 |
 |
FDA
Home Page | Search FDA Site |
FDA A-Z Index | Contact FDA
![]()
May 18, 2005
On February 18, 2004, FDA issued a Report entitled "Combating Counterfeit Drugs: A Report of the Food and Drug Administration." The comprehensive Report highlights several measures that can be taken to better protect Americans from counterfeit drugs. These measures address six critical areas:
Over the past year, we have worked with manufacturers, wholesalers, pharmacies, consumer groups, technology specialists, standard-setting bodies, State and Federal agencies, international governmental entities, and others to advance the measures outlined in the Report. Significant progress is being made in many of these areas. Although we continue to believe that the U.S. drug supply is among the safest in the world, more work needs to be done to further implement these measures and further secure our nation's drug supply.
In 2004, FDA's Office of Criminal Investigations (OCI) initiated 58 counterfeit drug cases, a significant increase from the 30 cases initiated in 2003. We believe that this is in part due to an increased awareness and vigilance at all levels of the drug distribution chain as a result of the Combating Counterfeit Drugs Report released last year. In addition, this increase in investigations is due to increased referrals from and coordination with other state and federal law-enforcement agencies and communication with drug manufacturers.
Fortunately, most of the counterfeit drugs at issue did not reach consumers because we focused our limited resources and developed proactive investigations that enabled us to identify components of counterfeit products and interdict finished counterfeit drug products before they entered domestic distribution.
Although the number of counterfeit drug cases has increased and the threat to the public health is real, most of the suspect counterfeits that we discovered in 2004 were found in smaller quantities, compared to those found in 2003. Most of these drugs were destined for the black market or internet distribution, rather than for widespread distribution in the nation's drug supply chain.
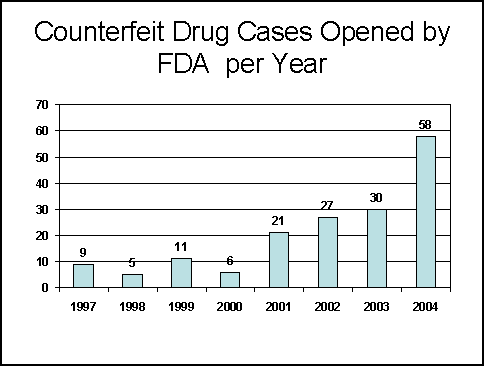
Previously reported data for FY 1997-2003 were revised due to new information
indicating involvement of counterfeit drugs in other previously uncounted criminal
investigations.
In the Report, we stated that it is critical to implement new technologies to better protect our drug supply. We concluded that a combination of rapidly improving track and trace technologies and product authentication technologies could be used to provide a greater level of security for drug products. These technologies are intended to secure the product, packaging, and movement of the product as it travels through the drug supply chain.
In the Report, we stated that adoption and wide-spread use of reliable track and trace technology is feasible by 2007. This would help secure the integrity of the supply chain by providing an accurate drug "pedigree," a record documenting that the drug was manufactured and distributed under secure conditions. We particularly advocated for the implementation of electronic track and trace mechanisms and noted that radio-frequency identification (RFID) is the most promising technology to meet this need. RFID technology uses a tiny radio frequency chip containing essential data in the form of an electronic product code (EPC). Implementation of RFID will allow supply chain stakeholders to track the chain of custody (or pedigree) of every package of medication. By tying each discrete product unit to a unique electronic serial number, a product can be tracked electronically through every step of the supply chain.
Over the last year stakeholders have made tremendous progress in the development and implementation of EPC/RFID. This is a huge endeavor that requires close collaboration among all constituents of the pharmaceutical distribution system. We have observed and supported this collaboration, and we continue to support it today.
A critical piece of this undertaking is the development of standards for the type of technology to be used and the systems for storing and sharing pedigree information. This activity will ensure that the electronic track and trace technologies adopted are comprehensible and data communication systems are interoperable. We have been present at and actively participated in many industry, standard-setting, and government meetings and workshops where implementation issues have been discussed. We appreciate the opportunities we have been given to participate in the discussions and provide input when needed.
