research is needed to understand the social and behavioral contributions in this area. NIA behavioral and social scientists are collaborating closely with the Institute's neuroscience program to encourage research that: (1) examines the influence of contexts (behavioral, social, cultural, and technological) on cognitive and day-to-day function of older individuals, (2) investigates the effects of age-related changes in cognition on activities of daily living, social relationships, and health status, and (3) develops strategies for improving everyday function through cognitive interventions. Such research will be in line with activities suggested by the National Research Council's recent report on The Aging Mind. In addition, studies will also look at the role that individual differences, such as motivation, self-efficacy, beliefs about aging, emotion, sensory limitations, experience, and expertise, may play in cognitive function.
Behavioral Medicine and Interventions. It is well known that a wide range of healthy and unhealthy behaviors influence health and well being at any age. Research in this area will look at the dynamic interrelationships among aging, health, and behavior, expanding traditional studies of behavioral medicine by adding an aging perspective. This area of research should encompass a wide range of health and illness behaviors, including healthy lifestyle practices, medical self-management, and coping with chronic illness and disability. Epidemiological and behavioral research identifying risk factors as well as influences on health will guide intervention studies in this area. Increasingly, interventions may be viewed as multilevel, focusing not only on older individuals, but on family and societal changes as well. An important aspect of this work will be an emphasis on strategies for disseminating and translating findings and information as new interventions are developed.
Reducing Health Disparities
The health status of racial and ethnic minority groups in the U.S. has improved steadily over the last century. Despite such progress, disturbing disparities in health persist between majority and minority populations. In 1997, for example, average life expectancy at age 65 was 16.1 years for African Americans and 17.8 years for Caucasians. Demographic projections predict a substantial change in the racial and ethnic makeup of the older population, heightening the need to examine and reduce differences in health and life expectancy. Research to date has shown that health disparities are associated with a broad, complex, and interrelated array of factors. Disease risk, diagnosis, progression, response to treatment, caregiving, and overall quality of life each may be affected by variables such as race, ethnicity, gender, socioeconomic status, age, education, occupation, country of origin, and possibly other lifetime and lifestyle differences. Understanding these relationships will require a thoughtful program of research. Toward that end, the NIA recently completed a year-long review of these issues and developed a strategic plan to address health disparities in the older population. The plan covers fiscal years 2000 to 2005 and sets goals for research, research training, research resources, and dissemination of health information, and attention is paid to health disparities across the full spectrum of NIA-supported research. This part of the narrative looks at selected research advances in AD, menopause, and osteoarthritis that are adding to knowledge about health in various groups. It also describes future research directions for reducing health disparities in the U.S.
AD in the African American Community
While the incidence and prevalence of AD generally and in specific groups remains to be clarified, a few reports have suggested that racial and ethnic minorities might be at greater risk of AD. Research on AD in special populations looks at a number of factors, two of which are described below:
Potential Environmental Risk Factors for AD in African Americans. Socioeconomic or environmental variables may affect the risk of developing AD, even within racial or ethnic groups. A longitudinal study of dementia and AD in a group of older African Americans included analysis of such variables, examining the effect of level of education and rural vs. urban childhood environment as potential risk factors for AD. For individuals who grew up in an urban setting, low education did not increase the risk of AD significantly. However, for those with rural residence to age 19, low education was a significant risk factor for the disease. Such studies are part of a growing body of research suggesting a link between socioeconomic or environmental variables and risk of developing AD.
Cholesterol May Be a Modifiable Environmental Risk Factor for AD. The APOE-ε4 allele, a form of the APOE gene, is a risk factor for development of AD in most populations; however, its role in the risk of AD among African Americans is unclear. APOE also plays a role in cholesterol transport and studies have suggested an interaction among serum cholesterol, APOE status, and AD. This interaction recently was evaluated in a group of older African Americans. Increasing total cholesterol was associated with increased AD risk in the group with no APOE-ε4 alleles, but total cholesterol was not associated with increased AD risk in the group with one or two ε4 alleles. The study results suggest that cholesterol may be a potentially modifiable risk factor for AD in some African Americans who do not carry any APOE-ε4 alleles. Interestingly, this is consistent with recent animal studies indicating that a high cholesterol diet may increase the levels of beta amyloid plaques in brains of transgenic mouse models of AD.
