|
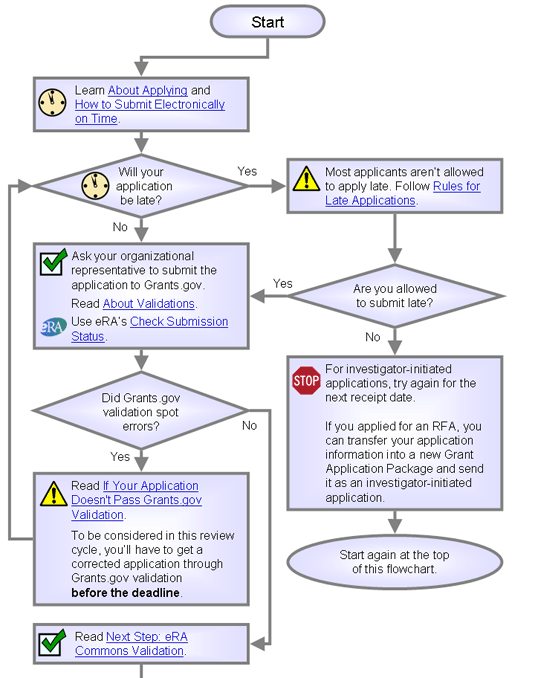 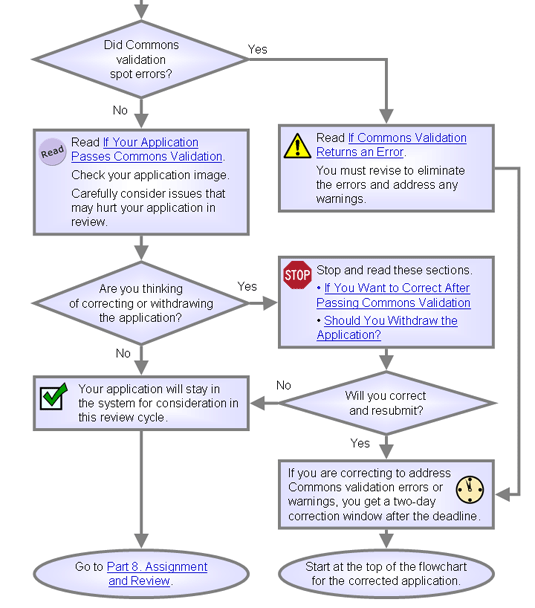
Table of Contents
Are You Ready for This Part?
Part 7. Applying describes the ins and outs of submitting your application.
Before reading this page, be sure that you . . .
About Applying
| Over 90 percent of R01 applicants successfully complete the application process on their first or second attempt. |
|
In this section, we explain the automated checks your application must pass and your action items for each stage.
Decision points below will help you analyze tricky timing you may face should you need to correct your application.
Although we focus on what to do when your application doesn't pass a step, don't be daunted by these hurdles. Over 90 percent of R01 applicants successfully complete the application process on their first or second attempt.
Find out if your institution wants you to apply using Grants.gov’s software or an alternative. We assume you will use the default: your organization submits applications directly to Grants.gov.
This document does not repeat the instructions from the Grant Application Guide or NIH's Electronic Submission site.
How to Submit Electronically on Time
| Most applications are due three times a year. Apply early to avoid crunch times. |
|
Your authorized organizational representative must submit your application to Grants.gov by the deadline in the funding opportunity announcement.
For step-by-step details on this process, read NIH's Submit Application. Then return here for more advice on timing your submission.
Here's how deadlines work:
- Most applications are due three times a year.
- NIH accepts investigator-initiated grants on three receipt dates.
- New non-AIDS R01 applications are due February 5, June 5, and October 5.
- AIDS, small business, renewals, and other grant types have different due dates.
- Go to the Standard Due Dates for Competing Applications for all NIH receipt dates.
- Each request for applications has its own, one-time receipt date.
- Your electronic application is on time if Grants.gov successfully receives it by 5:00 p.m. your institution's local time on the due date, except holidays -- see Federal Holidays.
- If a deadline lands on a weekend or federal holiday, it moves to the next business day.
- NIH strictly enforces schedules.
- Your application will get a time stamp and tracking number from Grants.gov. The time required for automated checks after that point does not affect the submission's official time of receipt.
