TABLE OF CONTENTS
EXECUTIVE SUMMARY
OVERVIEW OF TREATMENT PROGRAM
METHODOLOGY
Design and Analysis
Assessment
Clinical Factors
TYPES OF TREATMENT
Brief Interventions
Behavioral Treatments
Nonbehavioral Psychotherapy
Support Networks
Program Portfolio
Pharmacotherapy
Program Portfolio
SELECTED TREATMENT TOPICS
Adolescent
Special Populations
Alcohol and Smoking
Program Portfolio
Comorbidity
Self-Help Groups
Natural Resolution
REFERENCES
APPENDICES
A: Subcommittee for Review of Treatment Portfolio
B: Experts in Treatment
C: NIAAA Program Staff
D: NIAAA Staff and Guests
TREATMENT
REPORT OF A SUBCOMMITTEE OF THE NATIONAL ADVISORY COUNCIL
ON ALCOHOL ABUSE AND ALCOHOLISM
EXECUTIVE SUMMARY
The National Institute on Alcohol Abuse and Alcoholism's (NIAAA) Subcommittee for the Review of the Extramural Research Portfolio for Treatment met on November 8-9, 1999. The charge to the Subcommittee was to examine the appropriateness of the breadth, coverage, and balance of the treatment portfolio, identifying research areas that are well covered and others which are either under-investigated or which otherwise warrant significantly increased attention. The Subcommittee was asked also to provide specific advice and guidance on the scope and direction of the Institute's extramural research activities in the treatment area.
The Subcommittee for the Review of the Extramural Research Portfolio for Treatment consisted of two co-chairs, NIAAA Advisory Council member, and an advisory group of six individuals. Three of these individuals have demonstrated expertise in alcohol-related areas, and three individuals have demonstrated expertise in non-alcohol-related areas (see Appendix A).
The review process was initiated by having experts (see Appendix B) in treatment prepare written assessments of the state of knowledge, gaps in knowledge, and research opportunities. NIAAA program staff (see Appendix C) presented the current extramural portfolio, categorized into the areas of policy, problem areas, youth and media, special populations and basic research and methodology, and training and career development. All information was shared with experts, selected NIAAA staff, and the co-chairs and advisory group before the meeting.
A summary of FY 99 treatment awards is detailed below.
|
Treatment |
Percentage of
Treatment to Total |
No. Amount
(in thousands) |
No. Amount |
|
Research Project Grants1 |
72 |
$22,703 |
12% |
14% |
|
Cooperative Agreements |
11 |
5,844 |
92% |
49% |
|
Research Centers |
2 |
3,322 |
14% |
14 % |
|
Research Careers |
13 |
1,629 |
19% |
22% |
|
Research Training |
6 |
1,290 |
10% |
20% |
|
Total |
104 |
$34,788 |
14% |
17% |
1 includes SBIR awards and reimbursable funds.
On November 8-9, 1999, experts and NIAAA program staff made abbreviated presentations of their material followed by discussion among all of the participants, including representatives from other NIH Institutes and guests (see Appendix D). After completing this process, the co-chairs and advisory group, with input from the experts, delineated the following list of research priorities, in order of importance.
PRIORITIES RESULTING FROM REVIEW OF
TREATMENT PORTFOLIO
- (1) Mechanisms of Action of Treatments. Over the last decade, there has been considerable progress in identifying effective psychosocial and pharmacological treatments for alcoholism; however, the specific theory-driven mechanisms of action are often poorly understood. For continued progress in treatment development, it is critical to develop a better understanding of the biological, psychological, behavioral, social and environmental mechanisms of these interventions and to develop more accurate models of treatment and placebo effects. Examples of such research include: elucidation of causal chains underpinning treatment effects; testing of mediators and moderators of therapeutic processes; determining the relative contributions of skills training versus motivational enhancement to treatment outcomes; and clarifying the additive and subtractive effects of complex therapies. Development of new or enhancement of existing, theory-based behavioral interventions including psychotherapies, behavior therapies, cognitive therapies, and family therapies is needed.
(2) Combinations and Sequencing of Treatments. Current alcoholism treatments generally have been found to have modest effects. By combining and or sequencing treatments, it may be possible to enhance outcomes. Research is needed on theory-based therapeutic models of combined treatment or stepped care, particularly focused on tailored interventions for special populations. Combined interventions can include behavioral, cognitive, psychotherapy, support network or pharmacological treatments. Interactive mechanisms of action should be clarified. Combined treatments will first require efficacy testing and, at a later stage, studies to determine their transportability. Such studies may test whether a combined treatment shown to be efficacious in a controlled setting can still be effective when transferred to the community.
(3) Health Seeking Patterns and Processes. It is evident that the majority of individuals with alcohol-related problems change their drinking patterns and associated problems without intensive formal treatment intervention, either through natural resolution or brief motivational interventions in health care and other settings. It is critical to develop a better theory-based understanding of these natural change efforts and health-seeking behaviors. Key areas of research include factors that promote and sustain movement towards recovery, processes of care engagement (e.g., proactive versus reactive approaches), compliance with care recommendations, retention in change processes and maintenance of change. Research also is needed on the role of community-based recovery networks (e.g., self-help and mutual-help groups) in these processes, including their role in treatment, long-term effects and cost-effectiveness models.
(4) Concurrent Disorders. Individuals with alcohol use disorders have exceptionally high rates of co-occurring disorders, including medical comorbidity, nicotine and other drug disorders, and psychiatric disorders. Research is needed to: better understand patient characteristics associated with different concurrent disorders (e.g., diagnostic status, biological status, phenotyping, developmental staging); elucidate the effects of concurrent disorders on alcoholism treatment outcomes; understand the process of change in the non-alcohol comorbid disorder; and develop specialized interventions for comorbid patients. In addition, assessment instruments and methods need to be enhanced to measure the broader range of characteristics and outcomes relevant to this population.
(5) Help Agent Behaviors. Although it is well recognized that change processes and outcomes are affected by helping agents (e.g., therapist, health care provider, clergy, family member, concerned significant other), this area of research has received little attention within the alcoholism field. Theory-driven models for assessment of provider effects are needed and could include provider attributes (characteristics, cognition, and beliefs), processes between client and provider, and training or interventions aimed at the change agent. Design innovations (e.g., learning from expert providers) and statistical strategies must be developed to address the methodological problems inherent in this line of research.
Issues that cross-cut across the priorities:
Special populations (e.g., gender, minorities, developmental stage)
Measurement
Matching
Technology transfer
Additional issues related to mechanisms of funding:
Stage 1 psychotherapy development based on basic behavioral sciences
Effectiveness studies under health services research portfolio
K-25 awards to promote biostatisticans' interest in alcoholism treatment
Awards for meetings -- biostatistics
Trans-Agency funding (DOD, VA, CSAT)
Trans-Institute funding (NIDA, NIMH)
Pharmaceutical industry
Back to Top
OVERVIEW OF TREATMENT RESEARCH AT NIAAA
(Richard Fuller, M.D. and John Allen, Ph.D.)
Historical Perspective
There was not a separate Treatment Research Branch (TRB) until the Division of Clinical and Prevention Research (DCPR) was established in 1987. Dr. Allen was appointed Chief of the Treatment Research Branch in 1987, and Dr. Fuller was appointed Director of the Division of Clinical and Prevention Research in 1988. At the time that the Treatment Research Branch was established, the NIAAA portfolio was primarily focused on behavioral treatments and was small in dollar amounts by today's standard ($4 million).
Two reports were influential in guiding the development of the TRB portfolio for the next decade. One was the "Report of the Fifth Ad Hoc Extramural Science Advisory Board on Treatment" and the other was the Institute of Medicine's report entitled "Prevention and treatment of Alcohol Problems Research Opportunities." (1989)
The first report summarized the existing knowledge and identified new areas of research. The Executive Summary of this report urged the following research goals: 1) "...giving special emphasis to the reliable classification of subtypes of alcoholics who can be matched to treatments in ways that maximize effectiveness...," 2)"Develop better measures of treatment outcome that are sensitive to changes in biological, social, and psychological functioning...," 3) "Identify and clinically evaluate new pharmacological agents, ...", 4) "Evaluate the effectiveness of...behavioral, psychosocial, and mutual help approaches...through randomized controlled trials...", 5) "Conduct carefully controlled studies to measure cost-benefits and costs offsets of alcoholism treatment," and 6) "Descriptive studies of treatment systems and service networks..."
How close has TRB come in meeting those objectives during the past decade?
(1) In his first year as Chief of TRB, Dr. Allen wrote the RFA to solicit applications of a multi-site patient-matching study. This led to Project MATCH that vigorously tested the matching hypothesis in a large sample.
(2) Better measures have been developed. One example is the DrInC, which identifies and quantifies the consequences of excessive drinking. There are many more examples. However, there is still no consensus on what outcome measures should be selected if one is limited to one or two, although percent days abstinent is commonly used.
(3) A large medications development program has been developed.
(4) A landmark randomized clinical trial of Alcoholics Anonymous was done. This trial was begun before TRB existed under the guidance of Dr. Ernestine Vanderveen, but was completed, and the results published during TRB's tenure. Project MATCH also provided insight into mutual help groups by testing Twelve Step Facilitation therapy. Project MATCH also tested two behavioral therapies: cognitive behavioral therapy and motivational interviewing. Project MATCH and the other TRB-supported clinical trials of behavioral and psychological therapies for alcoholism give us a firm understanding of efficacy of non-pharmacological therapies for alcohol dependence. What was missing until recently was a similar set of randomized studies of therapies for alcohol abusing and/or dependent adolescents. This major void has recently been corrected.
(5-6) These areas are now the responsibility of the Health Services Research Program.
The Institute of Medicine (IOM) report also advocated studies of patient-treatment matching and studies of treatment costs, benefits, and cost-offsets. The IOM report was silent regarding medication development. The report did identify a critical topic absent in the Extramural Advisory Board report, i.e., brief interventions. At that time, TRB was not supporting any research on brief interventions. Since then it has stimulated the field and several studies have been supported. The results from three of those studies have been published and confirm that brief interventions in primary medical settings in North America are effective in reducing alcohol consumption as has been found in several other countries.
The IOM report also briefly discussed smoking and posed three questions on treating smoking in alcoholics as research opportunities. The Treatment Research Branch recognizing the frequent co-occurrence of smoking and alcohol dependence and the serious impact of smoking on the health of alcoholics developed a research program addressing smoking and alcoholism.