Racial/Ethnic Disparities in Women's Health
Menopause Symptoms in a Multiracial/Ethnic Population of Women. The Study of Women Across the Nation (SWAN) is a prospective longitudinal examination of the natural history of menopause in a large multiracial/ethnic sample of women, age 40–55. Participants include African American, non-Hispanic Caucasian, Chinese, Japanese, and Hispanic women. A survey of SWAN participants identified relationships between reported symptoms of menopause (including hot flashes and night sweats, heart pounding, urine leakage, and forgetfulness) and demographic and lifestyle factors (such as socioeconomic status, education level, difficulty paying for basic items, smoking behavior, body mass index and physical activity). Generally higher reporting levels for specific menopausal symptoms were associated with low socioeconomic status, smoking, low physical activity, and being overweight. The reporting of specific menopausal symptoms varied significantly among racial/ethnic and socioeconomic groups and by lifestyle. Scientists point out that research in this area may provide guidance to health care providers in assessing symptoms by increasing their sensitivity to racial and ethnic differences in reporting symptoms.
Knee Osteoarthritis More Prevalent in Younger African American Women. A recent study in women age 28–52 found that osteoarthritis (OA) of the hand and knee is common after the age of 40, and OA develops between 35 and 40 years for both African-American and Caucasian women. Knee OA was much more frequent in African-American women compared with white women of the same age. The frequency of hand OA was similar between African American and white women. This study provides evidence that primary prevention of OA might need to be attempted in young adulthood. And the striking difference observed in prevalence of knee OA between African-American and white women points to a need to identify factors that might contribute to the African Americans' increased risk of developing OA.
Selected Future Directions in Research on Health Disparities
Reducing Health Disparities. It is estimated that the percentage of racial minorities and Hispanics in the population of Americans over the age of 65 will increase from 16% in the year 2000 to about 36% in 2050.(11)
Although an array of factors has been associated with health disparities—including race, ethnicity, gender, genetics, socioeconomic status, age, education, and occupation—further research is needed to identify the factors involved in these differences. Research at the NIA-supported Resource Centers on Minority Aging Research and other activities are underway to address a range of measurement issues in the study of multiracial and multiethnic populations. As this basic work moves ahead, new NIA studies will focus on the influence of early and midlife health, nutrition, education, social and cultural factors and health care on the health of older people. Research will also expand understanding of how to prevent or lessen the effects of disease by designing more culturally appropriate interventions and modes of health information dissemination and by discovering means to enhance healthy behaviors in older racial and ethnic populations.
Specifically, there is urgency in identifying genetic and nongenetic risk and protective factors for age-related cognitive decline, AD, and other neurodegenerative diseases of aging in racially and ethnically diverse populations. The NIA is stimulating research on several fronts: to assess and compare prevalence and incidence rates for mild cognitive impairment and AD among different ethnic subgroups, using culturally appropriate instruments; to determine the importance of particular genetic risk and protective factors as well as potential nongenetic risk factors, including comorbid conditions such as cardiovascular and cerebrovascular disease; and to identify differences in factors conferring risk or protection, such as early development, diet, and education.
Other Selected Future Directions in Research
Developing and Distributing Research Resources. Physical resources—such as animal models, chemicals, tools, and other technologies—play a critical role in research. The NIA develops and distributes these high quality resources to investigators efficiently and at reduced cost. These resources include:
- Aging colonies of animal models necessary for research on aging processes and specific age-related diseases
- Cell cultures and tissue, cell, and blood banks for basic and epidemiologic research
- DNA resources for genetics
- Bioinformatics technologies to make, record and analyze findings on basic biological research
The NIA will continue to identify and evaluate opportunities for providing research resources and infrastructure development with advice from extramural and intramural researchers. The Institute is also working on information technologies to assure broad access to archived data vital to researchers and policymakers and to ensure protection of anonymity and confidentiality of participants in clinical studies. In conjunction with other NIH institutes, the NIA will support research on new mathematical and informatics methodologies and on improved instrumentation and computational techniques for modeling systems changes in aging.
AIDS
Cognitive Impairment May Be Specifically Increased in Older HIV Positive Individuals. A longitudinal study seeks to understand how aging affects the cognitive and motor disorders due to human immunodeficiency virus (HIV) infection—minor cognitive-motor disorder and HIV-1 associated dementia. Researchers used a battery of standardized tests to measure performance in learning and memory, language abilities, visuospatial processing, the speed of processing information, problem solving abilities, and fine eye-hand motor coordination. Older HIV-positive individuals tended to perform significantly less well than their younger HIV-positive counterparts on measures of verbal memory and the speed of visual scanning and discrimination, while these differences were not observed when younger and older HIV-negative individuals were tested. Older HIV-positive individuals also have a greater number of the symptoms of this minor cognitive-motor disorder than their younger HIV-positive counterparts. The investigators conclude that older HIV-positive individuals are more likely to have clinically diagnosed cognitive and motor disorders.