We received a number of questions over the past year regarding RFID and regulatory issues from members of the supply chain. In response to these common questions, on November 15, 2004, we issued a Compliance Policy Guide (CPG) for implementing RFID feasibility studies and pilot programs as an important and essential step in moving this technology forward. The CPG presents FDA's current thinking regarding several labeling, current Good Manufacturing Practices (GMP), and other regulatory issues that may arise by affixing an RFID tag to a drug product for a feasibility study or pilot program. Several members of the supply chain simultaneously announced their intention to move forward with pilot programs (joint programs across the supply chain or within an individual company) that will involve the tagging of products susceptible to counterfeiting. In fact, three major pharmaceutical companies said that they will incorporate an RFID tag into at least one of their products by the end of 2005. We have been in close communication with participants in these and other pilot studies and provided input when appropriate.
Also in November, we announced the creation of an internal, cross-agency "RFID Workgroup." This group is charged to monitor adoption of RFID in the pharmaceutical supply chain, pro-actively identify regulatory issues raised by the use of this new technology, and develop straightforward processes for handling those issues. We believe that the workgroup will improve communication with members of the supply chain on RFID related issues and will facilitate both the performance of pilot studies and the collection of data needed to formulate policy.
It is important to gain a better understanding of the effects of RFID on drug products, particularly biological products because they may be more susceptible to change in their environment. In the past year, we developed a protocol for the Product Quality Research Institute (PQRI) (a collaboration of FDA, academia, and industry) to evaluate the effects of radio-frequency on specific biological protein-based products. This study is in its very early stages. Also, a laboratory within FDA's Center for Devices and Radiological Health is conducting analyses of the heating and the radio-frequency field strengths induced in certain liquid pharmaceuticals by some RFID systems. We are encouraged by the response of individual companies informing us that they are conducting studies. In addition, the Health Research Initiative of the Auto-ID Laboratories is conducting additional studies on the effects of radio-frequency on various drug products and storage conditions. We look forward to the results of such studies.
Next Steps: FDA will continue to play an active role in public and private sector efforts toward developing an "electronic safety net" for our drug supply, including the adoption and widespread use of reliable track and trace technology by 2007. We will continue to facilitate and monitor standard-setting activities, including efforts by epcGlobal (an entity that has taken a lead role in developing standards) to establish standards for numbering systems, chip frequency, electronic pedigree, and data-sharing and security. In addition, we will continue to encourage and foster research on the use and potential impact of RFID on drug and biological products. Finally, we will regularly review the extent and pace at which RFID is being adopted.
In the Report, we noted that authentication technologies for pharmaceuticals (such as color-shifting inks, holograms, taggants, or chemical markers imbedded in a drug or its label) have been sufficiently perfected that they can now serve as a critical component of a layered approach to control counterfeit drugs. FDA's Report acknowledged the importance of using one or more authentication technologies for drug products, in particular those most likely to be counterfeited. Over the past year, we have worked with individual drug manufacturers who sought to incorporate such technologies into their product, labeling, or packaging. When asked, we have provided advice and suggestions regarding application and use of authentication technologies and worked with sponsors on the regulatory issues associated with making changes to approved product labeling.
In the Report, we said that in order to facilitate the use of authentication technologies on or in approved products, we would consider publishing a draft guidance on notification procedures for making changes to products, their packaging, or their labeling. We decided not to issue guidance in the past year because we would like to gain additional experience working with companies in their application and use of authentication technologies so the guidance can have appropriate general applicability.
Next Steps: We will continue to work with companies and organizations to facilitate use of authentication technologies in products, labeling, and packaging.
In the Report, we said that adoption of electronic track and trace technology would help stakeholders meet and surpass the goals of the Prescription Drug Marketing Act (PDMA). We said that we intend to focus our efforts on facilitating industry adoption of this technology. To allow stakeholders to move toward an electronic pedigree we said that we would further delay the effective date for certain provisions in a final rule that FDA promulgated in December 1999 to implement the Prescription Drug Marketing Act of 1987 (PDMA), as modified by the Prescription Drug Amendments of 1992 (PDA). On February 23, 2004, we published a notice in the Federal Register delaying the effective date until December 2006.
As stated above, we are pleased with the progress stakeholders, standard-setting bodies, and software and hardware companies have made thus far toward implementing an electronic pedigree for drug products. We recognize that there have been, and continue to be, challenges along the way. However, we are optimistic that this progress will continue in an expeditious manner toward meeting our 2007 goal. If it appears that this goal will not be met, we plan to consider the options regarding implementation of the PDMA provisions that are the subject of the stay.
Next Steps: We are closely monitoring the progress of widespread use of electronic pedigrees as we assess whether to lift, maintain, or pursue other options regarding the stay of implementation of the provisions in the PDMA final rule. We will continue to work with stakeholders to facilitate implementation.