In summary, we now have a better understanding of what treatment results can be expected from non-pharmacological treatments. An exciting medications development program now exists, the first fruit of which is the FDA approval of naltrexone to treat alcoholism. Studies done in North American now document the effectiveness of brief interventions. The efficacy of treatments for adolescents is now being addressed, as is the appropriate treatment for smoking in alcohol treatment programs. Finally, the methodology for doing treatment research has been improved, and these methods have become standard.
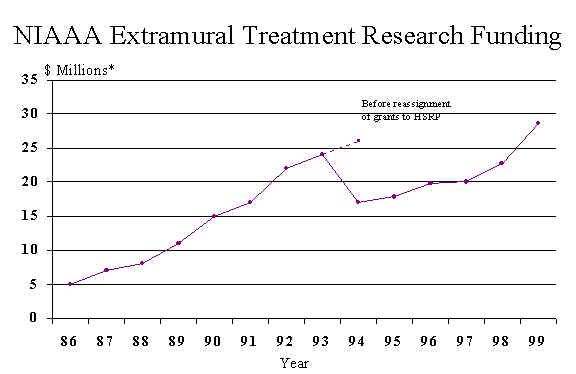
The primary activity of the Treatment Research Branch (TRB), like that of NIAAA's other extramural branches, is to support and monitor well-designed investigations related to alcohol abuse and dependence. Investigations are funded by mechanism of grants, cooperative agreements, and contracts. The graph on the next page reflects TRB financial support of projects since conception of the Branch to the present.
In addition to funding extramural investigations, the Branch performs three other important activities:
. Enhancement of Research Methodology
. Stimulation of Quality Research Proposals
. Dissemination of Research Findings
Enhancement of Research Methodology
Despite the extreme prevalence, societal cost, and suffering resultant from misuse of alcohol, until recent years research on alcoholism treatment tended to be of rather low scientific quality. Clearly this situation has now dramatically improved. Randomization, blinding, objective assessment, documentation of interventions, sophisticated data
analysis, etc., have now come to characterize studies in this field. TRB has attempted to contribute to the evolution of research methodology in such as:
- Publication of a primer addressing research design and statistical issues as they pertain to alcoholism treatment.
- Publication of a detailed handbook on alcoholism assessment instruments, including topical state-of-the-art reviews of the various domains of assessment, acceptable instruments, and presentation of psychometric and tests administration information by common data elements for each measure. (Much information from the handbook is also available on the NIAAA web site.) The assessment handbook is currently being updated and revised.
- Establishment with scientific community of guidelines for conducting investigations and reporting results on biochemical markers. These recommendations are encapsulated in an article published in Alcoholism: Clinical and Experimental Research.
- Through a Treatment Replication, Validation, Evaluation Program (TRVEP) contract explored Marlatt's taxonomy of relapse precipitants and developed methodology for studying relapse to drinking.
- Convened a workshop on methodological issues surrounding research on assessment and treatment of adolescents with alcohol problems.
- Based on two meetings with members of the alcohol treatment research community developed a core battery of instruments recommended for inclusion in all studies dealing with medication development.
- Developed of a manual reflecting on experience in Project MATCH and addressing the critical topic of compliance with treatment research outcome evaluation.
- Published manuals on the Drinker Inventory of Consequences (DrInC), a standardized measure of adverse consequences of drinking, and a computerized variant of the Timeline Follow-Back for assessing alcohol use (quantity, frequency, and peak intensity).
- Promulgated software and documentation for adaptive ("urn") randomization. This software can also assist researchers in realms of study outside of alcoholism.
Stimulation of Research Proposals
The Branch is constantly seeking to improve the number and quality of research proposals it can support. It has developed a large number of RFAs, PAs, and other solicitations identifying topics of particular concern. Equally important, staff members review and comment on concept papers submitted by candidate applicants. Additionally, members of TRB discuss research ideas and practical issues surrounding the grant application process by phone, via e-mail, as well as in public meetings.
To assure that there will be an abundance of quality proposals in the future, special efforts have been made to attract and assist promising young investigators. Strategies employed in this regard have included workshops to provide technical proposal writing skills, publication of R03 and R21 announcements for small awards to young investigators, and wide dissemination of SPIRIT, the NIAAA mentoring software to assist applicants in developing competitive proposals.
Additionally, Branch personnel consult extensively with applicants interested in refining grant proposals that have not yet earned scores in the fundable range.
Dissemination of Research Findings
As more is learned about effective treatment for alcoholism it has become incumbent on the Branch to disseminate findings to both the research community as well as to the treatment practice community.
The primary means of sharing findings with the research community are through scientific journal reports, monographs, and formal meetings with senior researchers in a topic area. Staff have published several important topical reviews of the literature and present regularly at national and international scientific meetings. Recent monographs published by TRB personnel have dealt with dynamics of relapse, adolescent treatment issues, and concurrent alcohol and nicotine dependence. A monograph in process focuses on craving. Formal meetings of late have dealt with issues such as assessment, medications, spirituality, biochemical markers, and craving.
It is more difficult to disseminate research findings to the treatment community due to its size and dispersion. Nevertheless, recent important contributions in this area have included two articles published in the Journal of Substance Abuse Treatment, a counselor-oriented periodical, and presentation of research findings at several national and international meetings, ASAM's state of science conference, and the grand rounds of several hospitals. Perhaps the most important initiative dealing with enhancement of treatment services based on research findings were meetings last year with Hawaii and New York State policy makers and treatment providers. It is expected that this latter meeting will prompt development of a demonstration of research-based treatment for alcoholism to be implemented in several community-based treatment programs.
Treatment manuals for the three interventions employed in Project MATCH as well as the Assessing Alcohol Problems, the assessment handbook noted above, have also been widely distributed throughout the treatment community in the U.S. and overseas and have received broad acceptance.
Back to Top
METHODOLOGY
RESEARCH DESIGN AND ANALYSIS ISSUES IN ALCOHOL TREATMENT
State of Knowledge (Robert L. Stout, Ph.D.)
There have been many developments in treatment research methods over recent years. The development of new therapies, often combining multiple components, has triggered a need for complex, ambitious studies. At the same time, there have been methodological developments in several areas that in principle should allow studies to be conducted more effectively and result in more accurate conclusions.
Research design and implementation: The most important issue in research design is the problem of replicability. Findings of "efficacy" do not always translate into improved performance in non-research settings ("effectiveness"), and Project MATCH found that the effects of well-controlled treatments can vary substantially from site to site. Determining the conditions under which replicability can, and cannot, be expected should be a high priority for the Institute. Since treatment research studies tend to be complex, multivariate enterprises, there is also a need for methodological research on: (1) methods for bias minimization [e.g., improved urn randomization]; (2) improved power analysis tools; (3) treatment process measurement; (4) longitudinal research design; and (5) outcome and utility estimation. There is also a need for debate on the tradeoffs between investigators' needs for data and the burden on research participants.
Analysis of Treatment Data: There is a major need to bring already-developed statistical methods into wider use in treatment research. These methods can help to solve a number of problems of relevance to treatment researchers. The Institute should explore funding mechanisms to encourage quantitative scholars to work on alcohol treatment research problems. Furthermore, conference grants or other mechanisms could be used to facilitate the application of methods such as latent growth, structural equation models, event history models, zero-inflated Poisson methods, and Generalized Estimating Equations techniques. There needs to be support for methodological research on improved methods for missing data (especially in longitudinal analyses), and better methods for multiple event analysis, applications of decision theory, and advanced quantitative modeling or simulation.
Specific recommendations:
- There should be K awards designated for quantitative researchers interested in entering alcohol treatment research. NIAAA should also sponsor conference grants to disseminate statistical methods to treatment researchers.
- It is important to delineate reasons for lack of replicability of treatment effects across sites/studies. This research should be targeted at site by treatment interactions (large variations in the effectiveness of nominally similar treatments from one site to another) and reasons for loss of effectiveness when treatments proven efficacious in research settings fail to be effective in standard clinical settings.
ASSESSMENT IN ALCOHOLISM TREATMENT
State of Knowledge (John P. Allen, Ph.D.)
Accurate and comprehensive assessment is fundamental to both treatment of and research on alcohol problems. Practitioners are primarily concerned with the clinical utility of the assessment, particularly how well it identifies the needs of a given patient and guides treatment planning. Researchers are more likely to be interested in a broader range of variables that may quantify and explain the overall impact of an intervention and may not be directly related to clinical care (Connors et al., 1994). Researchers seem to place a much higher premium on formal assessment than do many practicing clinicians, who appear to rely more heavily on interviews, review of past records, or clinical impression (Nirenberg and Maisto, 1990). Validity and availability of relevant norms for a particular assessment instrument are of considerable interest to the clinician. The clinician is also concerned about ease of administration, scoring, and interpretation of the instrument as well as cost, time, and acceptability of the measure to patients (Allen et al., 1992).
A number of characteristics have to be considered when selecting an assessment instrument.
- Purpose and clinical utility - instruments can vary according to the sequential process of assessment, i.e., screening, diagnosis, triage, treatment planning.
- Assessment timeframe - current or lifetime.
- Age or target populations - e.g., adolescents, adults, older adults, pregnant women.
- Norms available - allows test performance to be compared with that of a large, relevant group of individuals; few alcohol-related instruments have ethnicity-based norms.
- Administrative options - e.g., written, interview, computer.
- Training for administration
- Established reliability and validity
Specific recommendations:
- Development of computerized adaptive testing algorithms, capitalizing on advances in decision tree technology.
- Construction of subpopulation norms for individual assessment instruments.
CLINICAL FACTORS IN THE TREATMENT OF ALCOHOL
AND DRUG PROBLEMS
State of Knowledge (Lisa M. Najavits, Ph.D.)
Most alcohol and drug abuse treatment programs (97 - 99%) provide some form of psychotherapy or counseling, and yet the clinicians that deliver this treatment have been little studied. Substance use disorders (including alcoholism) are the only disorders in which a major psychosocial intervention, aside from psychotherapy, i.e., Alcoholics Anonymous or other non-professional self-help group, remains the dominant treatment (Najavits and Weiss, 1994).