Conclusion
One of the most remarkable achievements of the 20th century has been the extraordinary increase in life expectancy, both in the U.S. and worldwide. The added years offer new possibilities for personal achievement, family relationships, work, and contributions to community, which can benefit individuals as well as society. But in order to reap these benefits of longer life, the diseases and conditions associated with advanced age, which now affect millions of older Americans, need to be addressed. This mission is ever more urgent as the population rapidly grows older. Since the NIA's founding in 1974, groundwork has been laid for today's important advances in understanding basic aging, preventing disease and disability, including Alzheimer's disease, and defining special social and behavioral issues for older individuals, their families and caregivers, and clinicians. The latest studies provide additional basic understandings as well as new interventions to treat and even prevent some of the more devastating and disabling aspects of aging. With such research continued and intensified, we can move forward in meeting the promise of extended life by improving the health and well being of older people in America.
Overall Budget Policy
The Fiscal Year 2002 budget request for the NIA is $897,961,000, including AIDS, an increase of $93,509,000 and 11.9 percent over the FY 2001 level, and $191,992,000 and 27.9 percent over FY 2000.
A five year history of FTEs and Funding Levels for NIA are shown in the graphs below:
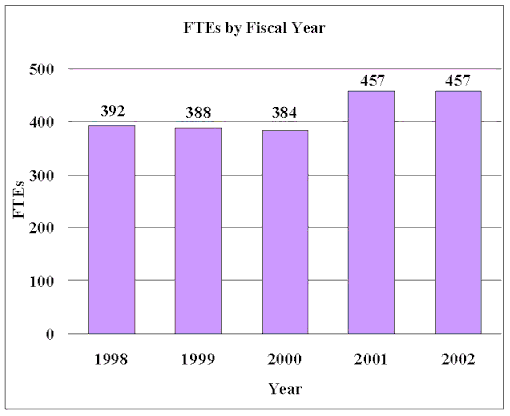
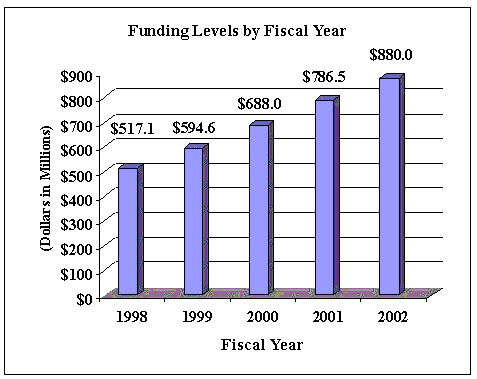
One of NIH's highest priorities is the funding of medical research through research project grants (RPGs). Support for RPGs allows NIH to sustain the scientific momentum of investigator-initiated research while providing new research opportunities. The Fiscal Year 2002 request provides an average cost increase for competing RPGs less than the Biomedical Research and Development Price Index (BRDPI) due to a one time adjustment for a major Alzheimer's Disease clinical trial funded in FY 2001. Noncompeting RPGs will receive increases of 3 percent on average for recurring direct costs. In FY 2002, total RPGs funded will be 1,344 awards, an increase of 69 awards over the FY 2001 estimate, the highest annual total ever awarded.
Promises for advancement in medical research are dependent on a continuing supply of new investigators with new ideas. In the Fiscal Year 2002 request, NIA will support 572 pre- and postdoctoral trainees in full-time training positions. An increase of 10 percent over Fiscal Year 2001 levels is provided for stipends and training-related expenses (e.g., health insurance, research supplies and equipment, and travel to scientific meetings).
The Fiscal Year 2002 request includes funding for 67 research centers, 205 other research grants, including 173 clinical career awards, and 65 R&D contracts. The R&D contracts mechanism also includes support for 13 contracts for the Extramural Clinical and Pediatric Loan Repayment Programs.
The NIA will invest additional Research Management and Support funds in the administration and oversight of its research programs and dissemination of health information to diverse populations. For example, NIA will dedicate significant program funds to search for new ways to prevent and treat Alzheimer's disease, with a special emphasis on testing interventions aimed at preventing the disease. Such efforts will require clinical trials on a scale never before attempted by the NIA, necessitating the creation of an oversight infrastructure of scientists who administer clinical trial programs and monitor data integrity. As special population groups grow at a faster rate than the population as a whole, unprecedented opportunities exist not only to increase the participation of diverse populations in research programs, but also to develop strategies to disseminate information for improving health behaviors. Outreach programs will also target enhanced education efforts for health professionals to increase their understanding of an aging population.
