 |
|
NTP Molecular Pathology Group Studies Robert Sills, D.V.M., Ph.D.
Group Leader Tel (919) 541-0180 Fax (919) 541-7666 sills@niehs.nih.gov P.O. Box 12233 Mail Drop B3-06 Research Triangle Park, North Carolina 27709 Delivery Instructions Research SummaryThe Molecular Pathology Group (1) reviews and prepares the pathology data for the NTP studies, (2) establishes biologic and mechanistic studies to complement the standard NTP toxicity and carcinogenicity studies, (3) extends the data base of the NTP Bioassay to include molecular biology studies, and (4) contributes to coordination/collection of tissues for the NTP Frozen Tissue Bank. The Molecular Pathology Group achieves its goals by a combination of program-related and institute-wide collaborative research, interagency collaborations and contract testing and support. The Molecular Pathology Group conducts interdisciplinary research in support of, and extends the mission of the National Toxicology Program (NTP)/NIEHS, to identify occupational and environmental toxicants/carcinogens and elucidate the pathogenesis of their effects. The Molecular Pathology Group is involved in two major areas of applied research. 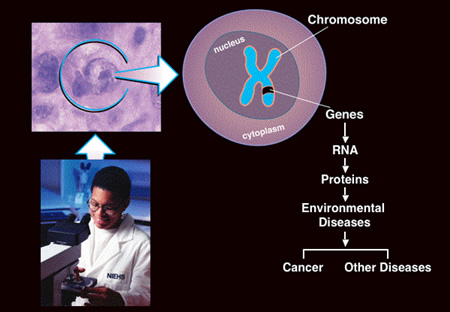
Figure 1: Molecular Pathogenesis - Environmental Disease
The first area of applied research focuses on the hazardous air pollutants, carbon disulfide which targets the central (spinal cord) and peripheral (nerves) nervous system, and carbonyl sulfide which targets the central nervous system (brain). The objectives of these non-cancer studies are to understand the toxicologic response in the nervous system to these compounds by a comprehensive evaluation of function, structure and mechanisms of disease. Through interdisciplinary research, the major finding in the carbon disulfide inhalation studies is that carbon disulfide is metabolized to dithiocarbamate intermediates which cause cross-linking of neurofilament proteins in long axons of the spinal cord and peripheral nerves. Of significance is that valine-lysine thiourea cross-linking on rat globin and lysine-lysine thiourea cross-linking on erythrocyte spectrin (similar mechanism as neurofilament cross-linking in the axon) have the potential to serve as biomarkes of carbon disulfide exposure and effect. In the ongoing carbonyl sulfide studies, using new imaging technologies (magnetic resonance microscopy), it was determined that carbonyl sulfide targets the auditory pathway in the brain. The significance of incorporating magnetic resonance microscopy in this study is that it allowed us to examine 200 brain slices, and made it possible to identify the most vulnerable sites of neurotoxicity, which would have been missed in our traditional neuropathology evaluations. Knowing the most vulnerable site of toxicity, it was possible to focus the electrophysiology studies on the auditory system and demonstrated decreases in auditory brain stem evoked responses. Future studies are to examine the pathogenesis of carbonyl sulfide neurotoxicity. The second area of applied research focuses on understanding the pathogenesis of tumors that develop in rodents following two years of exposure to environmental chemicals. Most of this research has focused on the tumor response in the B6C3F1 mouse. The B6C3F1 mouse strain has been used for carcinogenicity testing of over 500 chemicals in the National Toxicology Program. The objectives of this research are to evaluate spontaneous and chemically induced tumors for genetic alterations in major cancer genes, and to determine if the pathogenesis of rodent tumors is of relevance to humans. This research is driven by the chemicals which are nominated to the NTP for toxicity and carcinogenicity testing, and is based on the tumor outcome after 2-years of chemical exposure. During the pathology evaluation phase, the most predominant chemically related tumor responses are identified for examining genetic alterations in oncogenes and tumor suppressor genes. Generally, tumors are obtained from paraffin embedded blocks, and in some cases frozen tumors are available for evaluation. In these studies signature point mutations were identified for chemical classes such as 1,3-butadiene, chloroprene and isoprene. Also, for chemicals such as 1,3-butadiene, which are metabolized to epoxide and diepoxide intermediates, signature mutations were detected which were consistent with the adduct formed. The significance of this research is that the chemical specific mutations may serve as a biomarker of occupational exposure. Another major finding is that the molecular pathogenesis in mouse tumors following chemical exposure are of relevance to humans. The significance of this research was recently demonstrated in o-nitrotoluene induced large intestinal tumors. In both humans and mice, alterations in the β-catenin/Wnt signaling pathway, ras/map kinase pathway and cell-cycle dependent genes (cylcin D1, p53) were altered, suggesting environmental exposure may contribute to colon cancer. Major areas of research:
Current projects:
Robert Sills, D.V.M., Ph.D., received his Doctor of Veterinary Medicine degree from Tuskegee University (1984) and completed an internship in anatomical pathology from Tuskegee University (1985) and a combined residency/Ph.D. in toxicologic pathology from Michigan State University (1991). He is certified as a veterinary pathologist by the American College of Veterinary Pathologists. Sills heads the Molecular Pathology Group within the Cellular and Molecular Pathology Branch at the National Institute of Environmental Health Sciences, National Institute of Health. In addition, he is an adjunct associate professor in the Department of Toxicology at the University of North Carolina School of Medicine at Chapel Hill, North Carolina, and in the Department of Population Health and Pathobiology, College of Veterinary Medicine, North Carolina State University. His research activities include the study of molecular mechanisms of chemical carcinogenesis with focus on protooncogenes and tumor suppressor genes, neuropathology, and the inclusion of biologic based interdisciplinary research in NTP studies. He is a member of the American Association of Cancer Research, Society of Toxicologic Pathology (STP), and the American Veterinary Medical Association. He presently serves on the Executive Council for the Society of Toxicologic Pathology. Sills has served on the editorial board of Toxicologic Pathology. He chaired STP annual meeting sessions on the human genome, implications for toxicologic pathology and carcinogenesis, and the session on cellular and molecular neurocarcinogenesis, toxicologic pathology of the nervous system. Also, he co-chaired the International Life Sciences Institute (ILSI) seminar series on current issues in neuropathology. |
|

