| II. Accountability: Report on PEPFAR Partnerships for Prevention, Treatment and Care
|
Partnerships for Treatment
It was just a few years ago that many doubted that large-scale antiretroviral treatment programs could work in the world’s poorest nations. Now we know such programs can work. Hundreds of thousands of people are proving it.
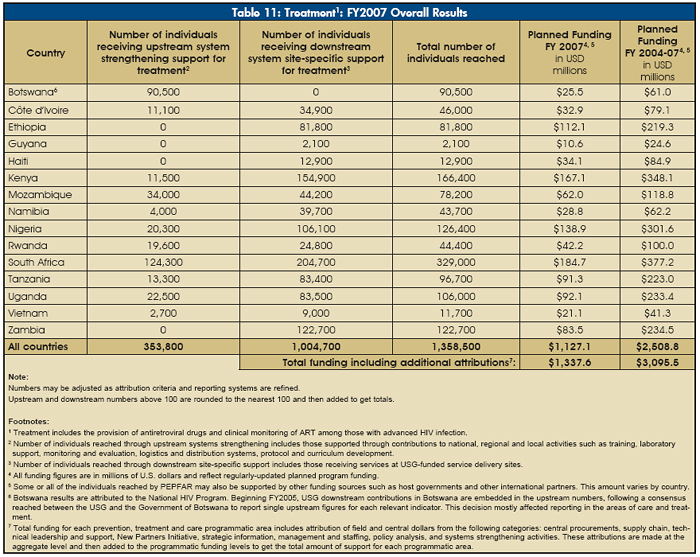
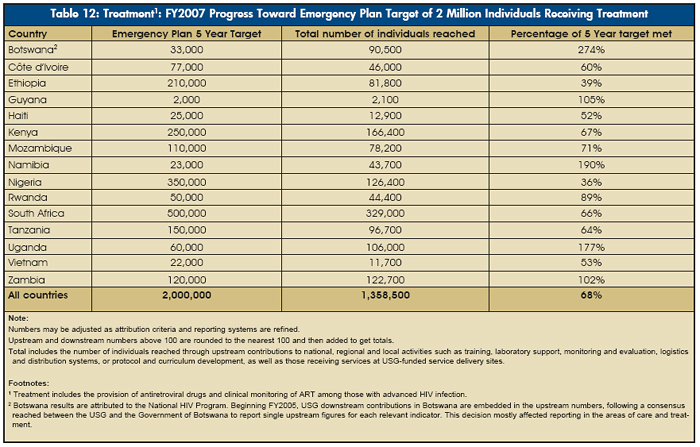
Approximately 1.45 million people — including nearly 1.36 million in the 15 focus countries — received treatment with support from rapidly scaled-up bilateral PEPFAR partnerships with host nations. In FY2007, PEPFAR provided $1.34 billion in support of treatment programs, including treatment for pediatric patients, or 48 percent of program funding in the focus countries. The striking growth of PEPFAR support for treatment in the focus countries is shown in Figure 21 and Table 11.
|

|
|
By September 2007 in the focus countries, approximately 45,000 individuals were being added to the number of people benefiting from PEPFAR support for life-extending treatment every month. The number of sites providing treatment increased by 55 percent from FY2006 to FY2007, and each month an average of about 87 new treatment sites came on line.
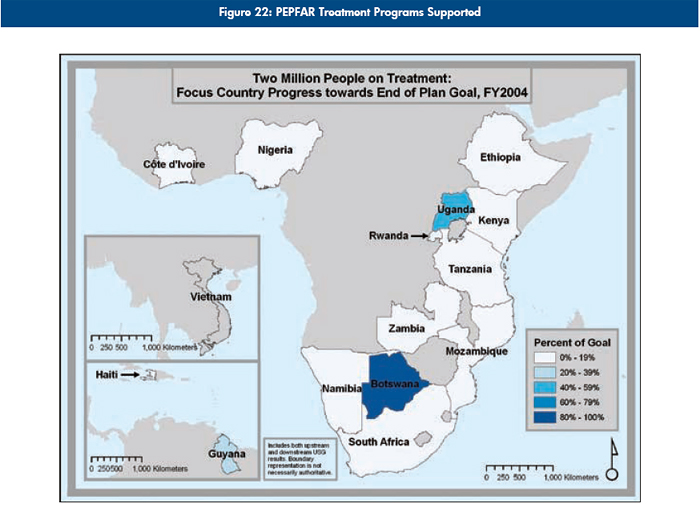
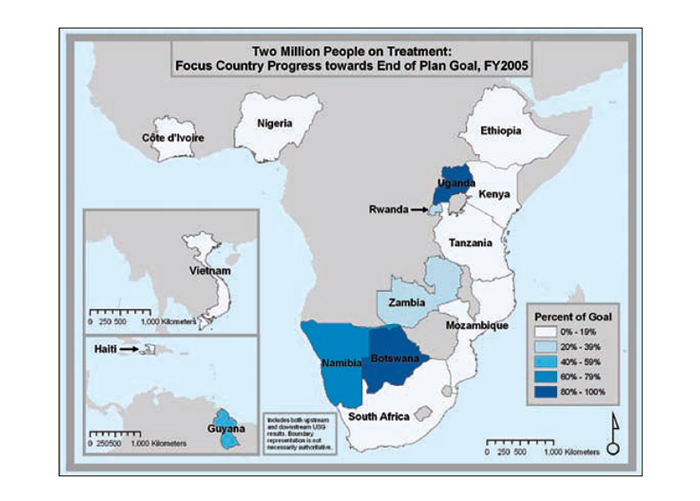
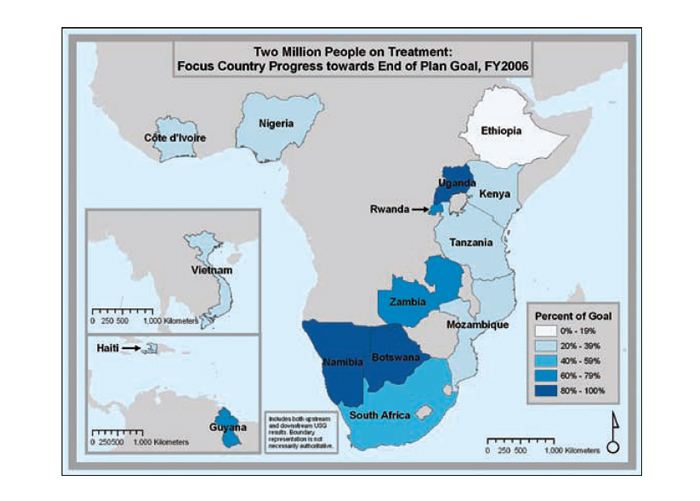
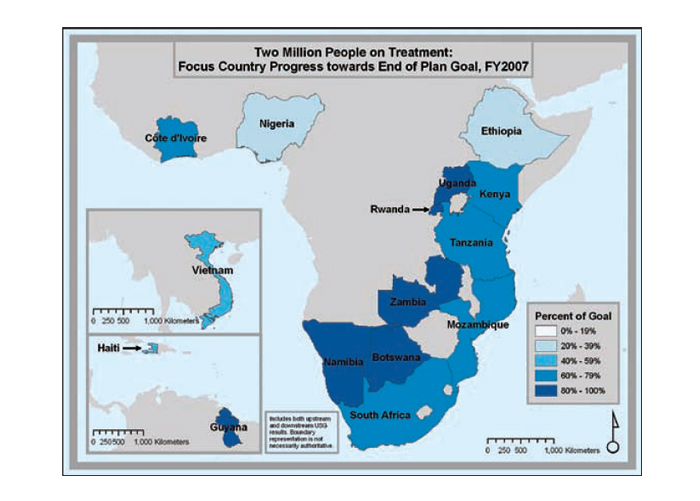
Progress in national scale-up
The series of maps in Figure 22 depicts the steady increase in Emergency Plan support for treatment coverage as programs scale up toward the five-year target of treatment support for two million people.