The mechanism distribution by dollars and percent change are displayed below:
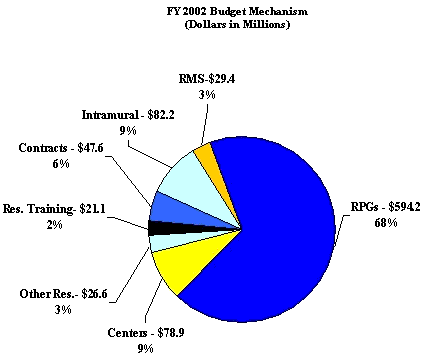
Total by Mechanism (Dollars in Thousands) |
| | FY 2000
Budget Authority | FY 2001
Estimate | FY 2002
Estimate | Percent Change from FY 2001 |
| Mechanisms | Number | Amount | Number | Amount | Number | Amount |
| Research Project Grants | | | | | | | |
| Noncompeting | 741 | $296,268 | 782 | $347,763 | 887 | $425,673 | 22.4% |
| Admin Supplements | (116) | 8,956 | (110) | 7,470 | (110) | 7,791 | 4.3% |
| Competing | 400 | 136,179 | 427 | 155,431 | 385 | 140,964 | -9.3% |
Subtotal | 1,141 | 441,403 | 1,209 | 510,664 | 1,272 | 574,428 | 12.5% |
| | 30 | 8,029 | | | | | |
| SBIR/STTR | 55 | 15,006 | 66 | 18,100 | 72 | 19,802 | 9.4% |
| Subtotal, RPG | 1,196 | 456,409 | 1,275 | 528,764 | 1,344 | 594,230 | 12.4% |
| Research Centers | 66 | 71,770 | 67 | 74,642 | 67 | 78,852 | 5.6% |
| Other Research | 188 | 21,474 | 190 | 24,538 | 205 | 26,586 | 8.3% |
| Training | 551 | 17,309 | 572 | 19,390 | 572 | 21,084 | 8.7% |
| R&D Contracts | 61 | 29,241 | 65 | 37,990 | 65 | 47,627 | 25.4% |
| (SBIR/STTR Contracts) | (1) | (497) | 0 | 0 | 0 | 0 | 0.0% |
| Intramural Research | | 68,232 | | 74,770 | | 82,193 | 9.9% |
| Rsch Mgmt & Support | | 23,534 | | 26,358 | | 29,389 | 11.5% |
TOTAL | | 687,969 | | 786,452 | | 879,961 | 11.9% |
Total amounts include funding for AIDS: FY2000-$2,068; FY2001-$4,143; FY2002-$4,298
About the NIA : Congressional Testimony & Budget Requests
References
1.Federal Interagency Forum on Aging Related Statistics. Older Americans 2000: Key Indicators of Well-Being. 2000.
2. Manton KG, Corder LS, Stallard E. Chronic disability trends in elderly United States populations: 1982-1994. Proc Nat Acad Sci USA 94: 2593-2598, 1997.
3. Evans DA, Funkenstein HH, Albert MS, et al. Prevalence of Alzheimer's disease in a community population of older persons; higher than previously reported. JAMA 262: 2551-2556, 1989.
4. Federal Interagency Forum on Aging Related Statistics. Older Americans 2000: Key Indicators of Well-Being. 2000.
5. Small, GW, Rabine, PV, Barry, PP, et al. Diagnosis and treatment of Alzheimer disease and related disorders. JAMA 16: 1363-1371, 1997.
6. Ernst, RL, Hay, JW, Fenn, C, et al. Cognitive function and the costs of Alzheimer's disease, Arch Neurol 54:687-693, 1997.
7. National Center for Health Statistics. Health, United States, 1999 With Health and Aging Chartbook. Figure 11, pg. 41. Hyattsville, MD: 1999.
8. Burt VL, Whelton P, Roccella EJ, et al. Prevalence of hypertension in the US adult population: results from the Third National Health and Nutrition Examination Survey, 1988-1991. Hypertension 25: 305-313, 1995.
9. Burt VL, Cutler JA, Higgins M, et al. Trends in the prevalence, awareness, treatment, and control of hypertension in the adult US population: data from the health examination surveys, 1960 to 1991. Hypertension 26: 60-69, 1995.
10. National Center for Health Statistics. Health, United States. 1999 With Health and Aging Chartbook. Table 33, pg. 156. Hyattsville, MD: 1999.
11. Federal Interagency Forum on Aging Related Statistics. Older Americans 2000: Key Indicators of Well-Being. 2000.