One of the most important findings from research is that clinicians are a key factor influencing alcoholism treatment outcome and retention (how long patients stay in treatment), typically accounting for more variance in outcome than do differences between active treatments or patient baseline characteristics (Najavits and Weiss, 1994). Clinicians' professional background characteristics, such as years of experience, training, etc., do not predict their effectiveness (Najavits and Weiss, 1994). Similarly, matching patients to clinicians produced null or mixed results (Sterling, 1998) as did the recovery status of the clinician (acknowledgment of substance use disorder) (McLellan et al., 1988). Effectiveness of individual clinicians becomes equivalent with adherence to a prescribed treatment approach according to some research (Crits-Christoph, 1991).
Topics of importance without clear conclusions include clinician personality characteristics and clinicians' beliefs about treatment of alcohol use disorders. The high rate of clinician burnout (Elman and Dowd, 1997) suggests the value of research into such topics as clinician-targeted interventions and clinician selection and training.
Specific recommendations:
- Promote research on clinicians' differences in effectiveness with alcoholic patients.
- Promote research on identification of variables that explain clinicians' differences in effectiveness with alcoholic patients.
Back to Top
TYPES OF TREATMENT
BRIEF INTERVENTIONS AND THE TREATMENT
OF ALCOHOL USE DISORDERS
State of Knowledge (Michael Fleming, M.D., M.P.H.)
The goal of brief intervention and brief counseling is to help people reduce or stop their alcohol use. While abstinence may be a long-term goal, the primary goal of brief interventions is to reduce alcohol consumption to low-risk levels and patterns of use. Brief intervention counseling can decrease alcohol use for at least one year in non-dependent drinkers in primary care clinics, managed care settings, hospitals, and research settings (Wilk et al., 1997), with similar effect sizes for men and women (WHO, 1996) and for all age groups over 18, including older adults (Fleming et al., 1997).
Brief intervention can reduce health care utilization as measured by reductions in emergency room visits and hospital days (Fleming et al., 1997), reductions in hospital readmissions (Gentilello et al., 1999), and reductions in physician office visits (Israel et al., 1996). Brief interventions may reduce mortality and health care and societal costs (Fleming et al., 1999) and can also result in improved clinical laboratory tests (Israel et al., 1996) and drinking and driving (Monti et al., 1999).
Specific recommendations:
- Conduct large multisite treatment trials that follow patients for at least five years (preferably 10 years) in order to assess morbidity (e.g., accidents, medical complications), mortality, and costs.
- Increase the number of effectiveness studies, e.g., how do we implement screening, assessment, brief counseling, pharmacotherapy, and referrals to therapists in the U.S. health care system.
BEHAVIORAL TREATMENTS
State of Knowledge (Ronald M. Kadden, Ph.D.)
This review of clinical research on behavioral and cognitive-behavioral approaches to the treatment of alcoholism covers cue exposure, contingency management, community reinforcement, coping skills training, relapse prevention, and patient-treatment matching. It identifies a number of gaps in knowledge and consequent research opportunities, which are summarized here. In the area of cue exposure research, there is need to identify the most potent cues, factors that moderate cue reactivity, and effective strategies to reduce the impact of drinking cues. Regarding contingency management methodology, encouragement should be provided to increase the application of this technique to alcoholism treatment, as well as to determine the most effective schedules of reinforcement and maintenance factors that would support sobriety after imposed contingencies are discontinued. With respect to the community reinforcement approach, the most effective elements of the intervention should be identified as well as means of sustaining their effects beyond completion of treatment. In the area of coping skills training, studies are needed to assess the relative effectiveness of its various components, and to foster the use both of functional analysis to guide the selection of targets for behavior change and of mastery criteria to assess the adequacy of skills training that is provided. In addition, further work is needed to substantiate the mediating role assigned to coping skills in the cognitive-behavioral conceptualization of recovery. With respect to the relapse prevention approach, further studies are needed to improve the system for classifying relapse episodes and to identify the most effective interventions for each type of episode. With respect to patient-treatment matching, despite a recent setback in research on this approach, it may nevertheless be the most effective way to identify which clients are most likely to benefit from each of the above treatment strategies. Initial steps in this direction would be to replicate the matching strategies that have the strongest support and to determine the minimum levels of client severity that are necessary for matching to be effective. Finally, there is considerable overlap among the various treatment approaches reviewed here and it is likely that further research along the lines suggested will impact several of them. This may lead to efforts to consolidate their most effective elements into a common treatment package.
Specific recommendations:
- Investigate ways to better maintain treatment effects (e.g., booster sessions, contingency management, role of environmental factors).
- Identify critical elements of behavioral treatments.
NONBEHAVIORAL PSYCHOTHERAPY
State of Knowledge (Carlo C. DiClemente, Ph.D.)
In the past 15 to 20 years, there has been a resurgence of interest in psychotherapies and psychosocial treatments that are not behavioral in nature.
There are few controlled clinical trials of psychodynamic treatments compared with either no-treatment controls or alternative alcoholism treatments, with the majority of outcome studies conducted in the late 1960s and 1970s. In general, there is little support for the utility of psychodynamically oriented treatments for alcohol abuse or dependence (Roth and Fonagy, 1996)
Interpersonal psychotherapy encourages participants to explore interpersonal relationships and problems through self-reflection, expression of emotions, and examination of current interactions within the treatment context; this treatment is most often delivered in a group therapy setting. Although controlled research does not support a unique role for this approach, there is some evidence that this type of treatment may be more effective for certain type of alcoholics (Litt et al., 1992).
The lack of controlled studies of cognitive techniques that are separate from cognitive behavioral techniques make it difficult to evaluate the unique effectiveness of cognitive therapy.
Motivational interventions are based on principles of motivational psychology, client-centered therapy, and the stages of change model and have provided a general and practical approach for changing behaviors associated with alcohol disorders. Responsibility for changing addictive behavior is assumed to lie within the patient, and ambivalence is recognized as a natural part of this change process. Studies over the past 10 years have demonstrated that motivational interventions are moderately successful in initiating change among a variety of individuals with alcohol-related problems (Miller et al., 1995). There are data to suggest that motivation and readiness to change are the best predictors of drinking outcomes throughout the posttreatment period for outpatients (PMRG, 1998). Motivational interventions appear to be particularly useful for individuals with higher levels of trait anger (PMRG, 1998).
Although there is a growing interest in alternative medicine approaches for the treatment of alcohol-related disorders, there are data available only for acupuncture. Overall, the findings of acupuncture treatment as an aid to rehabilitation and relapse prevention among alcohol dependent individuals are mixed suggesting that more controlled research should be conducted before this technique can be recommended.
Specific recommendations:
- Further examine motivation enhancement approaches in order to evaluate active ingredients (e.g., feedback, interpersonal style, length, setting, population) and how to best combine motivational interventions with other treatments with assumed different mechanisms of action and different targets of intervention.
- Encourage longitudinal studies of engagement in treatment and different paths of entry in order to evaluate the process of recovery across treatments and to better understand commonalties and differences in the paths and processes of recovery from alcoholism and associated problems.
Back to Top
INVOLVEMENT OF SUPPORT NETWORKS IN TREATMENT
State of Knowledge (Richard Longabaugh, Ed.D.)
Long-term studies have indicated that social factors are predictive of remittance and relapse in alcoholics and untreated problem drinkers (Humphreys et al., 1997). Moreover, managed-care alterations in treatment have reduced the importance of treatment factors in accounting for the variance evident in relapse (Humphreys, 1997). These observations suggest the importance of social network members in treatment.
Social networks may be conceptually divided into two subtypes, the pre-existing everyday social network in which the alcoholic may be embedded prior, during, and following treatment, and networks developed specifically for the purpose of facilitating the alcoholic's recovery, i.e., mutual self-help groups. Research on mutual self-help groups other than Alcoholics Anonymous is largely non-existent.
Most of what is known about involvement of the alcoholic's social network in treatment is limited to spouse and family involvement, with spouse involvement being much more prominent. Analyses across studies of the effectiveness of spouse and family involvement in treatment for alcoholism have demonstrated that their inclusion enhances treatment effectiveness (McCrady, 1989). This finding is somewhat mitigated by the observations that a significant number of alcoholics do not have spouses and a smaller percentage are without proximal families. Most martial therapy has been accomplished within the context of a cognitive behavioral conceptual model (McCrady and Epstein, 1995). The addition of behavioral marital therapy for alcohol abusers enhances the functioning of the couple, thereby enhancing outcome (Epstein and McCrady, 1998). Intervening with the spouse of an alcohol abuser who is unwilling to seek treatment has been shown to be highly beneficial (Miller et al., 1999).
When the focus moves from couple's therapy to family therapy, findings become more equivocal. Even less studied, is whether other members of the network outside of the family can be brought into treatment to enhance outcomes; an exploratory study by Longabaugh et al. (1995) suggests some beneficial effects. Early involvement by network supporters helps the alcoholic stay in treatment during the initial phase when they are likely to drop out (Galanter, 1993) and also helps to provide a long-term social context that is supportive of sobriety (Humphreys et al., 1997). Outside of couples and family therapy efforts, no studies have been identified that explicitly seek to accomplish the dual goals of support for reducing alcohol abuse and relationship enhancement within the broader social network.
Specific recommendations:
- Identify the conditions under which an intervention involving members of the network will be effective in short- and long-term in enhancing outcomes. These conditions include patient characteristics, network characteristics, and nature of the network intervention itself (with and without behavioral and pharmacological interventions), independently and in combination. This research should be theory-driven, and include testing the theory operationally.
- Emphasis should be placed on theoretically driven research that uses existing models of couples/family interventions for alcohol problems and tests whether the models can be extended to involve other members of the network, in lieu of the spouse/family member, and under what set of conditions.
NIAAA PORTFOLIO ON PSYCHOSOCIAL RESEARCH
(Cherry Lowman, Ph.D.)
The Treatment Research Branch addresses the primary problem of alcohol abuse and alcoholism through identification, development, and dissemination of effective treatment interventions. Three major types of intervention are developed or evaluated in treatment research supported by TRB: psychosocial interventions (for example, Cognitive Behavioral Therapy); pharmacological interventions (for example, naltrexone); and combined psychosocial-pharmacological intervention. This section of the Treatment Research Program Report presents information on psychosocial treatment interventions.
A number of advances have been made over the past decade in psychosocial treatment research on alcohol abuse and alcoholism. These include:
Continued development of the psychosocial research program. TRB has contributed to this program development by issuing Program Announcements and Requests for Applications, giving technical assistance, organizing workshops and symposia, and publishing monographs. For details on the portfolio, see the overview below.
Initiation in 1998 and intent to continue development of the Adolescent Treatment Research Program (see Category D below), a research grant program.