Beyond the focus countries, other bilateral PEPFAR treatment programs supported an additional 87,000 people (unlike previous years, this number includes only those reached with downstream PEPFAR support), for a total of 1.45 million receiving treatment with PEPFAR support worldwide.
|
|
 |
Reaching women and children
As part of its commitment to ensure treatment availability for children and women, PEPFAR bilateral programs have led international partners in supporting host nations in tracking clients by age and gender. Of those for whom PEPFAR provided downstream support for treatment in the focus countries, approximately 62 percent were women, which is higher than the estimated percentage of adults living with HIV in sub-Saharan Africa who are women.
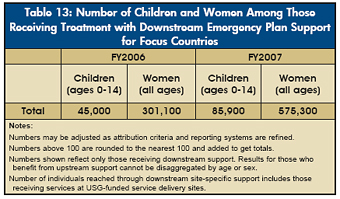
PEPFAR has also expanded access to treatment for children, with the number of children receiving antiretroviral treatment through downstream PEPFAR support increasing 77 percent from FY2006 levels (see Table 13). PEPFAR dedicated nearly $191.5 million to pediatric treatment in FY2006 and 2007 combined, reaching approximately 85,900 children with downstream support in FY2007, compared with only 4,800 in FY2004. |
Pediatric treatment has made steady progress, increasing the share of those receiving PEPFAR-supported treatment who are children from three percent in FY2004 to nine percent in FY2007. This percentage is above UNAIDS’ new estimate of the global share of HIV-positive people who are children (7.5 percent).
