April 30, 2002
Senator Harkin and Members of the Committee:
Thank you for inviting me to appear before you today to discuss Alzheimer's disease (AD), an issue of interest and concern to us all. I am Dr. Richard Hodes, Director of the National Institute on Aging (NIA), the lead federal agency for Alzheimer's disease research. I am delighted to be here this morning to tell you about the progress we are making toward understanding, treating, and preventing AD.
As you know, AD is a major public health issue for the United States, and it has a devastating impact on individuals, families, the health care system, and society as a whole. Approximately 4 million Americans are currently battling the disease, with annual costs estimated to exceed $100 billion. Moreover, the rapid aging of the American population threatens to increase this burden several-fold in the coming decades. However, despite the grim statistics, we have made, and are making, tremendous progress.
Until very recently, preventing or curing AD was considered, at best, a distant possibility. Our understanding of AD's underlying biology was limited, and for this reason it was difficult even to predict what might be effective as a treatment or preventive.
Today, the picture is considerably brighter. Through laboratory and population-based scientific studies, we have identified a number of risk factors for AD, including both genetic and possible lifestyle factors. Research supported by the NIA, the National Institute of Neurological Disorders and Stroke (NINDS), and the National Institute of Mental Health (NIMH) has identified several genes that can cause AD, thereby helping us identify pathways affecting its development or progression, which will lead to better molecular predictors of the disease even before it is clinically apparent. The development and refinement of powerful imaging techniques that target anatomical, molecular, and functional processes in the brain will give us an improved ability to diagnose AD early, while the patient can still take an active role in decision-making. These techniques, along with better neuropsychological tests, are also enabling us to identify people who are at very high risk of one day developing the disease and to determine just how the disease starts in the brain. This knowledge, in turn, may allow early intervention in persons long before the disease affects their level of functioning.
Most importantly, we are making significant advances toward effectively treating, or even preventing, AD. (Chart #1) NIA is currently supporting 18 AD clinical trials, seven of which are large-scale prevention trials. These trials are testing agents such as estrogen, anti-inflammatory drugs, and antioxidants for their effects on slowing progress of the disease, delaying AD's onset, or preventing it altogether. We eagerly await the results of these trials.
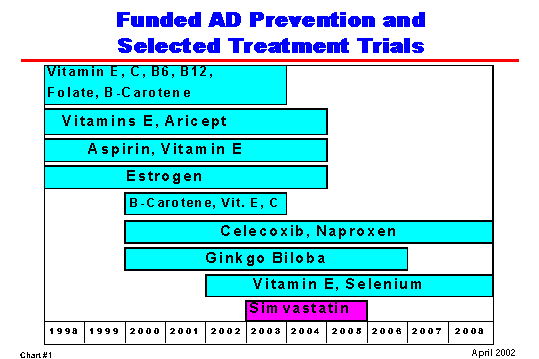 |
As we search for effective preventive interventions and treatments for AD, it is becoming clear that, rather than seeking only a "magic bullet” that will, by itself, prevent or cure the disease, we may be able to identify a number of potential interventions that together can be used to reduce risk. Several recent studies have highlighted this.
For example, a recent study in the New England Journal of Medicine [1] indicates that elevated blood levels of the amino acid homocysteine, already considered a risk factor for cardiovascular disease, are associated with an increased risk of developing AD. (Chart #2) The relationship between AD and homocysteine is of particular interest because blood levels of homocysteine can be reduced, for example, by increasing intake of folic acid (or folate) and vitamins B6 and B12. And, in fact, in a separate study in the Journal of Neuroscience [2], NIA researchers show that folic acid may protect AD transgenic mice against death of neurons in one of the brain regions most affected in AD. NIA has ongoing clinical trials of these substances to test whether supplementation can slow the rate of cognitive decline in cognitively normal men as well as in women at increased risk for developing heart disease. A pilot clinical trial to determine effective treatment levels of folate/B6/B12 for lowering plasma homocysteine levels in persons with AD is ongoing, and a full-scale clinical trial on people diagnosed with AD is due to start in 2003. Other studies have indicated that the use of statins, the most common type of cholesterol-lowering drugs, may lower the risk of developing AD (Chart #3). A clinical trial to determine whether statins slow the rate of disease progression in AD patients is planned for fall 2002.