 Our Advice: Get Your Application in Early Our Advice: Get Your Application in Early
You should submit your application at least two days before the deadline so you have time to get through validations.
However, two days is an absolute minimum and assumes your application will successfully pass the extensive validations process on the first try.
We recommend that you plan for additional time. If your application doesn't pass validations, you may need several more days to make corrections and get the corrected version through before the deadline. Read additional information below at About Validations.
Apply early to avoid crunch times. NIH applicants as well as those from other agencies can bog down Grants.gov's resources. There may be overlapping submission dates you don't know about.
For helpful information on timing an electronic application, see When are the submission deadlines? and Corrected or Late Electronic Applications questions and answers.
In some instances, NIH allows late applications. Read the next page for details.
Find more information online:
 |
Do you think your application might be late?
- No. Ask your organization to submit the application for you, then continue reading at About Validations.
- Yes. Continue reading here.
|
Rules for Late Applications
| NIH and NIAID accept late applications for certain reasons only. |
|
In some instances, NIH allows late applications.
- NIH's Center
for Scientific Review (CSR) and NIAID's Scientific
Review Program may accept an application late if you have a valid reason.
- Valid reasons include federal electronic systems issues, natural disasters,
personal tragedies, and some service on an NIH study section.
- NIH
issues special Guide notices after natural disasters.
- When submitting a late application, you must include a cover
letter describing the reason for the delay. See Do You Need a Cover Letter? for tips.
- Neither CSR nor NIAID guarantees it will accept a late application,
and both have a limited window for receiving them.
- Depending on who conducts the initial peer review, some
practices are different:
- CSR
- Reviews most investigator-initiated applications.
- Follows the NIH late application policy.
- NIAID
- Reviews requests
for applications (RFA), some program
announcements that identify location of peer review (PAR),
and others -- see What award types are peer reviewed at NIAID? for details.
- Decides whether to accept a late application case by case. Contact the scientific
review officer listed in the RFA or PAR to discuss
your situation.
- See the Late Applications SOP for more information.
For more information on NIH's policy on late submissions, read the January 4, 2008, Guide notice.
 |
Will your application be late?
- No. Ask your organization to submit the application for you, then skip the next question to continue reading at About Validations.
- Yes. Read the next question.
|
 |
Are you allowed to submit your application late?
- No. Try again for the next receipt date, if any. See How to Submit Electronically on Time above.
- Yes. Follow the instructions above and ask your organization to submit the application for you, then continue reading here.
|
About Validations
| Don't expect automated validations to spot content issues. |
| Don't wait for confirmation emails -- watch your application's status in the Commons. |
|
Validations are automated checks of an application's form data and attachments.
It's important to understand what validation does and doesn't do.
 Our advice: don't expect a validation to spot content issues, for example, a missing data table from your Research Plan. To bypass problems, plan ahead, and read NIH's Avoid Common Errors for examples. Our advice: don't expect a validation to spot content issues, for example, a missing data table from your Research Plan. To bypass problems, plan ahead, and read NIH's Avoid Common Errors for examples.
Your application must pass two validations: Grants.gov and eRA Commons. If it fails either one, you must go through the entire submission again.
If validations reveal problems or you spot other issues, you need to decide if and when to submit a corrected application. See the scenarios below, and talk to your mentors for their advice.
First Step: Grants.gov Validation
Grants.gov validation checks for very basic items.
After submitting your application, your organization's representative should receive a submission receipt and then a validation
confirmation or rejection email message from Grants.gov. For
a sample, see Email
notifications from Grants.gov.
This confirmation usually arrives within minutes, but it can take up to 48 hours during busy periods.
 |
Did your application pass Grants.gov validation?
|
If Your Application Doesn't Pass Grants.gov Validation
 Our Advice: Look at the Deadline When Deciding What to Do. Our Advice: Look at the Deadline When Deciding What to Do.