Improving infant diagnosis
Increasing the availability of pediatric treatment requires more than increased resources. Standard HIV tests, which test for HIV antibodies, cannot reliably identify children as being HIV-infected until after 18 months of age because of the presence of maternal antibodies. Thus, it is difficult to determine which young infants and children are truly infected and need treatment — and 50 percent of HIV-positive children will die by age two if they are not treated. In order to accurately diagnose HIV infection in young infants and children so they can access treatment, PEPFAR supports nations in expanding polymerase chain reaction (PCR) testing to identify the presence of HIV itself, and not just antibodies. To expand access to accurate diagnosis, PEPFAR-supported programs are performing these tests using dried blood spots on filter paper, which require less blood per test than older methods and easily can be transported to central laboratories for testing.
PEPFAR has supported country-level policy change to allow PCR-based dried blood spot testing in order to reduce the cost and burden of infant diagnosis. Most focus countries have now adopted such policies, making accurate diagnosis and management of pediatric treatment a growing reality.
Addressing ongoing barriers to pediatric treatment
Other barriers currently limiting the scale-up of pediatric treatment and care services include a lack of providers equipped with the necessary skills to address the special needs of HIV-positive children, the relatively high cost of pediatric ARV formulations, regulatory barriers to registering pediatric ARV formulations, weak linkages between PMTCT and treatment services, and limited information about pediatric doses of medicines at different ages and weights. To address these barriers, PEPFAR supports training programs that teach health care workers how to treat pediatric patients, and the development of dosing guides for children of various ages and sizes. PEPFAR also continues to work with pharmaceutical companies, implementing organizations, and multinational organizations such as UNICEF and WHO through a public-private partnership (announced by First Lady Laura Bush in 2006) to address these barriers. |
 |
Increasing the availability of safe, effective, low-cost generic medications
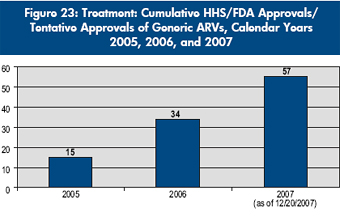
The Emergency Plan’s impact on treatment access extends beyond PEPFAR-supported programs to increased availability of safe, effective, low-cost, generic ARVs in the developing world. To meet the need for such ARVs, the Food and Drug Administration within the U.S. Department of Health and Human Services (HHS/FDA) introduced an expedited “tentative approval” process whereby ARVs from anywhere in the world, produced by any manufacturer, could be rapidly reviewed to assess quality standards and subsequently cleared for purchase under PEPFAR. As of December 20, 2007, 57 generic ARV formulations have been approved or tentatively approved by HHS/FDA under the expedited review, including eight fixed-dose combination products that contain two drugs in the same tablet or capsule, and four fixed-dose combination products that contain three drugs in the same tablet or capsule. Fourteen products are intended primarily for pediatric use. The steady increase in approvals is shown in Figure 23. As a side benefit, the process has also expedited the availability in the United States of seven generic versions of ARVs whose U.S. patent protection has expired. |
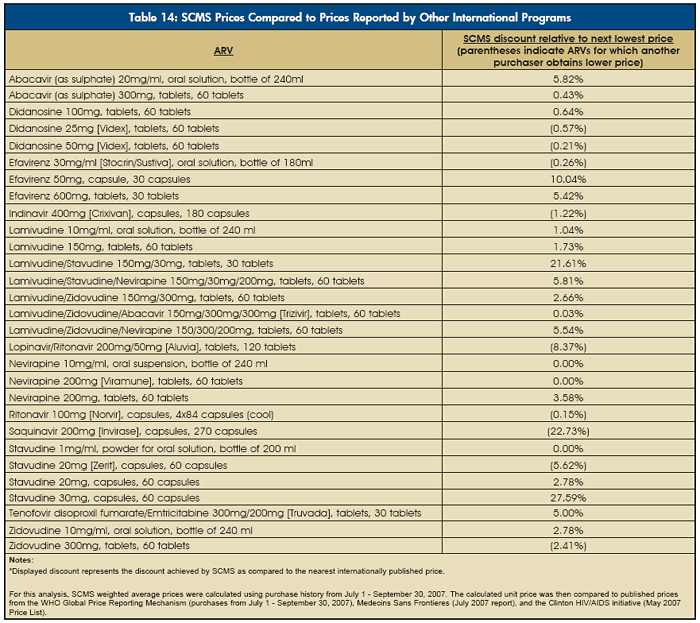
Table 14 is drawn from the work of SCMS, and shows that SCMS has been able to achieve the lowest price on nearly 70 percent of antiretroviral regimens for which internationally published prices were available (17 of 20 first-line regimens and three of nine second-line regimens).
While this level of savings is a considerable achievement, the use of generics within and between countries varies, and significant challenges remain:
- Prices for pediatric ARV formulations and second-line ARVs remain higher than first-line regimens.
- In countries where approval and registration of generic ARVs is slow, partners may still have to use innovator drugs.
- Some buyers continue to purchase branded ARVs, due to unfounded concerns that even HHS/FDA-approved generics are not as effective.
In addition, the U.S. dollar’s decline against other currencies and the increasing cost of raw materials for ARVs could slow or stop the decline of ARV prices. |
 |
Back to Report Main Page
|
| |  |  |



