|

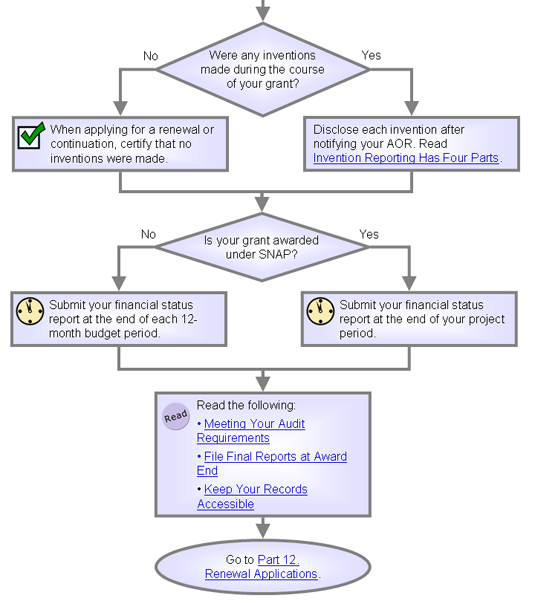
Table of Contents
Are You Ready For This Part?
Part 11a. Managing Your Grant tells you how to comply with the administrative, policy, legal, and reporting requirements of your grant.
Before reading this page, be sure that you . . .
Negotiation
Determines Your Terms of Award
| Know your options when negotiating your grant with NIAID. |
|
Congratulations! You are about to receive your grant award. You will work closely
with your institution throughout the entire grant period.
After you've completed the items above, a grants management specialist will contact you to negotiate the level of support for your project and other aspects of your award.
- Your support level will depend partly on NIAID's financial management plan for the current fiscal year, which is posted on Paylines and Budget once we have a budget.
- To see the budget that your reviewers recommended, check your summary statement through the eRA Commons.
- If you want to read more information on this topic now, see the Grants Negotiation SOP.
You will see the Notice of Award in your Commons account about six to eight weeks after the Council meeting. However, you may be able to start spending
funds before getting your Notice of Award.
- Your business
office must approve, since doing so is at your institution's risk.
- Find details in our Early Grant Awards questions and answers.
Find additional information in the Grants Negotiation SOP.
Your Award May Differ From Your Request
In your Notice of Award, the total budget, and Specific Aims may differ from those you requested for a few reasons.
- Initial peer review. Study section may recommend changes to your Research Plan or budget. For example, it may deem that you can achieve your Specific Aims with less money or time than you requested. Reviewers may have felt that some of your Specific Aims are not necessary.
- Overlap. A grants management specialist or program officer may modify your award based on overlap identified in the other
support in your just-in-time information. If
part of your Research Plan or scientific effort
has already been paid for by NIH or another organization, we will
reduce the funding level.
- Programmatic reduction. We may have to reduce your budget if our annual appropriation is not sufficient for us to fund applications at Council-recommended levels. Check our Financial Management Plan to see if there is a programmatic reduction for the current fiscal year.
If NIAID modifies your Research Plan or budget, a grants management specialist will contact you to begin negotiating your terms of award. In this negotiation, you may revise your project or request a restoration of funds, years, or Specific Aims. If you wish to pursue any of these options, get advice from your program officer.
If your budget was reduced by 25 percent or more due to a programmatic
reduction (not peer review), you must modify the scope of the project, timeline, and budget. Get advice from your
program officer and approval
from your grants management specialist.
Please note that NIAID may decide to award an investigator-initiated clinical trial or epidemiology study as a cooperative agreement. We need your permission for the conversion, but if you refuse, you would not get the award.
If you agree, you will negotiate new terms with your grants management specialist. For details, see the Conversion of Grants to Cooperative Agreements SOP.
Information for Multiple PIs
If you requested to allocate funds among PIs, we will include the allocation in a footnote.
Make Sure
Your Institution Has Negotiated F&A Rates
In
most cases, your grant support will pay for direct
costs plus facilities
and administrative costs (previously called indirect costs) negotiated
for your institution.
Depending on the type of organization you work in, you can find the following information on direct and F&A costs that may be charged to
your grant.
If your organization is non-profit, it may have negotiated
F&A costs rates before you receive your Notice of Award. Most for-profit and new organizations negotiate
F&A rates after they receive their notice.
If your institution has to negotiate F&A rates,
your Notice of Award will direct it to the Division
of Financial Advisory Services, which will ask your institution to
submit a proposal for F&A cost rates. Once NIH approves a rate, it notifies you
and NIAID.