Your next step depends on whether the deadline has passed.
| Timing |
Suggested Action |
| Before the deadline |
Correct the issues and have your AOR submit
a new application at least two days before the deadline. Get details at NIH's Submitting Changed/Corrected Applications, then return here for advice. |
| After the deadline |
If your application does not get through
Grants.gov by the deadline, it will be late. NIH rarely accepts
late applications -- see the Rules for Late Applications -- so, more likely, you will have to correct and wait four
months for the next receipt date (if any). |
Your corrected application must pass Grants.gov validation to move on to the next step.
 |
Did your corrected application pass Grants.gov validation?
- No. See the table above. Send a corrected application to address the issues.
- Yes. Continue reading here.
|
Next Step: eRA Commons Validation
After passing Grants.gov validation, your application data moves to NIH for the more thorough Commons validation, which may take up to 24 hours. The Commons check may result in errors, warnings, or both.
- Errors -- inaccuracies, inconsistencies, omissions, and some formatting problems that cause your application to be unacceptable.
- Warnings -- potential issues that won't stop your application from moving forward but can reflect serious problems you should correct.
You can see NIH's complete list of potential Errors and Warnings.
 Our Advice: Check Application Status, Know What Errors and Warnings Mean for You Our Advice: Check Application Status, Know What Errors and Warnings Mean for You
Don't wait for the confirmation email Commons sends you and your signing official after validation. You, your signing official, or any assistants should check the application in the eRA Commons Status module. For multiple PI applications, any PI can do this. Get details at NIH's Check Submission Status.
Commons validation leads to one of three results:
- Passed -- no errors or warnings.
- Passed -- no errors, with warnings.
- Failed -- errors.
 |
Did your application pass Commons validation?
|
If Commons Validation Returns an Error
 Our Advice: Ask Yourself, "To Send or Not to Send" a Corrected Version Our Advice: Ask Yourself, "To Send or Not to Send" a Corrected Version
Errors stop your application from moving forward. If you get one or more errors, you must decide when to send a corrected version.
In the table below, counts of "days" include only business days.
| Timing |
Suggested Action |
| Before the deadline. |
Ask your organization to submit the corrected application. Get details at NIH's Submitting Changed/Corrected Applications and our Corrected or Late Electronic Applications questions and answers; then return here.
Corrected applications sent before the deadline may include any kind of edit. |
| After the deadline. |
You can use the two-business-day correction window to address only errors or
warnings from eRA Commons validation.
- NIH
may reject your application if you use the correction
window to make other revisions.
- NIH will compare the application to your cover letter to
confirm.
For your corrected application to be on time, it must pass Grants.gov
and Commons validation by 5:00 p.m. your institution's local time on the second business
day after the submission deadline.
Get details at NIH's Submitting Changed/Corrected Applications and our Corrected or Late Electronic Applications questions and answers; then return here. |
| After the end of the correction window. |
NIH rarely accepts
late applications -- see the Rules for Late Applications -- so, more likely, you will have to correct and wait four
months for the next receipt date (if any). |
Your corrected application must pass Commons validation without errors to move on to the next step.
 |
Did your corrected application pass Commons validation by the end of the correction window?
- No. See the table above for your options.
- Yes. Continue reading here.
|
If Your Application Passes Commons Validation
Once your application passes Commons validation, the
system generates an application image.
NIH gives you two business days to review the application image in the eRA Commons; use the Status module. During this viewing window, decide if you want your signing official to reject it.
If you're happy with the application image, you need take no other action at this point and let your application continue to peer review.
 Our Advice: No Matter What, Check the Image to Determine If You Want to Correct Our Advice: No Matter What, Check the Image to Determine If You Want to Correct
Do not assume your application is in good shape.
- You may want to correct your application to address warnings -- they can reflect serious problems you should correct.
- If there's time before the deadline, you may also want to correct to address issues not reflected in warnings. Perhaps you omitted something important or inserted the wrong version of an attachment.
Before midnight on the second day of the viewing window, decide if you're happy with the current application image.
 |
After viewing the application image in the Commons, do you want it to continue to peer review?
- Yes. Take no action. The application will automatically move forward at midnight on the second day of the viewing window. Skip ahead to Part 8. Assignment and Review.
- No. Read the next question.
|
 |
Are the problems in the application image due to technical issues with NIH's software?
- Yes or maybe. Contact the eRA Commons Help Desk immediately.
- The Help Desk will help you diagnose the problem. They will advise you on the next steps, including whether to reject the application image.