In the Report, we recognized the important role that the states have in regulating the drug supply chain, and we stated that adoption and enforcement of strong, proven anti-counterfeiting laws and regulations by the states would help in our collective effort to detect and deter counterfeit drugs. FDA strongly supported the efforts taken by the National Association of Boards of Pharmacy (NABP) in revising the Model Rules for Licensure of Wholesale Distributors for states to adopt. These Model Rules make it difficult for illegitimate wholesalers to become licensed and then to transact business. Four states have laws in place that are similar to the Model Rules ( Florida, Nevada, California, and Indiana), and other states are considering adoption (e.g., New Jersey, Iowa ). FDA has provided advice and input on a few state legislative proposals and we recommend that more states move in this direction in the coming year.
NABP last year also announced the creation the Verified-Accredited Wholesale Distributors™ (VAWD) program as a complement to the Model Rules. Applicants for VAWD accreditation undergo a criteria compliance review, licensure verification, an inspection, background checks, and screening through NABP's clearinghouse. It is intended to provide assurance that the wholesale distribution facility operates legitimately, is validly licensed in good standing, and is employing security and best practices for safely distributing prescription drugs from manufacturers to pharmacies and other institutions. Recently, Indiana was the first state to pass a law that requires VAWD accreditation for all drug wholesale distributors who do business in Indiana.
In the Report, we said that there would be great value in the creation of a national list of drugs most likely to be counterfeited based on factors that are likely to contribute to counterfeiting risk. The Model Rules called for such a national list as a starting point for application of pedigree requirements in the short term so that there would not be 50 different state lists. In December 2004, NABP convened a National Drug Advisory Coalition, which included industry and state and national government representation. FDA has served in an ex-officio role on this Coalition. The Coalition developed criteria for inclusion or removal from such a list and created a national list that includes 31 drugs. FDA applauds NABP on this accomplishment.
We recognize that states have implemented and are considering provisions requiring a pedigree (in some cases electronic) for drug products. We are pleased that these efforts complement federal requirements and believe that rapid and uniform implementation of a pedigree that starts at the point of manufacture and accompanies the drug product until it is dispensed would be beneficial. As stated in the Report, adoption and enforcement of the Model Rules by all states would have the greatest impact on protecting the nation's drug supply.
In the Report, we also said that increased penalties would help deter counterfeiting and more adequately punish those convicted. As we continue the efforts on the Federal level, it is equally important that states adopt stronger penalties (like those outlined in the Model Rules) so the penalties associated with counterfeiting drugs are commensurate to the significant threat they pose to the public health.
Next steps: FDA will continue to support efforts by the states to adopt and enforce stricter laws and to pursue increased Federal penalties for drug counterfeiting.
In the Report, we described the important role that all participants in the drug supply chain have in adopting secure business practices. Around the time the Report was issued several trade associations for wholesale distributors issued guidelines for their members regarding best practices for drug d istribution system integrity. In fact, in the past year, the Healthcare Distribution Management Association (HDMA) released new membership rules that require active members to adopt best practices that include extensive regulatory, financial, security, and due diligence processes and procedures.
It is also important to note that many of the secure business practices outlined in these trade associations' best practices guidelines are included in the Model Rules for Licensure of Wholesale Distributors for adoption by the states.
Next Steps: We will continue to work with stakeholders who would like to develop secure business practices.
In the Report, we indicated that we would encourage and educate health professionals to use the MedWatch form as a mechanism to report suspect counterfeit drugs to FDA. To make the reporting of suspect counterfeits easier, we changed the instructions for the MedWatch reporting form, both paper and electronic versions, so reporters will know how and when to report suspect counterfeits. We have also amended the MedWatch website description of product problems and added "suspect counterfeit" to the list of product problems to report to FDA using the MedWatch form. FDA staff has promoted the use of MedWatch for reporting suspect counterfeits in numerous speeches to health professional organizations over the past year. A small number of such reports are starting to come in using the MedWatch form.
Next steps: FDA will continue to educate health professionals to use the MedWatch form to report suspect counterfeit drugs.
In the Report, we stated we would create a Counterfeit Alert Network (CAN) and partner with health professional and consumer groups to provide timely and effective notification to their members or constituents of a verified counterfeit event. By signing the CAN co-sponsorship agreement, organizations become CAN partners and agree to deliver time-sensitive messages and information on specific counterfeit incidents and educational messages about counterfeits in general, as well as information about how and when to report suspect counterfeit drug products. In the past year, we have formed the CAN and currently 13 organizations have signed the CAN co-sponsorship agreement.