If you want to know how your
institution calculates F&A costs, ask your
sponsored research office.
Accepting the Award
Accepting
you grant isn't
a formal process.
By this point, we will have set up a method of payment with your institution so
you can receive funds. At the start of your project
period -- the period of time we agree to fund you -- we begin sending
you money. By using the money, you've accepted the award.
Read more about accepting a grant award and
getting funds sooner in our Early Grant Award questions and answers.
Read Your Notice of Award, Know Your Terms
| You'll receive a Notice of Award for every budget period (usually one year) of your award. Read it carefully. |
|
When
NIAID issues a Notice of Award, we have accepted all your application information. You can find the Notice of Award in the eRA Commons; use the Status module or Issued Notice of Award query.
Your Notice of Award
Read your Notice of Award -- it houses a lot of helpful information. It tells you the funds you will receive for current and future years,
start and end dates, terms
and conditions of award, and the name of your program
officer and grants
management specialist.
You'll get a Notice of Award for every budget
period (usually one year) but just one for a multiyear grant.
You may also want to read the NIH Grants Policy Statement on Notices of Award. If this is your institution's first NIH award, your notice will contain a link to our Welcome
Wagon Letter, which contains a wealth of helpful information.
Know Your Terms of Award
When
you accept a grant award from NIH, you agree to be bound by its terms
and conditions, which take effect as soon as you receive grant funds. Find them in section III of the Notice of Award. To see general terms and conditions for all grants, visit NIH's Award Conditions and Information for NIH Grants.
Read the terms before you begin your research, so you do not
unknowingly break them.
In that section, you may find that NIAID placed a temporary restriction on your award.
- For example, if you're
working with select
agents, we'll add a Select Agent Terms of Award for NIAID Grants that prohibits you from using grant funds for select agent research until
you've registered with CDC or USDA.
- We can put a restriction on your grant at any time, for various reasons, including if you fall behind in reporting requirements.
- Until we lift the restriction, you legally cannot use grant funds to do the research or publish any data resulting from that research.
Find more information online:
Don't Forget to Keep Up With Policy Changes
At any point during your grant, NIH can institute a new policy that affects you. To stay abreast of the news, read
NIH Guide notices and Subscribe to Email Alerts.
We also maintain a list of NIH policy changes during the past year at Top Policy Changes. Get more tips on staying informed at Keep Up With Policy Changes.
Public Access
Here's an example. You must submit to PubMed Central (PMC) an electronic version of any final peer-reviewed manuscript resulting from NIH-funded research that is accepted for publication on or after April 7, 2008. This requirement applies even if the full text of the article is on a public Web site.
Plus, NIH requires you to include PMC ID numbers in all applications, proposals, and progress reports when citing a paper that results from NIH funding.
Read Other Project
Information Form: Bibliography and References Cited and the Public Access of Publications SOP for details.
Grantees Can
Take Many Actions Independently
| Without NIAID approval, most grantees can rebudget
funds and carry over unobligated balances from one budget period to the next. |
|
Grantees have
a lot of flexibility to make changes in their approved project or budget
without NIAID's approval. These privileges are derived from laws known
as the expanded
authorities, which give greater autonomy to grantees.
Without NIAID approval, grantees can:
- Extend a project
period without additional funds, with some exceptions -- read the No-Cost Extension SOP for details.
- Carry over unobligated balances from one budget period to the next unless the award says prior
approval is required. See the Carryover Requests SOP for details.
- Transfer work to a third party through consortium agreement,
contract, or other means.
- Make cost-related changes, including rebudgeting of
funds, such as patient care or equipment,
unless these changes constitute a change
in scope.
Expanded authorities apply to all grant types (except individual fellowships). You still
need to pay attention to your Notice of Award to see whether any specific actions are not
allowed.
Find more information online:
Some Actions
Require Our Approval
| You need our approval when changing key
personnel or the grantee organization. |
|
While
you can make certain
changes that don't require NIAID approval, we always
need to approve some actions first. Here's the list.
- For new or renewal awards, spending money more than 90 days before the start
date.
- For noncompeting grants, you do not need prior approval for charging preaward costs before the start date.
- Changing key
personnel. For PIs, see the Change of Principal Investigator SOP.
- Changing the grantee organization. See Change of Grantee Organization below.
- Changing the status of the grantee organization.