Initiation in 1989 and completion in 1997 of a cooperative agreement to conduct a multisite randomized clinical trial to assess the effectiveness of treatment-patient matching strategies for three types of psychosocial intervention(motivational enhancement treatment, cognitive behavioral therapy, and Alcoholics Anonymous facilitation therapy). P>Initiation of the Treatment Research Validation and Extension Program (TRVEP) in 1991, a program of contracts to assess the validity and reliability of widely used methodologies in alcohol treatment research (see discussions under Screening and under Relapse below).
Continued development of the treatment services research program (1990 - 1994), a research grant program with foci on treatment services and financing, treatment effectiveness, and primary care intervention studies; transferred to the newly formed Health Services Research Program in 1994.
Overview of FY 1999 Psychosocial Program
In FY 1999, the NIAAA Treatment Research Program funded 38 active extramural research project grants (30 R01s, 1 R37, 3 R29s, 2 R21s, 2 R03s), four Small Business Innovative Research (SBIR) grants (2 R43s, 2 R44s), five K awards (1 K01, 1 K02, 1 K05, 2 K23s), and four small contracts, making a total of 51 funded projects at a total cost of $13,160,228. These projects are described below under five general areas of psychosocial treatment research:
|
Topic |
Total Costs |
Description |
| (A) Controlled clinical trials |
$1,954,390 |
Pages 21 - 23 |
| (B) Relapse |
$1,970,639 |
Pages 23 - 29 |
|
(C) Family transmission and intervention |
$2,787,554 |
Pages 29 - 32 |
|
(D) Adolescent treatment |
$4,228,770 |
Pages 32 - 36 |
|
(E) Screening and brief intervention |
$2,218,875 |
Pages 36 - 39 |
Discussion under each of these headings provides background, brief descriptions of the projects, and results when available.
Back to Top
A. Controlled Clinical Trials
The keystone of alcoholism treatment research is the use of randomized controlled clinical trials to systematically assess the impact of behavioral, psychosocial and pharmacological interventions on behavioral outcomes. Such treatments are usually delivered in specialized medical or psychotherapeutic treatment settings, to patients who have self-referred or have been referred or recruited for treatment and who agree to participate in the study, by providers who have received professional training. The typical clinical trial employs a randomized controlled design in which treatment duration is 12 weeks and treatment outcomes at 12 months from the experimental condition are contrasted with 12-month outcomes from treatment as usual. They also provide an opportunity for extensive patient assessment along multiple concurrent dimensions postulated to predict outcomes.
Controlled Clinical Trials Funded in FY 1999
The 23 clinical trials discussed in the psychosocial section are of two types: research project grants independently initiated by the extramural research community (n=13) and research project grants submitted in response to a Request for Applications (n=10). Three clinical trials are discussed in this controlled clinical trials category; another two are discussed under "Relapse" (depression and social phobia); two under "Familial interventions"; 12 under "Adolescent treatment"; and four under "Screening and brief interventions."
Following are descriptions and findings, when available, of the research grants assigned to this category.
Alcoholics Anonymous. Dr. Kimberly Walitzer (AA11529) assesses the effectiveness of two strategies designed to facilitate involvement in Alcoholics Anonymous in the context of a 12-session outpatient alcoholism treatment program. Clients are assigned to one of three conditions: a minimal approach, a directive-confrontive approach, and a motivational approach.
Aftercare. Dr. James R. McCay (AA10341) compares outcomes over two years of follow-up from three types of aftercare intervention: (1) minimal aftercare (referral to self-help groups and brief telephone case management); (2) standard disease-model aftercare counseling (two group therapy sessions a week), or (3) one individual cognitive-behavioral relapse-prevention session and one group therapy session per week. Relative cost-effectiveness of the three different approaches will also be determined. Preliminary findings from this project, based on a partial sample, indicate that short-term outcome may be related to whether or not patients reside in structured abstinent living situations such as half-way houses or sober houses. Those who live in structured situations appear to enjoy better outcomes and may not require the most intensive intervention. Initial cost effectiveness evaluations suggest that costs of telephone case management such as locating subjects may be no less expensive than standard aftercare treatment. Confirmation of these findings requires comprehensive data analyses of the full study sample and all long term follow-ups.
Technology transfer. This project, under the direction of Dr. Jon Morgenstern (AA10268), represents the first systematic, controlled effort to transfer a treatment determined to be efficacious under experimental conditions to a real-world treatment practice. The two broad research questions addressed by the project are: (1) Can "front-line" treatment counselors be trained to deliver a standardized Cognitive Behavioral Coping Skills Treatment in real-world practice? and (2) Can research-based alcohol treatments achieve more effective outcomes than practice as usual in real-world settings? The project has successfully trained a cadre of counselors, using cognitive behavioral therapy (CBT) manuals and professional trainers, and patients have been assigned to one of three treatment conditions: manualized CBT (high standardization), Real World CBT (low standardization), and traditional treatment. Two studies were completed during FY1999.
Study 1. Feasibility of knowledge transfer. The feasibility of training front-line counselors to deliver CBT using treatment manuals was examined in order to determine if this technology transfer strategy might aid in dissemination efforts. The sample comprised 29 substance abuse counselors drawn from two community-based treatment programs. CBT counselors reported high levels of satisfaction with the training, intention to use CBT interventions and confidence in their ability to do so. Supervisor and rater ratings indicated that 90% of counselors were judged as having attained at least adequate levels of CBT skillfulness. This study demonstrates the feasibility and promise of using psychotherapy technology tools - manuals, videotape monitoring, and supervision - as means of disseminating empirical knowledge to the substance abuse practice community. Results of this study have been submitted for publication.
Study 2.CBT treatment effectiveness as delivered by front line counselors. Preliminary analysis of within treatment outcomes of a randomized trial testing the effectiveness of CBT was conducted in a sample of 134 outpatients at 3 months follow-up. Participants were randomly assigned to three conditions: high standardization CBT, low standardization CBT, and a treatment-as-usual condition. In addition, the study took place in two settings: a) individual CBT therapy was added to intensive outpatient treatment as usual (n=90), and b) individual CBT therapy was offered as a stand-alone outpatient treatment (n=44). Using hierarchical linear modeling, neither treatment condition nor a condition-by-setting interaction was significant. Thus, participants appeared to experience equivalent good within-treatment outcomes across treatment conditions. Future analyses at 15-months follow-up may, of course, yield very different findings.
Computerized treatment interventions. Three Small Business Innovative Research (SBIR) Program grants are devoted to development of computerized treatment interventions which may be used as experimental or control conditions in future clinical trials and therefore have been included in this section. Two of the SBIR grants are in Phase I, development of the prototype.
Dr. Reid Hester (R43 AA11703) will develop and evaluate an interactive computer software program that provides a brief motivational intervention for individuals who range from at-risk drinkers to alcohol dependent individuals. The program will utilize the "FRAMES" elements common to effective brief motivational interventions.
The interactive voice response (IVR) telephone system is being utilized by James C. Mundt (AA12366) to develop a computerized aftercare IVR system for self-monitoring of daily drinking with automatic feedback mechanisms for posttreatment relapse intervention and prevention.
Dr. Emil Chiauzzi (AA11694) has developed a computerized program to be offered through substance abuse and alcohol treatment programs to prevent relapse in early recovery. Dr. Chiauzzi's approach utilizes an important new technology for teaching complex skills with computers. This project is in Phase II, field-testing.
Back to Top
B. Relapse
Despite the discomfort and danger of acute withdrawal from alcohol use, most alcoholics initially achieve a period of sobriety with or without formal treatment. Unfortunately, many return to drinking within a short period of time. Alcoholism has therefore come to be conceptualized as a chronic relapsing disorder similar to other chronic health conditions. Understanding the nature of relapse is fundamental to the identification and development of interventions that will contribute to positive long-term outcomes.
There are several types of studies that facilitate research on the addiction recovery process. These include both treatment outcome studies and natural history studies both of which assess the impact of multiple domains of variables (the hypothesized relapse predictors) on long-term outcomes. These also include studies that focus on elucidating or experimentally manipulating specific phenomena posited to influence long-term drinking outcomes such as psychiatric comorbidity, craving, and neurocognitive characteristics. Finally, secondary analyses of existing databases may enable testing of relapse hypotheses and modeling of recovery processes. Research project grants in TRB's psychosocial portfolio relevant to relapse prediction are discussed below under one of these six categories.
Relapse-Related Projects Funded in FY1999
Treatment Outcome Studies
Although most treatment research is concerned with treatment outcomes, the term in this context refers to clinical studies of long-term outcomes (usually 12 months or more) of patients treated in "real world" rather than experimental settings. Since it is difficult to study variability in a specific natural treatment process (the "black box"), the emphasis in studies of this type usually is on identification of patient characteristics that predict long-term behavioral change.
Replication and Extension Research. The three contracts (N01AA1006, N01AA1007, N01AA10011) under TRB's Treatment Research Validation and Extension Program (TRVEP), awarded to Brown University, the Research Institute on Addictions, and the University of New Mexico, were treatment outcome studies in TRVEP's first program of research, the Relapse Replication and Extension Program (RREP). The purpose of this study was threefold: to replicate and assess the predictive validity of Marlatt's taxonomy of relapse precipitants (i.e., high risk situations); to contrast alternative taxonomic conceptualizations such as those represented by the Inventory of Drinking Situations and the Reasons for Drinking Questionnaire; and to assess the predictive validity of other putative predictors of relapse in addition to Marlatt's taxonomy of high risk situations. The focus of the three treatment outcome studies was prediction of behavioral outcomes and posttreatment functioning from an array of domains hypothesized to predict relapse, including Marlatt's high risk situations, negative affect, testing personal control, coping behavior, outcome expectancies, self-efficacy, and craving.
The primary conclusions of this study were: (1) although the methodology utilized to collect data for the taxonomic method of relapse classification clearly serves several important clinical functions, predictive validity and cross-site reliability is insufficient to justify its use for scientific research in its present form; (2) the Reasons for Drinking questionnaire, which extends Marlatt's original method by replicating the questions in self-report Likert scale format, did appear in the one site tested to have moderate predictive validity; (3) both effectiveness of coping behavior and belief in the disease model of alcoholism were strong predictors of long term outcomes; (4) adverse events, stressors, self-efficacy and outcome expectancy did not predict most long-term outcomes examined in the RREP studies. These latter variables, however, may represent relatively transient conditions, ones proximal to relapse, that would not be consistently captured in the bimonthly assessments conducted in these studies. The results of this relapse research program, including theoretical and methodological articles pertinent to relapse research, were published in a December 1996 Supplement to the journal Addiction (Lowman et al.).