 |
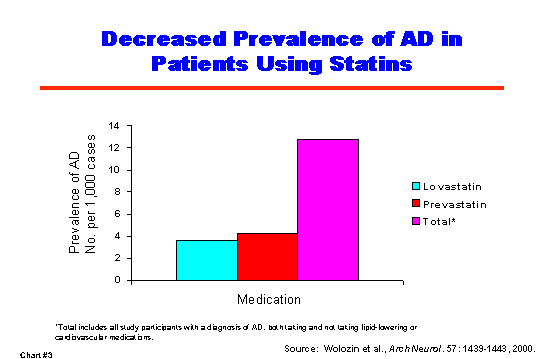 |
Another promising area of study is the role of mentally stimulating activities throughout life as a factor capable of maintaining cognitive health or even reducing the risk of cognitive decline or AD. Through its Advanced Cognitive Training for Independent and Vital Elderly (ACTIVE) study, NIA is currently exploring whether three specific interventions (on memory, reasoning, and speed of processing) can maintain or improve functioning in unimpaired, community-dwelling older adults. In addition, NIA-supported researchers recently found that more frequent participation in activities such as reading, doing crossword puzzles, or playing card games is associated with a reduced risk of later developing AD. [3]
In addition to these exciting clinical findings, NIA-supported investigators are beginning to unravel AD's complex etiology. For example, until very recently, just four of the approximately 30,000 genes in the human genome were conclusively known to affect the development of AD pathology. Three of these genes cause early onset AD, and only one is associated with the more common form of the disease, late-onset AD (LOAD). Recent genetic studies suggest that as many as four additional and as yet unidentified genes may also be risk factors for LOAD, and regions in several different chromosomes have been identified as likely locations for these genes. Finding new risk factor genes will help identify pathways affecting the development or progression of AD and may eventually lead to better predictors of the disease even before it is diagnosed.
To facilitate the identification of the remaining AD risk factor genes, NIA is planning an expansion of its National Cell Repository. A national resource for research on AD, the Repository was created to collect and distribute DNA, cells, and information from families with multiple members with AD and related dementias. Its activities include the production of a catalog of cell lines and DNA samples that are available for qualified scientists to study. The expansion will allow researchers to more rapidly identify the underlying genetic mechanisms and environmental risk factors that interact to cause the more common late-onset form of AD. Understanding these mechanisms will provide opportunities for the design of effective diagnostic, therapeutic, and preventive interventions.
The process of translating basic science findings into clinical interventions is a challenging but critical component of AD research. (Chart #4) For example, a promising finding gained through basic research efforts was the ability of an immunization strategy to prevent or reverse formation of amyloid plaques in mouse models of AD. In collaboration with NINDS, NIA has issued a Request for Applications (RFA) and funded a number of studies to better understand the science underlying the vaccine approach. Similarly, NIA-funded studies are providing exciting new evidence on the identity of the snipping enzyme that cuts the amyloid beta molecule out of its precursor protein, and ways to blunt its activity. These interventions may be capable of preventing the formation of amyloid plaques.
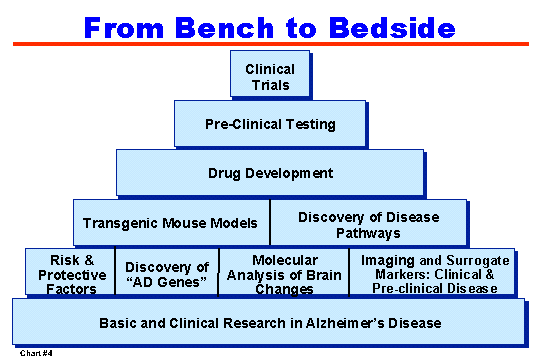 |
 |
In addition, NIA-supported researchers have recently made a surprising discovery about the role of amyloid plaques in AD pathology. (Chart #5) In one study, investigators found that amyloid beta oligomers, or small precursor components of amyloid plaques, inhibited brain mechanisms thought to be involved in memory formation in rats. [4] In another, scientists used an immunization strategy to treat plaque-containing AD transgenic mice. Although the amount of plaques in the mice's brains remained constant, the mice very quickly regained cognitive functioning. [5] These findings suggest that amyloid plaques themselves may not be responsible for AD's cognitive symptoms, and that a related pathology – perhaps a precursor molecule such as the amyloid beta oligomer– is the true culprit. This insight, in turn, may lead to the development of new and effective treatments for the disease.
Although the findings are still preliminary, these studies illustrate the importance of continued basic research to help us understand the mechanisms behind AD development and pathology, and the ways in which basic research findings can suggest new prevention and treatment strategies.
Scientists funded by NIA, NINDS, and NIMH are also developing and refining powerful imaging techniques that hold promise of earlier and more accurate diagnosis of AD, as well as improved identification of people who are at risk of developing the disease and a more complete understanding of normal and abnormal age-related changes in the brain. (Chart #6) For example, recent studies suggest that positron emission tomography (PET) scanning of metabolic changes in the brain and magnetic resonance imaging (MRI) scanning of structural brain changes may be useful tools for predicting future decline associated with AD and other neurodegenerative diseases.
 |
Researchers have also developed a new way of using functional MRI (fMRI), a technique for visualizing activity of brain structures, that is both easier on the person being tested and capable of imaging smaller structures in the brain than has been possible in the past. Using this new technique, investigators assessed the hippocampus, an area of the brain involved in memory formation, in people between 20 and 88 years of age. They found that activity in certain regions of the hippocampus declines normally with age, but that decline in a specific region, the entorhinal cortex, is abnormal and may reflect an illness or condition such as AD. They conclude that some age-related memory loss is normal, due to ordinary hippocampal changes, but that individuals with dysfunction in the entorhinal cortex may be at increased risk of progressing to full-blown AD. [6] These studies, if confirmed by ongoing longitudinal observation of the patients, hold the promise, for the first time, of being able to distinguish between the subtle brain changes that occur with normal aging and those that are a harbinger of clinical AD.