- Add, remove, or transfer a foreign subaward or foreign site.
- Transfer
a subaward from a foreign organization to a U.S. organization, or vice versa.
- Taking a second extension of a final budget period.
- Changing award terms and conditions, or undertaking any activities
disapproved or restricted as a term
of award.
- Using salary funds in a career
award for a new purpose.
- Performing alterations
and renovations exceeding $300,000 in total
costs. Regardless of cost, foreign grants need preapproval before spending on alterations
and renovations.
- Carrying over unobligated balances from one budget period to the next if the award says prior
approval is required. See the Carryover Requests SOP for details.
- Making any change in your project that constitutes a change
in scope -- see What
Constitutes a Change in Scope? below.
See the Prior Approvals for Post-Award Grant Actions SOP for a complete list.
Contact Us Early
Be sure to request approval from NIAID well in advance -- some changes
in scope require up to two months' notification. If you have questions,
contact the grants
management specialist or the program
officer listed in your Notice of Award.
See how to email a request for an approval at Prior Approvals for Post-Award Grant Actions SOP. You may also want to read the NIH
Grants Policy Statement on Prior Approval Requirements.
Change of Grantee Organization
If you change institutions, your institution can either relinquish the award to your new one, including to a foreign institution, or nominate a replacement PI.
NIAID does not pay additional costs, such as salary changes, caused by a transfer. However, we do pay for higher facilities and administrative costs, based on the availability of funds. When negotiating with a new institution, keep any added expenses in mind.
If your organization agrees, you may take equipment to
a new site. Ask your institution to submit an Official
Statement Relinquishing Interests and Rights in a Public Health Service Grant (form PHS 3734). Grants management and program staff will review the form, then send it
to the NIAID Council for approval.
See Change of Principal Investigator SOP.
Information for Multiple PIs
Grants with multiple principal investigators have other actions that require prior approval:
- When a PI wants to withdraw from a grant, NIAID will evaluate the request considering how the withdrawal would affect the project, especially its scope of work and leadership plan.
- If a PI changes institution, the grantee may request to establish a subaward with the PI's new institution. Alternatively, the grantee may transfer the award to the new institution; the new grantee would then need to establish a subaward agreement with the former grantee.
- If the contact PI moves to a new institution, the remaining PIs must request a new contact PI, since this role must be filled by someone at the grantee institution. NIAID will not automatically transfer the grant if the contact PI moves.
You may also want to read the NIH
Grants Policy Statement on Changes in Project and Budget.
What
Constitutes a Change in Scope?
| Get your grants
management specialist's approval before changing your Specific Aims or using a new technology. |
|
Changes
in the scope of the research significantly alter your peer-reviewed and Council-approved
project. You need to obtain approval from your grants
management specialist before making this type of change. Here are some examples that might change the scope of your research:
- Changing the Specific Aims.
- Changing to a different animal model.
- Using research animals or human
subjects in a way other than approved.
- Shifting the research emphasis from one disease area to another.
- Including new research animals or human subjects.
- Using a new technology.
- Rebudgeting funds
in or out of a single budget category by more than 25 percent of the total
costs of the award.
- Rebudgeting funds so that an alterations and renovations project exceeds $300,000. Regardless of cost, foreign grants need preapproval before spending on alterations
and renovations.
- Changing the principal
investigator.
- Having the principal investigator on a leave of absence for more
than 90 days.
For a list of all actions that constitute
a change in scope, see NIH
Grants Policy Statement on Prior Approval Requirements.
If transferring a grant from one organization to another, read NIH
Grants Policy Statement on Change of Grantee Organization.
Pay Attention
to How You Spend Your Money
Your
expenditure rate is important. When working with your grant funds,
ask yourself: Am I pacing myself? Am I spending all my money in the
first month or two of the project? Am I not spending the money fast
enough?
Don't depend on your institution to monitor your expenditures.
We expect you to have reasonable monthly expenditures.
In reviewing your quarterly reports, we take your expenditures
into account when considering whether to continue funding your
project.
Some grantees can use grant monies to pay for the costs of inventions,
licensing and patents. Items include licensing fees, attorney's
fees for preparing or submitting patent applications, and fees
paid to the U.S. Patent Office.
Your Reporting Requirements
| You play a large role in preparing reports,
though your business office submits them. |
|
Once
your project is underway, you and your institution have ongoing requirements, described below:
- Quarterly reports
- Financial status reports
- Invention reports
- Progress report
- Audit requirements
In some cases, you may have additional
requirements.