The three RREP contracts have been extended to complete integration of the three site-specific individual databases into a single multisite database in order to replicate the original analyses on a larger sample. In addition, the three sites will complete analyses of data on craving and treatment outcomes.
Meta-Analysis of Treatment Outcome Research (Finney, AA08689). This project extends a previously developed database to provide a meta-analytic review of outcome and effect size in alcoholism treatment research published in English between 1970 and 1998. With the data from this project, it will be possible to describe the nature of alcoholism treatment research over the 29-year period and the extent to which it reflects various components of methodological quality. It will also be possible to determine whether the methodological quality of alcoholism treatment research has improved over time.
Longitudinal Natural History Studies
These types of studies differ from treatment outcome studies in that they do not usually include a treatment component. Natural history studies examine over time the unfolding of the in vivo course of alcohol use and abuse in a specific population. Such studies make major contributions to knowledge of alcohol use disorders by illuminating etiological processes, familial transmission, mechanisms underlying post-treatment functioning, factors contributing to spontaneous recovery, and factors contributing to the emergence and remission of alcohol-related problems in specific populations. There are three natural history studies in TRB's psychosocial portfolio. Two are discussed here; Robert A. Zucker's longitudinal study of children of alcoholics is discussed in the section below on familial transmission.
Alcohol Use Among the Elderly. Dr. Rudolf H. Moos (AA06699) is conducting a longitudinal study on problem drinking and life stress among older adults. This project currently has three components: (1) a ten-year follow-up, now completed, of a community sample of late-life problem drinkers and non-problem drinkers and of spouses included in earlier follow-ups, now completed; (2) a 4 - 5 year follow-up of a nationwide sample of more than 5,000 treated late-life alcoholic patients; and (3) analysis of Medicare data to calculate readmission and mortality rates, and to identify predictors of readmission and mortality in late-life VA substance abuse patients. Results from the community study show that late-onset problem drinkers may be more reactive to health problems and people's reactions to their drinking than early-onset problem drinkers who may require more structured health care to successfully recover from alcohol problems. Older problem drinkers have relatively flexible coping styles, which suggests to the PI that their adaptation can be enhanced either by improving their social resources or by reducing their reliance on avoidance coping. "The findings suggest that the best treatments for alcohol use disorders are those that help patients manage their life circumstances, that low intensity treatment for longer intervals may be most helpful, that most alcoholic patients can be treated in outpatient settings, and that a supportive, well organized treatment regimen may be especially important for older patients." For a full report, see Chapter 15 In Gomberg, E.S., Hegedus, A.M. and Zucker, R.A. (Eds.), 1998, Alcohol Problems and Aging, pp. 261-279, NIAAA.
Recovery and Help-Seeking Processes in Problem Drinkers. Most problem drinkers do not seek help from professional treatments or Alcoholics Anonymous, yet many resolve their problems without it. In order to understand the natural resolution process and to develop interventions to reach problem drinkers who do not seek help, Dr. Jalie A. Tucker (AA08972) has designed a project to distinguish factors that influence help-seeking for alcohol problems from factors that influence problem resolution. Studies 1 and 2, completed in the first 5-year period, recruited problem drinkers from the community using media advertisements.
Study 1 involved a retrospective assessment of events during a 4-year period that surrounds the initiation of stable abstinence that had lasted 2 years or more. Across help-seeking groups, resolution was associated with decreases in negative events from the pre- through the post-resolution period and with increases in positive events during the first year of maintenance. Interventions, especially treatment, facilitated positive changes after resolution onset, but these help-seeking differences overlapped the more general pattern of events found to surround resolutions achieved through any one of several pathways. Tucker et al. (Experimental & Clinical Psychopharmacology, 3, 195-204, 1995) report a pilot version of Study 1.
Study 2, still under analysis, was a prospective follow-up of untreated problem drinkers who had recently initiated abstinence or moderation drinking and was guided by behavioral economic theory. In the second project period, two additional studies are being conducted. Studies 3 and 4 were initiated in the current five-year project period.
Study 3 is a prospective replication of Study 1 and involves a two-year follow-up of problem drinkers who recently initiated a resolution attempt with or without interventions.
Study 4 is a one-year follow-up of recently resolved, untreated problem drinkers and involves intensive study of the natural resolution process through daily participant self-monitoring by means of a computerized interactive telephone system. Studies 3 and 4 will yield detailed information about the dynamics of resolution and the role of contextual variables and interventions promoting it.
Psychiatric Comorbidity
Among persons with alcohol use disorders in the general population, lifetime prevalence for any psychiatric disorder may be as high as 44 percent. Most psychiatric disorders have been found to precede alcohol use disorders in onset, with the exception of mood disorders, which frequently appear to develop after onset of alcoholism. Research indicates that psychiatric comorbidity predicts poorer outcomes in alcoholic patients and has indicated that treatment for both disorders should improve outcomes for dually diagnosed patients. Treatment interventions, however, need to be tailored to the specific psychiatric condition. This is still an underdeveloped area of alcohol treatment research. There are only three independently initiated psychosocial research projects on this topic: one is a treatment outcome study and two are clinical trials.
Treatment Outcome Study: Post-Traumatic Stress Syndrome (PTSD). Following are preliminary findings from Dr. Pamela J. Brown's R29 research project (AA10011) to study PTSD in a clinical sample of substance abuse patients: (1) PTSD and traumatic events are common in substance abuse patients (94% had experienced an average of 3.5 traumatic events in their lifetime and over half met diagnostic criteria for PTSD); (2) PTSD patients relapsed more quickly than non-PTSD patients (at 35 versus 69 days, respectively); (3) improved PTSD was associated with better substance use disorder (SUD) outcomes but sustained remission from SUDs was not associated with improved PTSD; (4) daily drinking records indicate that PTSD patients tend to have a more volatile substance use course, cycling frequently between "illness" and "wellness" states; (5) for female patients, number of PTSD re-experiencing symptoms is a significant predictor of substance use outcomes, but no baseline measure of substance use predicts PTSD outcomes (Brown, P.J & R.L. Stout, Alcoholism Treatment Quarterly, in press); and (6) PTSD patients had a greater number of hospital overnights for addiction treatment compared to non-PTSD patients (Brown, P.J., Stout, R.L. & Mueller, T., 1999, Psychology of Addictive Behaviors, 13, 115-122.).
Clinical Trials: Depression. This clinical trial (R.A. Brown, AA10958) tests the hypothesis that addition of cognitive behavioral treatment (CBT) for depressive symptoms will improve treatment outcomes among comorbid alcoholics in a standard, partial-hospital alcohol treatment program. The control condition for the CBT-depression condition is adjunctive relaxation training.
Clinical Trials: Social phobia. In this project (Randall, AA09751), the objective is to determine whether or not concurrent treatment of psychiatric co-morbidity leads to improved treatment outcomes for alcoholics. In the experimental condition, a subgroup of alcoholic outpatients who suffer from social phobia receive concurrent manualized cognitive behavioral treatment (CBT) for their alcoholism and for their social phobia. In the comparison group, only the alcoholism is treated with CBT. All data have been collected (baseline, 3, 6, 9, 12 months) for 110 outpatients with alcoholism and social phobia, 72 of whom are men, 38 of whom are women. The database has been prepared for analysis and findings will be available in 2000.
Neurocognitive Predictors
More than half of patients entering treatment for substance use disorders evidence mild to severe neuropsychological impairment (e.g., in executive control, memory, new learning, and visual/perceptive skills). It has been assumed that neuropsychological impairments such as these would be responsible for poor treatment outcomes either by leading providers to interpret impaired individuals' dysfunction as noncompliance behavior or by the limited ability of impaired patients to acquire and integrate new information with previous learning, or to plan and maintain behavioral alternatives to substance use.
Impaired Executive Function and Treatment Outcomes. Contrary to these expectations, Drs. Jon Morgenstern and Marsh E. Bates, in a recently completed study partially funded by TRB grants (AA10268 and AA11594, respectively), found that impaired executive function had no direct effect on treatment outcome among patients admitted to two 12-step treatment programs. The authors did find, however, that impairment significantly moderates the relationship between intermediate change processes and outcome. Change processes considered therapeutically positive (e.g., increasing self-efficacy, maintaining high levels of motivation to abstain and facilitating connection to AA) were strongly related to treatment outcomes in unimpaired individuals but were only weakly related in impaired individuals. These findings suggest to the authors that (1) outcomes for unimpaired and impaired patients may be mediated by different factors that sustain two different pathways to the same outcome; impaired patients may have reduced risk of relapse through an as yet unidentified protective factor; and (2) "inclusion of cognitively impaired individuals in substance use clinical trials might obscure treatment effects because processes targeted by treatments do not appear to operate in the same fashion for impaired and unimpaired individuals..." (Journal of Studies on Alcohol, Volume 60, 1999).
Alcohol, Aging, and Dementia: Neuropsychological Effects (Saxton, R29 AA10981). This project is a five-year cross-sectional and prospective study to validate the taxonomy of Alcohol Dementia, delineate cognitive profiles associated with the disorder, differentiate the disorder from Alzheimer's disease, and to investigate the validity, specificity, etiology and natural history of the taxonomic category of Alcoholic Dementia and the long term implication of abstinence on the disease process.
Craving
Although craving, as currently measured, has failed as a consistent and strong predictor of relapse in psychosocial research, interest in the construct has survived in clinical as well as basic science circles and has recently been revived with the advent of research in medications development (Monti et al., in press). There are two recently funded projects in the TRB psychosocial portfolio that examines, in controlled laboratory experiments, mediators of cue reactivity.
Information-Processing Theory. Dr. Tibor Palfai in anR29 project (AA11534) utilizes information-processing theory as the framework for research design. The project investigates, through an experimental laboratory study, the effects of cognitive urge control strategies (i.e., Distraction, Suppression, Acceptance, or no instruction) on subsequent processing of alcohol-related information such as anticipatory responses to alcohol cues. In addition to this primary objective, the project will examine mechanisms by which efforts to control urges to drink may increase subsequent reactivity to cues. A third objective is to examine conditions under which specific urge coping strategies are most effective.
