 |
|
Chemistry & Toxicology
Tel (919) 541-4667
Fax (919) 541-4632 P.O. Box 12233 Mail Drop B3-10 Research Triangle Park, North Carolina 27709 Delivery Instructions Research SummaryResearch, testing and analytical resources in the areas of chemistry and toxicology are provided for the Intramural and the National Toxicology Programs (NTP) by the three sections of the Chemistry Group: Chemistry Group Resources, Chemical Metabolism and Toxicokinetics and Chemical Toxicology. Chemistry Group Resources are provided primarily in the areas of analytical, bioanalytical, and synthetic chemistry through contracts directed by Chemistry Group staff. Research is primarily in the areas of chemical metabolism, disposition, toxicokinetics and mechanisms of chemical toxicity and is achieved through both intramural and contractual resources. 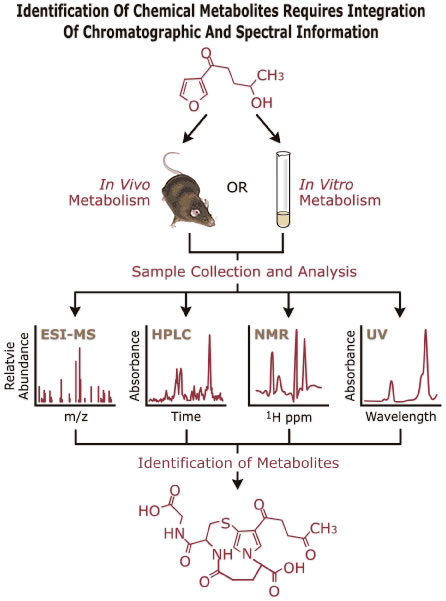 Figure 1. Metabolism of furans leads to reactive species associated with their toxicity. The GSH adduct recently identified results from formation of 3 new covalent bonds. Identification of chemical Metabolism requires integration of chromatographic and spectral information.
Testing and research are designed to address deficiencies in the literature and needs identified for the individual chemicals of interest to the NTP and the Intramural Program. Senior group staff act as consultants and collaborative investigators to provide expertise in the areas of chemical structure/activity relationships, analytical and synthetic chemistry, and the biological fate and toxicity of chemicals. Chemistry Group members also serve as an interface between extramural resources for chemistry and intramural scientists to achieve research, chemical analysis and testing not available in-house. Chemical Metabolism and ToxicokineticsResearch interests include investigations of the fate of chemicals in intact animals and mechanisms of chemical toxicity. These studies include investigations of the acute and chronic interaction of chemicals and/or their Metabolism with subcellular macromolecules to result in toxicity and/or carcinogenicity. Research also includes studies of the disposition of chemicals in intact animals including all phases of absorption, distribution, metabolism and excretion and the kinetic parameters that describe these processes. This research is conducted both in-house and through contractual resources directed by Burka. The objective of this work is directed toward quantitation of those chemical/biochemical interactions that occur as a result of acute and repeated exposure to xenobiotic chemicals. Chair of the Toxicokinetics FacultyThe Toxicokinetics Faculty’s mission is to facilitate the use of ADME (absorption, distribution, metabolism and excretion), Toxicokinetic and Modeling (ADME/TK/M) data in the design, conduct, interpretation and reporting of NTP toxicity/carcinogenicity studies. Toxicity studies may be those associated with a chronic 2-year bioassay or studies directed toward specific systems. The Faculty meets this mission by the assisting the Project Leader in incorporation of ADME/TK/M studies in the overall study design by a core group of discipline leaders and discussion of study design by the Faculty. The Faculty also provides a forum for discussion of studies, in progress or recently completed, to assist in the interpretation of those studies as well as provide background for future study design. In addition, recent advances in ADME/TK/M and general concepts may be discussed for use in study design. Major areas of research:
Current projects:
|
|