- For example, if you are working with human subjects, you need to get your certification of institutional review board approval
re-approved every year of your award.
- If you're working with research animals, you need to get your certification of institutional animal care and use committee approval
re-approved every three years.
As PI, you play a large role in preparing the reports,
though you don't actually submit them. You give information
to your business office so it can send us the reports.
Along with the reports
described above, your institutional business official will need
to submit an Annual
Report on Possible Research Misconduct to the Office
of Research Integrity. ORI will impose a bar on your award if it does not
receive this report.
It's good practice
to keep abreast of your due dates. That way you'll know when your
business office will need information from you and when to check that your business office has sent it to us.
Find more information online:
Specify Expenditures
In Your Quarterly Report
| Find out your institution's internal due dates. |
|
Your
quarterly Federal Cash Transaction Report, PSC 272, covers the financial information related
to your project. Go to the DPM Secure Systems Login to get the form.
Before your business office sends each report, it will ask you for information
that outlines your monthly expenditures. Contact your business office to find out your institution's internal due date.
Your business office submits
the PSC 272 to NIH within 45 days after the end of each quarter of the fiscal year and at the end of the grant's
project period.
| Quarter Begins |
Quarter Ends |
Due to NIH |
| October 1 |
December 31 |
February 14 |
| January 1 |
March 31 |
May 15 |
| April 1 |
June 30 |
August 14 |
| July 1 |
September 30 |
November 14 |
Find more information on the Division
of Payment Management Web site.
Know When
to Submit a Financial Status Report
| Submit all financial status reports electronically through eRA Commons. |
|
If
your award uses a streamlined noncompeting award (SNAP), your business office must send
NIAID a financial
status report at the end of your grant's last year.
For non-SNAP awards or
those requiring more frequent reporting, your institution needs
to submit a financial status report annually -- within
90 days of the end of each 12-month budget
period and at the end of the project
period.
Submit all financial status reports electronically through eRA Commons.
Invention
Reporting Has Four Parts
| Use NIH's iEdison to comply
with some invention reporting requirements. |
|
You
must report any inventions made during your grant.
Use NIH's iEdison to comply
with two of your four reporting requirements:
- You need to fully disclose an invention to us in writing within two months
after the inventor provides written disclosure to your institutional official.
- Provide
the grant, name of the inventor, and a
complete technical description.
- Your business office sends this information through iEdison.
- When applying for a renewal or noncompeting continuation
support of your grant, include either:
- A list of all inventions conceived or brought to practice during
the preceding budget period.
- Certification that no inventions
were made during the period.
- You also must submit an annual utilization report when you've
elected title to an invention or begin to receive royalties or
licensing fees from inventions that are not patented. Use NIH's
iEdison to comply
with reporting requirements.
- At the close of your project, submit a final invention
statement and certification, HHS
568. For more information, see File Final Reports
at Award End.
Find more information online:
Send Us an Annual Progress Report
| If you created a new model organism, include the number
of sharing requests received and fulfilled. |
|
To
continue NIH support of your research each year, do the following:
- Your business
office will submit your progress
report and other required documents.
- Submit to NIAID two months before
the beginning of your next budget
period.
- Use the PHS 2590 and read the instructions
carefully.
- Your program
officer reviews your progress report to determine
whether NIAID will continue funding your project. Grants
management specialists evaluate your grant's administrative
and fiscal status.
- Do not sign the progress report.
- For more
information, read the section below on multiple PIs and the next
two sections, What Forms to Fill Out for Your Annual Progress Report and Submit
Your Report Electronically.
You can search a list of due progress reports and pre-filled face
pages in the eRA
Commons at Progress
Report Search by IPF Number. Use your institutional profile number to get
this information for your grant.
NIH limits the kinds of information that may be
placed in an Appendix for all application types, including progress reports. For rules on including
images, publications, and other content, read If You Need an Appendix.
If you plan to create a new model organism, your program officer will assess how you have shared it, so be sure to include the number
of requests you've received and fulfilled. See What Resources
Do You Need to Share? and the Sharing
Model Organisms SOP for more information.
Be on Time
Remember to turn in progress reports on time! Late or incomplete
progress reports often result in late awards.
Public Access
When citing a paper you author or co-author that resulted from an NIH-funded award, you must cite PubMed Central (PMC) identification numbers in your progress report. Read the Public Access of Publications SOP for details.