Extinction Performance. Dr. Paul R. Stasiewicz (AA12033) will conduct an initial test in a human clinical sample of an animal model of extinction performance. Specifically, the project will examine the effect of context on measures of craving and alcohol cue reactivity in treatment-seeking alcoholics. The first four of six groups will be compared to test if post-extinction context affects renewal of reactivity to alcohol beverage cues. Group 5 will be utilized to test whether or not the presence of an extinction cue (i.e., one that was paired with the extinction context) can reduce craving and alcohol cue reactivity during the post-test. Group 6 will examine whether or not a procedure designed to increase saliency of the extinction cue can further enhance this effect.
Secondary Analyses
Relapse onset and its prevention have been the major focus of relapse interventions whereas antecedents of relapse termination and mechanisms of abstinence maintenance, although they may contribute equally important insights to treatment research, have been largely neglected.
Relapse Onset, Relapse Termination and Abstinence Maintenance. Dr. William H. Zywiak, in an exploratory study (R21 AA12302), will utilize the Project MATCH database to conduct secondary analyses on these three aspects of the recovery process. A primary aim of the project will be to determine the interrelationships of relapse onset, relapse termination and abstinence maintenance characteristics and the relationships of these three domains to temporal and alcohol-related parameters of relapse and general treatment outcomes.
Back to Top
C. Familial Transmission and Intervention
Family studies in TRB's psychosocial portfolio focus on families in which at least one member is alcoholic, usually a spouse or parent as in the projects discussed below. Families affected by alcoholism are as heterogeneous as alcoholics are in general and the risks to spouses and children of alcoholics will vary with the substance use status of the alcoholic (remission or relapse), density of family history of substance abuse, and psychiatric comorbidity of substance abusing or other family members. Some alcoholic families may be buffered from the negative effects of alcoholism by social environments and cultural attitudes and rituals that serve as protective factors. There are three types of family studies in the TRB portfolio: (1) transmission studies to examine factors that influence recurrence of alcohol/drug problems and associated comorbidities (for example, conduct disorder and depression) within families across generations, (2) a clinical trial that assesses the effectiveness of couples therapy on alcoholics' treatment outcomes and on marital quality, and (3) a treatment outcome study.
Familial Transmission and Intervention Projects Funded in FY 1999
Transmission Studies
There are five transmission studies in the TRB portfolio: three natural history, youth and development studies and two studies that conduct secondary analyses of major twin databases.
Natural History Studies: Alcoholic Families and their Children
Risk and Coping in Children of Alcoholics (Zucker, AA07065). This project is a longitudinal developmental study of children of alcoholics from the age of three years on and of characteristics of their parents. Both alcoholic and control families were recruited from a community sample of homes in which the father was alcoholic at baseline. By the time these children mature and the data are analyzed, there should be findings of major significance on the etiology of alcoholism and the familial mechanisms that underlie its emergence. Study findings are likely to suggest new strategies for early preventive intervention. Even now, as the children enter early adolescence, the project has demonstrated that the already known markers of adolescence and later life that presage greater prevalence of alcohol use disorders are showing up first in the highest risk families. Thus, 82 percent of the 9 to 11 year old boys who have had their first alcoholic drink and 75 percent of 12-14 year old boys (based on a partial sample) who are already drinking and have had their first drunkenness experience are children of alcoholics. These differences in early drinking experience of children in alcoholic homes parallel earlier study findings that by ages 3 to 5, children of alcoholics were better at identifying and labeling alcoholic beverages (that is, they already had a better idea of what this substance is, as a drug).
The study continues to document that behavioral markers of later adverse outcomes, and possibly also of higher risk for alcohol use disorder, are not uniformly distributed among children of alcoholic parents. Although the strong association of alcoholism and antisocial personality disorder in adulthood has been known for some time, the manner in which this has impact on family functioning, and even the way in which this set of adult characteristics can serve as an indicator of family risk has been less well understood. This project has found evidence that families with antisocial fathers have more children who manifest behavioral disturbance of both mood and aggressiveness/undercontrol, even at ages 3 to 8, than do other COAs in the sample.
At this point it is not possible to determine how much the observed parallelism between life damage in the antisocial alcoholic parents and poor behavioral outcome in the children is regulated by genetic factors, environmental factors, or some combination of the two, or more importantly, to identify what the specific gene-environment interactions are that are involved in these transactions. A small offshoot study from the project has indicated that serotonergic system differences may regulate some of these effects; a peripheral measure of this system, whole blood serotonin, was lower in the children of alcoholics who were higher in problem behavior. Other recent work has shown that the family rearing structure is different in the high risk, antisocial alcoholic families, and the emergence of risky externalizing behavior develops at a different rate in these family environments than it does in the lower risk families. Plans continue to call for the examination of a number of interactive and mediation hypotheses as the long-term study continues.
Biological Risk Factors in Relatives of Alcoholic Women (Hill, AA08082). This is a five-year developmental study of a cohort of high-risk male and female children of women with a severe form of alcoholism (i.e., early age of onset and high familial density). The objectives of the study are to model (1) age of onset for regular drinking and (2) severity of psychopathology in the high-risk cohort as compared with a control group of low-risk offspring. This research has already demonstrated that reduction in P300 occurs in offspring of alcoholic women as it does in children of alcoholic men and affects girls as well as boys. The investigators also found that psychopathology among the children of these alcoholic women was five to six times higher than control children and tended to increase as they matured but this trend was not apparent when the custodial father was not alcoholic.
Familial Transmission of Alcohol and Related Problems (Johnson, AA11699). In a longitudinal study of adolescents, followed from ages 12 to 31, a broad range of variables and 13 years of data are utilized to study the relationship of developmental outcomes to characteristics of alcoholic families as compared with nonalcoholic controls. The PI will: examine the predictive value of subtyping alcoholic families (based on timing and onset of alcoholism in parents); assess developmental outcomes of familial risk (based on psychiatric comorbidity and transgenerational drinking histories, including those of grandparents) as compared with risk-free families; and will evaluate within group variations in resilience and vulnerability among 25-31 year olds as influenced by the relationship between drinking outcomes and potential mediating and moderating variables.
Twin Studies
Genetics of Alcoholism and Depression (Bierut, AA00231). This project, funded by a K23 award, utilizes secondary analyses of Australian twin and COGA databases to examine the genetics of alcoholism and depression. In completed analyses of Andrew Heath's Australian twin study, Dr. Bierut found that shared genetic effects more fully explained familial aggregation of depression for both men and women than did a shared family environment. Her COGA analyses will focus on the genetic overlap between alcoholism and habitual smoking. In addition, the PI is recruiting subjects for a prospective study to further elucidate the relationship between alcohol dependence and major depressive disorder in a sample of siblings of alcoholics.
Familial transmission of Antisociality and Alcoholism (Slutske, AA00264). Funded by a K01 award, this project analyzes three twin databases (Andrew Heath's Australian database, the Vietnam Era Twin Registry, and the Dutch twin registry) with a focus on identification of mechanisms responsible for familial transmission of antisociality and alcoholism. In addition, Dr. Slutske is collaborating with Australian survey researchers to recruit an additional generation to Heath's twin database, the young adult offspring (1964-1976) of previously interviewed Australian twins and spouses. The enhanced database will have a total of 873 parents and 1,913 offspring.
Treatment Intervention Studies
There are three family/significant other intervention studies.
Alcoholic Couples -- Two Models of Heterogeneity (McCrady, AA07070). This project is a randomized controlled clinical trial that assesses in a clinical sample of alcoholic women the efficacy of alcohol-related Behavioral Couples Therapy with Relapse Prevention, adapted for women, as compared to an individually-oriented women's treatment. A secondary aim of this project is to assess the contribution to treatment outcome of individual psychopathology, partner psychopathology, quality of the intimate relationship, and functioning of other parts of the woman's social network.
Domestic Violence among Male Alcoholics in Treatment (O'Farrell, AA10796). This treatment outcome study has as its primary objective replication and extension of earlier research on the impact of Behavioral Marital Therapy for alcoholic men and their wives on domestic violence. This extension will determine whether or not standard individual alcoholism treatment for alcoholic men will have comparable effects among remitted alcoholics; that is, domestic violence will return to the low levels characteristic of nonalcoholic couples. Dr. O'Farrell will further explore these subjects through analyses conducted under a K02 award (AA00234).
Intervention with Significant Others (Miller, AA09774). The long-term objective of this study is to develop effective methods for counseling concerned significant others (CSO's) that will improve outcomes for them and for the problem drinkers about whom they are concerned. Toward this objective, three alternative strategies for counseling CSO's are being compared in a randomized trial: (a) Alanon-oriented approach, (b) Johnson Institute intervention, and (c) community reinforcement approach (CRA). The results of this trial have been striking. CSOs who received the community reinforcement and family training intervention succeeded, in 67% of cases, in engaging their identified patient (IP) in treatment. CSOs assigned at random to the Al-Anon facilitation condition engaged their problem drinker in treatment in only 13% of cases, and for the Johnson Institute intervention condition the comparable figure was 23%. In all three conditions, CSOs at 3-month follow-up showed significant improvements in psychosocial and emotional functioning. This is one of the scientific activities funded by Dr. Miller's K05 award (AA00133).
Back to Top
D. Adolescent Treatment
Alcohol is often abused by adolescents with consumption and risk for alcohol-related consequences increasing with each grade in school. By the 12th grade, as many as one-third of high school students have reported intoxication at least once during the past 30 days. National high school survey data (grades 8-12) show that some indicators of excessive drinking have increased over recent years (e.g., the prevalence of heavy drinking--five or more drinks during the past two weeks). Consequences of adolescent alcohol abuse may include: fatal injuries such as traffic deaths, suicides, homicides; impaired social and academic functioning; psychiatric problems such as antisocial, mood, and anxiety disorders; and high-risk problem behaviors such as polydrug use and the serious risks for increased problem behaviors and morbidity that it entails.
According to Martin and Winters (1998), as many as 13% percent of high school students and up to 30 percent of adolescents who are regular drinkers drawn from clinical and community sources may be "diagnostic orphans;" that is, they report no symptom of alcohol abuse and only one or two symptoms of alcohol dependence without satisfying full diagnostic criteria. This subgroup of adolescents typically reports symptoms such as tolerance, drinking more and longer than intended, and unsuccessful attempts or persistent desire to quit or cut down. These and other data indicate that the adolescent alcohol problem is a mounting and serious one.