These methodologies may also be useful for evaluating the efficacy of drugs in stemming the progression of AD or preventing its onset altogether. However, these and other emerging imaging techniques, while promising, require further testing and analysis before they can be routinely adopted in the clinical setting.
Another very important area of research involves easing the burden on caregivers of AD patients. In a sense, the AD "patient” is not only the person with the disease, but the entire family unit. Most Americans with AD are cared for outside the institutional setting by an adult child or in-law, a spouse, another relative, or a friend. The financial costs of this care can be devastating to families; the average lifetime cost per person for patients with AD is approximately $174,000. [7] In addition to these financial burdens, caregivers frequently experience emotional stress and physical strain.
NIA is investing in new approaches to assist these caregivers. A first priority is to assess the magnitude of the problem. For example, the ongoing Aging, Demographic, and Memory Study (ADAMS) has been designed to assess dementia and AD among Americans, the burden on caregivers, the economic cost of dementia to families and to society, and the burden of dementia over the course of the illness.
NIA is also supporting a study of a combined behavioral and drug intervention on patients with mild AD. In this study, caregivers will be key participants in the behavioral intervention, and the researchers hypothesize that this participation will reduce caregivers' psychological stress. In addition, NIA is supporting a large, multi-site clinical trial, REACH (Resources for Enhancing Alzheimer's Caregiver Health), to examine the effectiveness of various interventions to strengthen family members' capacity to care for individuals with AD. Thus far, the study has recruited over 1,200 caregiver/care recipient pairs at six different sites across the country to participate in 12 different interventions. REACH is designed to show us what works to support caregivers and at what cost; we anticipate that the first findings from this trial may be available within the next several years. The NIMH is supporting a major project called the Clinical Anti-psychotic Trial of Intervention Effectiveness for Alzheimer's Disease (CATIE-AD) designed to help identify effective treatments for behavioral problems in AD, to help reduce the burden of care for both providers and families.
Fifteen years ago, we did not know any of the genes that could cause AD, and we had no idea of the biological pathways that were involved in the development of brain pathology. Now, we know the 3 major genes for early-onset disease and one of the major risk factor genes for late-onset disease, and we have extensive knowledge of pathways leading to the development of AD's characteristic amyloid plaques in the brain. Ten years ago, we could not model the disease in animals. Today, transgenic mice are an invaluable resource for modeling amyloid plaque development in the brain and in testing possible therapies. Five years ago, we did not have any prevention trials funded and had no ways of identifying persons at high risk for the disease. Now, we have seven ongoing prevention trials, and scientists are identifying persons at high risk for developing AD by imaging, neuropsychological tests, and structured clinician interviews. And as recently as one year ago, we did not understand anything about how plaques and tangles relate to each other. Now, through the creation of the first double transgenic mouse to produce both plaques and tangles, we know that plaques in the brain can influence the development of tangles in brain regions susceptible in AD. Recent findings also suggest that there are some common mechanisms of disease in a number of neurodegenerative disorders, which will further inform research in AD.
It is difficult to predict the pace of science or to know with certainty what the future will bring. However, the progress we have already made will help us speed the pace of discovery, unravel the mysteries of AD's pathology, and develop safe, effective preventions and treatments, to the benefit of older Americans.
Thank you for giving me this opportunity to share with you our progress on Alzheimer's disease. I would be happy to answer any questions you may have.
S. Sesdradri, A. Beiser, J. Selhub, et al., "Plasma Homocysteine As A Risk Factor For Dementia and Alzheimer's Disease,” N Eng J Med. 346:7, pp. 476-483, 2002.
I. Kruman, T.S. Kumaravel, A. Lohani, W. Pedersen, R.G. Cutler, Y. Kruman, N. Haughey, J. Lee, M. Evans, and M.P. Mattson, "Folic Acid Deficiency and Homocysteine Impair DNA Repair in Hippocampal Neurons and Sensitize Them To Amyloid Toxicity in Experimental Models of Alzheimer's Disease,” Journal of Neuroscience. 22:5, pp. 1752-1762, 2002.
Wilson RS, Mendes de Leon CF, Barnes LL et al., "Participation in Cognitively Stimulating Activities and Risk of Incident Alzheimer Disease,” JAMA. 287: 742-748, 2002.
Walsh DM et al. Naturally secreted oligomers of amyloid b protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 416:535-539, 2002.
Dodart J-C et al. Immunization reverses memory deficits without reducing brain Ab burden in Alzheimer's disease model. Nature Neuroscience. 2002: Advance online publication.
Small SA et al. Imaging hippocampal function across the human life span: Is memory decline normal or not? Annals of Neurology. 51:290-295, 2002.
Ernst, RL and Hay, JW The US economic and social costs of Alzheimer's disease revisited. American Journal of Public Health. 84:1262-1264, 1994.