- In the rare event of a technical issue caused by NIH's software, you won't be penalized for delays.
- No. Continue reading here.
|
If You Want to Correct After Passing Commons Validation
 Our Advice: If You Plan to Correct, Consider the Timing and Severity of the Issue Our Advice: If You Plan to Correct, Consider the Timing and Severity of the Issue
To make corrections, choose the relevant scenario below. In the table below, counts of "days" include only business days.
| Timing |
Suggested Action |
| Before the deadline, during two-day viewing window. |
If you have enough time to get a corrected application through Grants.gov before the deadline, have your signing official reject the application image before the two-day viewing window expires.
Then ask your organization to submit the corrected application.Get details at NIH's Submitting Changed/Corrected Applications and our Corrected or Late Electronic Applications questions and answers; then return here.
Corrected applications sent before the deadline may include any kind of edit. |
| After the deadline, before the two-day viewing window ends. |
You can use the two-business-day correction window to address only errors or
warnings from eRA Commons validation.
- NIH
may reject your application if you use the correction
window to make other revisions.
- NIH will compare the application to your cover letter to
confirm.
For your corrected application to be on time, it must pass Grants.gov
and Commons validation by 5:00 p.m. your institution's local time on the second business
day after the submission deadline.
Get details at NIH's Submitting Changed/Corrected Applications and our Corrected or Late Electronic Applications questions and answers; then return here. |
| After the deadline, after the two-day viewing window ends. |
Your application has moved to CSR. It will be reviewed unless you ask your signing official to withdraw it. Read the next section for more on withdrawing an application. |
Should You Withdraw the Application?
There are two ways to stop the application from moving forward for peer review. You can withdraw it, or if the viewing window hasn't expired, you can simply reject the application image.
After the viewing window ends, your application will undergo peer review as is unless your scientific review officer allows you to send additional information or you have withdrawn it.
| Your application must be truly outstanding to get funded. Don't annoy reviewers with a mediocre application -- if it has problems, withdraw it. |
|
 Our Advice: Consider Withdrawing Our Advice: Consider Withdrawing
You should consider withdrawing your application in the following circumstances:
- You feel the application is not up to snuff.
- You've run out of time for corrections and can't resolve the issue with the scientific review officer. For more detail on that now, see You May Be Able to Send in Additional Data.
Remember that your goal is to impress your reviewers with the best possible application.
Balance the severity of the problems with the amount of time you have left to correct them in the same review cycle. Compare with the time lost if you wait for the next due date. You may want to get advice before deciding.
- Don't use up one of your two allowed resubmissions and squander the goodwill of reviewers on a sub-par application.
- For resubmission advice, see Part 11b. Not Funded, Reapply.
To withdraw your application from consideration, ask your organization to fax a signed letter to CSR's Division of Receipt and Referral at 301-480-1987. Specify the NIH accession number for the version you want to withdraw.
 Our Advice: Proceed With Caution When Deadline Is Imminent Our Advice: Proceed With Caution When Deadline Is Imminent
- If you plan to withdraw the application and resubmit for the same deadline, be careful. After your application is withdrawn, you no longer
have an active application in the system.
- As the deadline approaches,
you have the same disadvantages as anyone who applies at the
last minute!
- Allow at least two days to get your corrected application
into the system.
For more information, see Investigator Withdrawal of an Application SOP.
 |
Are the issues severe enough to harm your application in initial peer review?
- Yes. Consider withdrawing the application. If you decide to withdraw and revise, see the relevant sections of the NIH Grant Cycle for application advice.
- No. Your application will continue to CSR's administrative review. Continue reading.
|
Help us improve our outreach to you by emailing deaweb@niaid.nih.gov. |

 Our Advice: No Matter What, Check the Image to Determine If You Want to Correct
Our Advice: No Matter What, Check the Image to Determine If You Want to Correct 

 Our Advice: If You Plan to Correct, Consider the Timing and Severity of the Issue
Our Advice: If You Plan to Correct, Consider the Timing and Severity of the Issue  Our Advice: Consider Withdrawing
Our Advice: Consider Withdrawing  Our Advice: Proceed With Caution When Deadline Is Imminent
Our Advice: Proceed With Caution When Deadline Is Imminent 