NIAAA and the Center for Substance Abuse Treatment (CSAT/SAMHSA) collaborated in FY1998 in issuing a Request for Applications for research on Treatment for Adolescent Alcohol Abuse and Alcoholism. NIAAA earmarked up to $2 million and CSAT up to $1.9 million, annually for five years, to fund this program. The RFA was published in the NIH Guide on March 13, 1998; 25 applications were submitted by June 12 and were reviewed in August. As of October 1, 1999, NIAAA and CSAT had jointly funded a total of 14 new grants related to adolescent treatment research for alcohol problems. Of these, ten are clinical studies and four are questionnaire development projects. Overall, the quality and scope of these projects provide opportunities to identify effective approaches to treatment for adolescent alcohol problems (family therapy, cognitive behavioral therapy, brief motivational enhancement therapy, and self-guided change) in diverse groups of adolescents (high school students, juvenile delinquents and street youth, Hispanic, African American, and Native American youth). Only the clinical studies are described here under "Adolescent Treatment;" the screening studies are described below under "Screening and Brief Intervention."
Awarded Prior to FY 1998
Until NIAAA and CSAT issued the joint RFA on adolescent treatment, there were only two grants in this category. One is a clinical trial, conducted by Dr. Eric Wagner (AA10246), to assess the effectiveness of a high school-based, early intervention Student Assistance Program (SAP). A SAP intervention (group counseling) is compared with assessment/referral-only. Dr. Deborah Deas, under a K23 award (AA00247), will conduct an RCT to evaluate the effectiveness, in treatment-seeking adolescents who abuse alcohol, of two standard adults treatments--cognitive behavioral therapy and twelve-step facilitation therapy.
Awarded in FY 1998 and FY 1999
Ten clinical trials have been funded under the RFA on adolescent treatment: seven (1 R21 and 6 R01s) were funded in FY1998 and three (all R01s) were funded in FY1999. Because these research project grants have just been funded, there are currently no findings to report. A brief description of the projects is provided in order to illustrate the scope and diversity of the program.
Family Therapy
Four of the ten clinical trials assess the effectiveness of family therapy in treating adolescent alcohol use disorders. These are: (1) a comparison of the effectiveness of two primary therapies for adolescent alcohol abuse or dependence--Transitional Family Therapy which integrates nuclear family with multigenerational issues and Adolescent Group Therapy, a standardized form of community-based treatment-as-usual (Stanton, AA12178); (2) a comparison of the effectiveness of three interventions for alcohol problems among runaway youth recruited through a shelter for runaways--an intensive home-based family intervention, a traditional office-based family intervention, and "practice as usual" (Slesnick, AA12173); (3) assessment of the relative effectiveness and cost effectiveness of multisystem family therapy, combined with features of the Community Reinforcement Approach, as compared to community services in treatment outcomes of adolescent juvenile offenders with serious alcohol and other clinical problems, recruited through the criminal justice system (Henggeler, AA12202); and (4) comparison in a randomized controlled trial of the relative efficacy of four intervention approaches for youth problem drinking--functional family therapy, individual cognitive-behavioral therapy (CBT), combined family therapy and CBT, and, for comparison, a comparison skills-focused psychoeducational intervention (Waldron, AA12183).
In addition to these four family intervention investigations, there are two additional projects that involve family interventions which are described below: Wagner's guided-self change project, one version of which involves a parent, and Dishion's aftercare family intervention culturally tailored to Native Americans.
Cognitive-Behavioral Therapy
Two of the ten clinical trials evaluate cognitive-behavioral interventions. One is a pilot study to contrast the relative effectiveness of individual CBT and treatment-as-usual among adolescent girls who meet criteria for both alcohol use disorders and post traumatic stress syndrome (Najavits, R21 AA12181). In the second study, CBT is assessed both as a stand-alone individual therapy and as CBT therapy combined with functional family therapy (see No. 4 under family intervention studies above).
Guided Self-Change
Two of the ten studies consider the effectiveness of guided self-change in relatively brief interventions. One of these compares the efficacy of Individual Guided Self-Change Treatment (GSC-I) and Family-Involved GSC (GSC-F) in a randomized controlled trial that targets Hispanic/Latino and African-American juvenile offenders with alcohol problems (Wagner, AA12180). The second weighs the effectiveness of three low-cost, developmentally appropriate, voluntary self-change interventions (Brief Intervention, Guided Self-Change, and Peer Group Counseling) made available to 9,000 ethnically diverse students in four high schools (Brown, S.A., AA12171).
Motivational Enhancement
One of the ten projects explores the benefits of brief motivational enhancement intervention for alcohol problems among street youth recruited through drop-in centers and shelters (Peterson, AA12167).
Native Americans
Two of the ten clinical studies focus on working with community-based organizations to enhance treatment of alcohol use disorders in adolescents from Native American communities. One of these provides a brief aftercare intervention that engages parents in monitoring the posttreatment functioning and behavior of their adolescent children (Dishion, AA12170). The second project (Marlatt, AA12321) examines the effectiveness of interventions with Native American adolescents is a two-phase project. Phase I has been funded to (a) evaluate treatment services and a prevention program provided by a community health center that serves Native Americans exclusively, (b) test a brief screening instrument, and (c) develop a standardized protocol for pilot testing two adolescent interventions (life-skills approach and Native American 12-step approach), to be delivered and assessed at the Native American health center in Phase II of the project.
Future Research Needs on Adolescent Treatment
While NIAAA is committed to encouraging and awarding scientifically competitive clinical trials and pilot studies to improve treatments for adolescents, there are two additional research areas that need to be seriously addressed: (1) pretreatment identification and brief intervention and (2) enhancement of posttreatment functioning.
Pretreatment Identification and Brief Intervention
According to data from the National Household Survey of Drug Abuse (NHSDA), less than 10 percent of community adolescents with symptoms of dependence have ever been seen in treatment. Using NHSDA data, Dennis, Godley and Titus (in press) also discovered that adolescents are less likely than adults to perceive their substance abuse to be a problem and therefore are unlikely to seek treatment. These authors suggest that more early intervention is needed to increase problem awareness and access to treatment through activities such as outreach, screening, and brief interventions for substance abuse. Brief motivational enhancement counseling, for example, could be an effective means for heightening awareness of high-risk adolescents, thereby potentially motivating them to take action. Examples of possible studies to meet this need:
Develop effective screening instruments that target different settings (e.g., clinical, school, or criminal justice) and adolescent subgroups (e.g., those defined by gender, ethnicity or minority status).
Identify effective approaches for motivating adolescents to assume personal responsibility for resolving their alcohol and drug problems.
Enhancement of Posttreatment Functioning
Although initiation of abstinence in treatment is common among adolescents, sobriety is frequently challenged as soon as they return to earlier social environments. Outcome studies report that as many as 80% of adolescents in treatment may relapse in the first 12 months, primarily in response to peer pressure. Participation in support groups, compliance with aftercare programs, coping skills training (especially problem-focused coping), and family involvement in posttreatment interventions are associated with improved long-term treatment outcomes among adolescents. Thus, there is a pronounced need to develop or assess promising aftercare interventions that increase their prospects for maintaining treatment gains. These might include:
Assessment of family-based aftercare interventions.
Development and evaluation of aftercare programs that focus on training in coping skills to enhance practical management of and efficacy expectancy in salient environments for drinking and drug use.
Identification and evaluation of community-, school-, and/or family-based activities that provide pro-social alcohol- and drug-free alternatives to deviant peer activities;
Back to Top
E. Screening and Brief Intervention
Medical settings provide important venues to identify alcohol-related problems in individuals who present for treatment of other health problems. Objectively assessed prevalence rates of alcohol-related problems have been reported to range from 11% to 47% in primary care and emergency room injury patients and in hospital inpatients. Once identified, a brief intervention can be delivered to patients who are positive on the standardized screening test or the patient can be referred for alcoholism treatment. Medical patients with moderate alcohol problems have been shown to respond fairly well to brief intervention. Meta-analyses of brief intervention studies indicate that this approach is widely applicable and effective (Bien, 1993). Thus, it is not surprising that we have seen a research trend in the past decade toward brief interventions as primary treatment strategies. Brief interventions in public health settings provide a cost effective means for reversing alcohol problems; they not only cost little to provide but they have the potential to reduce harmful drinking in a large number of individuals. Because the cost effectiveness of brief interventions is dependent upon effective screening strategies, grants that focus on evaluation and development of screening questionnaires are also included in the psychosocial portfolio.
Over the past few decades, a variety of questionnaires have been developed to screen for problematic alcohol use in clinical populations. The goal of these screening tests is to identify those people who may benefit from brief intervention for heavy drinking or alcohol abuse or from referral for treatment of alcoholism. Among the most widely used alcohol screening tests in clinical practice as well as research are the CAGE, MAST (or SASST), and the AUDIT. It is only recently that the sensitivity and specificity of these instruments have been contrasted with each other within the same study population in order to determine which perform best in which subgroups (e.g., Cherpitel, 1994, 1998; Steinbauer et al., 1999). Although little screening work has been done with adolescents, Chung et al. (in press) recently studied the CAGE, TWEAK, and AUDIT with adolescents in a hospital setting. They found that the AUDIT demonstrated the best performance across the range of its cut-off scores relative to the TWEAK and CAGE. The study emphasized the need for routine screening of teens in a hospital setting.
Two new approaches to screening for alcohol problems have been developed in the past decade. These approaches were components in clinical trials designed to assess the effectiveness of brief intervention for alcohol problems in family practice settings. Both trials, now completed projects, were funded by TRB. In one, Michael Fleming utilized the Health Screening Survey (HSS) to identify alcohol problems among patients in rural family practice settings. The HSS is a five-minute health behavior questionnaire containing sets of questions on exercise, smoking, weight, and alcohol use. The alcohol questions collect information on weekly quantity and frequency of alcohol consumption over the past three months, number of episodes of binge drinking, and patients' perceptions of whether or not they have an alcohol problem. The HSS also includes the four CAGE questions. The second screening approach was developed and tested by Yedy Israel. This involved administration in the family practice waiting room of the Trauma Questionnaire (TQ), developed by Harvey Skinner, a self-report questionnaire with only one of the five questions related to alcohol. Those who scored positive on the TQ were then asked the four CAGE questions by their physician in his/her office. The sensitivity and specificity of the HSS and TQ in identifying patients with alcohol problems have not yet been compared with those of the standard screening tests discussed above.