Also, in the Report, we stated we would develop internal guidelines for the informational contents of outgoing FDA messages that would be useful to communicate a counterfeiting incident to CAN partners. In the past year, we have developed these guidelines, in the form of a template, in collaboration with CAN partners. This template will allow for the efficient preparation and delivery of uniform counterfeit alert messages for partners to further disseminate.
Next Steps: FDA will encourage stakeholders to become members of the CAN and continue to work with CAN partners to be ready to disseminate effective and appropriate counterfeit alerts when needed.
In the Report, we said that we would streamline our internal processes to respond quickly to reports of suspect counterfeits by improving coordination and communication among all initial responders in the agency. In the past year we amended our internal standard operating procedures and developed a protocol for more efficient internal communication and coordination when a suspect counterfeit drug is reported to the agency, regardless of where the report is received (e.g., MedWatch, an FDA field office, call to the FDA hotline).
Next Steps: No additional action is required.
In the Report, we noted that educating consumers about the risks of counterfeits is a critical piece of the effort to stop counterfeits from entering the stream of commerce. In the past year we have taken many steps towards educating consumers. First, we developed two public service announcements (PSAs) geared to consumers. These PSAs ran in 4.5 million magazines. In addition, 4.6 million medication leaflets distributed by retail pharmacies with patient's prescriptions also carried these PSAs along with additional consumer information about counterfeit drugs. Also, FDA drafted an article about counterfeit drugs that was printed in several local papers nationwide, with an estimated readership of about 9.5 million consumers.
We also set up a webpage on the FDA website for consumers to obtain information about counterfeit drugs, FDA initiatives, and educational information. This website can be found at www.fda.gov/counterfeit. In addition, the National Consumers League (NCL) developed a highly informative website containing useful consumer information about counterfeit drugs.
In the past year, FDA partnered with the National Health Council (NHC) to jointly create and disseminate educational messages on counterfeit drugs. NHC is a private, non-profit organization of over 100 national health-related organizations. Under this partnership, messages to raise awareness of the dangers of counterfeit drugs and how to avoid them will be developed and tested to measure their effectiveness. In addition, products will be created to deliver these messages to the target audience.
In addition, FDA is developing educational messages to inform pharmacists about how to recognize counterfeits, counsel patients on how to minimize the risk of exposure to counterfeits, and on how to notify FDA if a counterfeit drug is suspected. These efforts are in the early stages.
In the Report, we said that we would re-launch our safe online buying practice campaign. In March 2005, we launched a new campaign with tips for consumers on how to buy drugs safely on the Internet and minimize their risks of getting a counterfeit or otherwise substandard drug.
Next steps: We will increase dissemination of the PSAs and counterfeit drug messages. We will continue to update and post relevant information on the counterfeit drug webpage. We will also continue to work with the NHC to finalize educational messages and develop a dissemination strategy for those messages. In the coming months, we will also work with pharmacy organizations to finalize educational messages for pharmacists and develop a strategy to disseminate these messages.
In the Report, we recognized that counterfeit drugs are a worldwide concern, and we stated that we would collaborate with foreign stakeholders to develop strategies to deter and detect counterfeits globally. In February 2004, the World Health Organization (WHO) hosted a meeting to discuss an approach for developing global strategies for combating counterfeit drugs. FDA participated in this meeting and supports WHO's efforts in this area. It was decided at the WHO meeting that a concept paper would be drafted with a proposed strategy to address this problem. In March 2005, we attended the 4 th Pan American Conference on Drug Regulatory Harmonization held by the Pan-American Health Organization (PAHO) where a report was presented and recommendations were discussed regarding combating counterfeit drugs in the Americas. FDA's counterfeit drug initiative is consistent with the recommendations of the PAHO report.
FDA's Office of Criminal Investigations (OCI) continues to work with foreign law-enforcement agencies directly and through Interpol on individual international counterfeit cases.
OCI also has provided training on counterfeit drugs to foreign law-enforcement, customs and judicial officers from various parts of the world through the U.S. Patent and Trademark Office (PTO) Intellectual Property Enforcement Academy. In addition, in the past year, several individual countries have sought FDA's insights, advice, and/or training on combating counterfeit drugs. Although the approaches that we outlined in the Report were specific to the U.S. drug distribution system, many of the principles outlined in the Report are applicable generally.