More Information for Multiple PIs
For applications with multiple
PIs, note
the following:
- Submit only one PHS 2590.
- List the contact PI on the PHS 2590 Face
Page and additional PIs
on Form
Page 1 Continued.
- Ensure all PIs have a signature assurance on file with their
institutions.
- For eSNAP, the sign-off function can
serve as the signature assurance for the contact PI.
- Other
PIs must provide a separate assurance.
- For changes to the leadership
plan, mark “change” on
the Progress
Report Summary.
- Describe changes to communication
plans, procedures for resolving conflicts, and the administrative,
technical, and scientific responsibilities for the PIs.
For further details on multiple PIs, see Take Heed -- You Might Want to Avoid a Multiple PI Application. Find more information online:
What Forms to Fill Out for Your Annual Progress Report
Streamlined Progress Reports
| Most people send their noncompeting progress report electronically using eSNAP. |
|
If your grant has automatic carryover authority,
you will likely submit your progress report using
the streamlined noncompeting award process (SNAP).
Check your Notice
of Award to see if you may use SNAP.
With SNAP, you fill out only certain parts of the PHS
2590 form. See the Noncompeting
Progress Reports and Program Officer Approval SOP for details and read the PHS
2590 instructions.
Most people are now using an electronic SNAP. See the eSNAP SOP.
 |
May you use SNAP?
|
Non-Streamlined Progress Reports
If your award does not have automatic carryover authority, submit a non-SNAP 2590. Program project grants, cooperative agreements, and institutional research training grants require non-SNAP reports.
A non-SNAP report includes all the information in a SNAP as well as form pages 2 and 3 of the PHS
2590. For information about sending it in, see Submit Your Report on Paper.
See the Checklist for Part 11a. Managing Your Grant.
Submit
Your Report Electronically
| NIH plans to phase out the paper approach, and most noncompeting progress reports are already sent through eSNAP. |
|
With eSNAP, your program
officer and grants
management specialist have simultaneous access to the noncompeting progress report, speeding processing and letting you see the status of your report.
NIH plans to phase out the paper approach, and most noncompeting progress reports are already sent through eSNAP.
Ask your business office whether eSNAP is enabled for your institution. For more detail on how to enable eSNAP, your institution should read NIH's Electronic Streamlined Non-competing Award Process. If you're not eligible for eSNAP participation, see Submit
Your Report on Paper.
eSNAP reporting uses the PHS 2590. Requirements are the same as SNAP except:
- eSNAPs are due 45 days before the grant anniversary date, instead
of 60 days for paper submissions.
- You can include citations by providing
links to the online journal instead of sending a reprint.
- You don't need to provide institutional
review board and institutional
animal care and use committee approval dates. Your institution will provide this information;
read more in Human Subjects Certifications: IRB
or IEC SOP and Animals in Research SOP.
- Institutions may delegate the authority
to submit an eSNAP progress report to a PI.
- NIH stores the data in your key
personnel report in the eRA Commons so you can retrieve
and update it when you wish.
Be sure to read the PHS
2590 instructions for more information.
For eSNAP, the sign-off function of the Commons can
serve as the signature assurance. If you are part of a multiple PI grant, see the section on multiple PIs at Send Us an Annual Progress Report.
To learn more about eSNAP, see the eSNAP
(Electronic Streamlined Noncompeting Award Process) SOP, the Commons
Demonstration Site, and the eSNAP
User Guide.
Submit
Your Report on Paper
| You must
submit a paper progress report two months before the budget start
date, four months for training awards. |
|
If your institution is not enabled for eSNAP, send a paper streamlined
or non-streamlined progress report to the centralized mailing
address:
Division of Extramural Activities Support, OER
National Institutes of Health
6705 Rockledge Drive, Room 2207, MSC 7987
Bethesda, MD 20892-7987 (Use this ZIP code for the U.S. Postal Service, including express mail.)
Bethesda, MD 20817 (Use this ZIP code for commercial carriers such as FedEx and UPS.)
Remember that to continue grant support, you must
submit your progress report two months before the budget start
date, four months before the start date for training awards.
Meeting Your
Audit Requirements
| Annual audits are required for any institution that spends $500,000 or more
a year of federal grant money. |
|
Your institution will have an audit if it spends $500,000 or more
a year of federal grant money.