Next Steps: To the extent that resources permit, FDA will continue to work with international organizations, foreign law enforcement agencies, and individual governments to provide training and advice concerning drug counterfeiting and to collaborate on coordinated strategies to combat the problem of counterfeit drugs globally.
Significant progress has been made towards implementing the measures outlined in FDA's Combating Counterfeit Drugs Report issued in February 2004. Although the use of electronic track and trace technology is still in the implementation stage, adoption and widespread use is closer to becoming a reality as stakeholders work diligently to find solutions to the challenges faced along the way. The use of authentication technologies is gaining acceptance as manufacturers realize that steps should be taken to protect their products from sophisticated counterfeiters. States are starting to adopt stricter laws and harsher penalties to ensure that only legitimate wholesalers do business in their state and they are taking measures to do their part in protecting supply chain integrity. Trading partners in the drug supply chain are also taking steps to ensure secure business practices are adopted and utilized as drug products are bought and sold. Educational efforts have been undertaken to help health professionals and consumers develop a greater awareness and knowledge about counterfeit drugs and how to minimize the risks of exposure. In addition, efforts are underway to tackle counterfeit drugs on a global level.
Despite the progress made, there remains a viable and concrete threat of counterfeit drugs entering the U.S. drug distribution system. We must all continue to work together to expeditiously pursue the measures outlined in the Report to further protect the safety and security of the U.S. drug supply.
![]()
Below are a number of significant counterfeit drug cases that were closed in the past year:
During the first quarter of 2005, three men pled guilty to federal criminal charges in a multi-million dollar Lipitor smuggling and counterfeiting conspiracy. The pleas are a result of an ongoing OCI investigation involving the manufacturing, smuggling, and interstate distribution of counterfeit pharmaceuticals that was initiated by OCI in April 2003. To date, eight people have been indicted; four have pleaded guilty, and another was convicted by a trial jury.
In another counterfeit Lipitor case, an OCI undercover operation resulted in the arrest and conviction of a Belize citizen for violating Title 21, U.S.C. § 331 (a) – Introduction into Interstate Commerce of a Misbranded Drug. In September 2004 the defendant was sentenced to 10 months incarceration and 1 year probation.
On March 9, 2004, an Austin, Texas man pled guilty to four counts of conspiracy to introduce misbranded and unapproved new drugs into interstate commerce, counterfeiting human growth hormone, and possessing controlled drugs with intent to distribute. Two other persons involved in these offenses were previously convicted and sentenced.
On June 23, 2004, an individual pled guilty to charges of conspiracy, trafficking in counterfeit goods, and a felony violation of the Federal Food, Drug and Cosmetic Act. In pleading guilty, the defendant admitted that he conspired with a manufacturer in Beijing to import thousands of counterfeit Viagra tablets into the United States, which he would then resell. The defendant was sentenced on March 25, 2005 to 18 months in prison, followed by 3 years probation and was fined $6000.
On June 16, 2004, an indictment was unsealed in San Diego that charged an individual with conspiring to unlawfully distribute human growth hormone and trafficking in counterfeit goods. According to the indictment, this individual obtained counterfeit Serostim and sold it to bodybuilders who did not possess lawful prescriptions for the drug. Another individual involved in this investigation pled guilty to similar charges on February 19, 2003. Serostim is a prescription drug containing the active ingredient "somatropin," a form of human growth hormone. Serostim is approved by the FDA for use in the U.S. to treat AIDS wasting disease.
An Alabama drug wholesaler was convicted for violating Title 21, U.S.C. § 331 (i) (3) – Selling and Holding for Sale a Counterfeit Drug. In October 2004 the company was sentenced to 5 years probation and fined $24,000.
In January 2005, a Southern California man pled guilty to importing counterfeit Viagra from China and manufacturing 700,000 counterfeit Viagra tablets at a lab in the U.S. An accomplice was convicted of similar charges in September 2004. The total value of the counterfeit Viagra in this case is more than $5.65 million.
In January 2005, a San Diego man was sentenced to serve a 51-month prison term and forfeit substantial cash proceeds for his role in operating a large Internet pharmacy scheme. The drugs distributed included a variety of products counterfeited in Mexico, smuggled into the U.S. and sent throughout the country. Some of the ingredients for the drugs were shipped from India and China. In other instances, unapproved and counterfeit drugs made in India and Pakistan entered the U.S. via the Bahamas. At least 14 other individuals are also being prosecuted in California or Florida as part of this international conspiracy.
![]()