Educational or nonprofit institutions
are subject to the requirements of OMB
Circular A-133. For-profit organizations can satisfy
audit requirements with either of
two audit types:
Audits are required annually. Grantees usually
have 30 days after the receipt of the auditor's report to respond
to
audit findings.
Your institution should submit audit reports to:
Federal Audit Clearinghouse
Bureau of the Census
1201 East 10th Street
Jeffersonville, IN 47132
Even if your institution is exempt, you still need to maintain your grant records in case NIH wants to review or audit them. Find more information online:
File Final Reports
at Award End
| There are three final reports: financial, progress, and invention. |
|
At
the end of an award, your institution submits three closeout reports:
- Final financial status report.
- Final
progress
report. See Preparation of a Final Progress Report below (PHS 2590).
- Final invention report (HHS
568).
You have 90 days after the expiration or termination of your grant
to complete these final forms. Failure to submit final reports on time may affect future funding to your institution.
For closeout requirements, see the NIH
Grants Policy Statement on Closeout.
Prepare a Final Financial Status Report
Your Final Financial Status Report should indicate the exact balance of unobligated funds, which must match the amount listed in your Payment Management System's Federal Cash Transaction Report (SF-272).
Prepare a Final Progress Report
The Final Progress Report helps NIAID staff evaluate your research and should include the following:
- Complete heading listing the grant number,
PI, grantee institution, project title,
and date of the entire period of the grant (include any authorized
extension of the final budget period).
- Statement of progress made toward the achievement
of the Specific Aims. List the results, positive or negative, direct
or indirect, and those you considered to be significant.
When possible, tie these results to the report's publication
list (see next bullet).
- List of publications originating from the grant and those already in print, in press, or planned as a result
of the grant.
- You must cite PubMed Central (PMC) identification numbers when citing a paper you author or co-author that resulted from an NIH-funded award. The PubMed Central ID and PubMed ID numbers are different. If you have the PubMed ID, you can use the National Library of Medicine's PM ID: PMC ID Converter to find out the PMC ID or vice versa.
- Also be aware that you must submit to PMC an electronic version of any final peer-reviewed manuscript that resulted from an NIH-funded award and was accepted for publication by April 7, 2008. Some publishers submit the article for you. For more information, see the Public Access of Publications SOP.
- For more information, including what to do if a PMC ID is not ready, the Public Access of Publications SOP.
- Documentation of sharing of model organisms, including
the number of requests you received and fulfilled. See the Sharing
Model Organisms SOP.
- Description of data, research materials, and other information resulting from research and how they may be shared with other investigators. See the Data Sharing for Grants: Final Research Data and Data
Sharing for Grants: Genome-Wide Association Studies SOPs.
- For human subjects, report on the inclusion of gender and minority study subjects using the PHS 2590's Inclusion Enrollment Report. If children were involved, indicate how the study was relevant for them.
If you are applying for a renewal and get funded, you do not need to submit a final progress report since you will document progress in your renewal application.
If your renewal application does not get funded, you need to submit a final progress report.
Submit Final Reports -- Electronic
Submit final reports electronically through the eRA Commons. Query the Commons Status system for a list of ended grants, then enter the Closeout screen to submit the reports.
Submit Final Reports -- Paper
Submit financial status reports electronically through eRA Commons.
Include one copy of reprints not previously submitted, when available.
If you have submitted a publication to the PubMed Central archive, you may send a PMC identification number instead.
Send your final progress report and invention report to:
NIH Centralized Processing Center
6705 Rockledge Drive
RM 2207, MSC 7987
Bethesda, MD 20892 (Use this ZIP code for the U.S. Postal Service, including express mail.)
Bethesda, MD 20817 (Use this ZIP code for commercial carriers such as FedEx and UPS.)
E-mail: DeasCentralized@od.nih.gov
Fax: (301) 480-2304
Go to the Checklist for Part 11a. Managing Your Grant.
Keep Your Records
Accessible
| Keep records for three years after the
grant ends. |
|
You
must keep your project records accessible for three years after the
grant ends.
If any issue arises, we need to be able to verify the records,
which must include all data and fiscal information. For very detailed information,
read Retention and Access Requirements for Records, 45 CFR Part 74.53.
Through the Freedom
of Information Act, other people can gain access to information
concerning your grant. If other scientists formally request non-proprietary
information from your application, our FOIA office
will provide it. See the Privacy, Conduct, Conflict of Interest, and Clinical Research Ethics questions and answers.
Help us improve our outreach to you by emailing deaweb@niaid.nih.gov. |