Screening and Brief Intervention Projects Funded in FY 1999
Five screening studies are described in this section: a TRB contract developed to investigate which screening approach, including the HSS and TQ, performs best under which circumstances; a computerized version of the AUDIT, and three grants to assess primary care screening approaches in medical settings serving adolescents. Also described are four RCTs of brief interventions delivered in two types of medical settings (ER and primary care) and in a community sample.
Screening
Sensitivity and Specificity of Alcohol Screening Questionnaires for Primary Care Settings (Volk, N01AA81015). This contract is the second study funded under TRB's Treatment Research Validation and Extension Program which funds projects to assess issues related to the performance of widely used approaches to screening, diagnosis and assessment. The objectives of the contract are: conduct a literature review to identify all approaches to screening for alcohol problems published from 1971 to 1999; identify the most widely used and/or promising of these for screening in medical settings; design a screening study to compare the sensitivity and specificity of the selected approaches; adapt the design to a military setting; reanalyze the Contractor's data on the AUDIT in order to determine which of three cut-off points is most efficient to use in which types of patient subgroups.
Computerized AUDIT for Primary Care. This project (Chiauzzi, R44 AA11052), funded under the NIH Small Business Innovative Research (SBIR) Program, is an SBIR Phase II field test of a multimedia, interactive program known as "Computer Assisted Alcohol Screening and Treatment for Primary Care" (CAAST-PC), a bilingual assessment tool for detecting early indications of alcohol problems in primary care settings, developed in Phase I of the project. The CAAST, derived from WHO's AUDIT, will be made available in both Spanish and English.
Screening Adolescents for Alcohol Use Problems in Medical Settings. Three adolescent screening studies were funded under the joint NIAAA/CSAT program on Treatment for Adolescent Alcohol Abuse and Alcoholism (see section above on Adolescent Treatment for background). One of these involves assessment of factors influencing the validity and accuracy of a Minnesota state-sponsored questionnaire for identifying adolescent alcohol problems in primary care settings (Harrison, AA12179). The American Medical Association (Chicago) is developing a screening protocol to identify adolescent problems in medical settings based on literature review, a medical advisory board, and feedback from focus groups (Yoast, AA12186). Dr. John Knight (AA12165) is testing and validating a shortened form of the CRAFFT, a questionnaire designed to screen for adolescent alcohol problems in the medical office.
Brief Intervention
Emergency Rooms
There are currently two emergency room studies in the TRB portfolio.
Tailored Alcohol Messages in the Emergency Department (Blow, AA11629). The goal of this study is to test the effectiveness of computerized tailored messages to reduce alcohol use and alcohol-related problems among injured hazardous drinkers 18 and over admitted to the Emergency Department (ED) within the past 24 hours. This is the first ED study to collect screening data on patient characteristics with a personal digital assistant. This personal information is then uploaded into a computer program that prints a booklet tailored to the specific characteristics of the patient. This RCT compares outcomes of four conditions: tailored computerized messages in booklet format vs. generic messages in booklet format with or without brief advice.
Brief Intervention for ETOH Positive Injured Patients (Longabaugh, AA09835). This RCT's objectives are (1) to contrast the main effects (i.e., reduction in subsequent alcohol-related negative consequences) of two types of intervention delivered to alcohol positive patients in a Level 1 Trauma Center, and (2) to determine if there is an interaction between intervention type and prognostic factors. The "Immediate Intervention" takes place at the time of the ER visit; it aims to elicit from the patient a goal and a plan to reduce future hazardous drinking and its consequences. The second intervention, a "Booster Session," attempts to consolidate the effects of the intervention initiated in the initial intervention. These two interventions are compared with one another and with standard practice. Analyses of three month outcome data from a partial sample of patients indicate that the brief intervention plus booster session results in significantly better outcomes than brief intervention alone which does not differ significantly from the standard care condition. Further, the data suggest that patients who are likely to benefit from the brief plus booster condition are those who report no readiness to change drinking prior to the initial intervention. These findings must be tested on the full sample before being regarded as conclusive.
Primary Care
HMO Setting. Dr. John E. Helzer (AA11954) is conducting an RCT to determine the efficacy of a low cost intervention for heavy and problem drinking appropriate for use in a primary care practice. The Interactive Voice Response (IVR) computer-based telephone technique will be tested in a primary care practice as a treatment tool to enhance physicians' brief alcohol interventions with heavy and problem drinkers. IVR allows the caller to respond to a recorded voice asking scripted questions by using the telephone touch pad to input brief numeric answers. The study design involves office-based delivery by the primary care physician of a brief intervention using the FRAMES motivational model. The patient is then assigned to one of four experimental conditions, three of which involve daily responding to the IVR for six months: IVR with no feedback, IVR with monthly feedback via the physician, IVR with monthly feedback and financial compensation for making the calls, and a control group with no IVR intervention. The two major objectives of the study are to assess: (1) the feasibility of using IVR as an intervention in primary care patients including call compliance rates (will financial compensation make a difference?) and the validity of consumption reports, and (2) whether IVR, with or without feedback to patients, enhances the effect of a brief alcohol intervention by a physician.
Community
Self-Help Interventions. Dr. John A. Cunningham, under an R03 (AA11928), is conducting an RCT to assess the efficacy of two types of self-help interventions--self-help books to guide problem drinkers through the change process, and personalized assessment feedback interventions designed to increase individual's motivation for change. The project will compare four conditions in a community sample: books, feedback, books and feedback, and no intervention. Research participants will be recruited through a general population survey.
NIAAA PORTFOLIO ON MULTISITE CLINICAL TRIALS
(Margaret E. Mattson, Ph.D.)
Since 1989 the Treatment Research Branch has supported two multisite cooperative agreements. Project MATCH, initiated in 1989, and Project COMBINE, initiated in 1997. Project MATCH, involved 9 clinical sites and a Coordinating Center and recruited 1600 patients. It was designed to be a test of the matching hypothesis that stated that outcomes for alcoholics could be significantly improved if patients were assigned to alternative psychosocial treatments based on their particular needs and characteristics. Since its conclusion the study has published widely and has been the subject of numerous commentaries. Highlights of major findings are summarized below. Project COMBINE, is currently in its third year and consists of 11 clinical sites and a Coordinating Center. It aims to discover the effects of two pharmacologic agents (naltrexone and acamprosate) and two psychosocial therapies alone and in various combinations. The background and major features of the study are described in part B below.
Back to Top
A. PROJECT MATCH
The initial results of Project MATCH were published in early 1997, with additional reports on main findings released later in 1997 and 1998. The findings from Project MATCH, the largest and most statistically powerful clinical trial of psychotherapies ever undertaken, were awaited eagerly. Three treatments were studied, Twelve Step Facilitation, Motivational Enhancement Therapy, and Cognitive Behavioral Therapy. The findings of MATCH were surprising to many and challenged the role of patient-treatment matching as a major benefit in clinical management of alcoholism.
Ten promising primary matching hypotheses, based on results from several decades of previous research, were reported in the initial publication. The results supported only one of the primary matches, i.e. patients with low psychiatric severity receiving Twelve Step Facilitation had a relatively small advantage (12% difference in abstinent days). It was thus concluded that for the three well-defined and manual guided therapies employed in the study matching does not significantly improve outcomes. It was noted that these findings do not rule out the possibility that client-treatment matches interact with other patient characteristics and other treatment approaches may exist.
Subsequent analyses on three-year matching outcomes for clients who had been treated in the five outpatient sites of Project MATCH showed that client anger demonstrated the most consistent interaction in the trial. There were significant matching effects evident at both the 1-year follow-up and 3-year follow-up, with clients higher in anger doing better in MET than in the other two treatments.
Additional significant matching effects emerged in the 3-year outcome analysis. Regardless of treatment group, AA attendance had a beneficial effect on drinking outcomes for those high on the social network variable. The effect was especially marked for the high network patients from the Twelve Step Facilitation treatment group who achieved 91% abstinence versus 60% in the comparison group, and 1.5 drinks per drinking day versus 4 drinks.
Apart from matching effects, the study also shed light on outcomes in drinking, overall and for the three treatments individually. Clients in all three treatment conditions showed major improvement in drinking, as well as other areas of functioning such as depression, use of illicit drugs, and liver enzyme status. As a whole, MATCH clients were abstaining on over 85% of the days throughout the year following treatment, with overall alcohol consumption decreased fivefold. Even for those not successful in maintaining abstinence who continued to drink, there was a substantial reduction in consumption. In general, effects for the three treatments were quite similar, with small differences favoring increased abstinence in the Twelve Step Facilitation group.
No matching effects were found during the treatment period. However, during the 12 week treatment period, it was found that there was a difference between the 12 session treatments (Cognitive Behavioral Therapy and Twelve Step Facilitation) and the 4 session treatment (Motivation Enhancement Therapy) in the outpatient arm such as 41% of CBT and TSF clients were abstinent or drank moderately without alcohol-related consequences, compared with 28% of MET clients.
The progression of men and women from the MATCH sample through landmark events in the development of alcoholism was analyzed. Women exhibited shorter average progression times than men (i.e. "telescoping"), between several pairs of successive age-of-onset variables, such as getting drunk regularly and first encountering drinking problems.
High rates of patient compliance to both therapy and research protocol were achieved in Project MATCH. A number of baseline predictors of small magnitude were found, with sociopathy and previous treatment history being the only patient characteristics predictive of both treatment and research follow-up. Strong, single-characteristic baseline predictors of compliance did not emerge. Treatment attendance was favorably related to drinking outcomes, suggesting that retention of clients in treatment for as long as possible may enhance chances of good outcomes.
A report on the clinical implications of the Project MATCH findings appeared in the Journal of Mental Health and addressed the clinical relevance of the results in terms of matching effects, main effects, compliance to therapy and therapist training. The journal Addiction published a series of critiques on Project MATCH from an international panel of 14 commentators, along with a response by The Project MATCH Research Group.
A report on the effects of therapist characteristics was published. Perhaps because of the standardization of the therapists' training, therapist characteristics did not exert major overall effects on client outcomes, except in a few individual cases.
Seven methodology manuals were published as the Project MATCH Monograph Series. These include the three therapy manuals, Form 90, DRINC, and two manuals on compliance. The Project MATCH Monograph Series has been very popular among practitioners, especially the three therapy volumes, for which over 30,000 requests have been received.
A survey of users of the three treatment manuals was conducted among a sample of users. Overall satisfaction scores were high, with the predominant users being masters' level counselors. An interest in receiving manual-specific training was expressed. Almost half of the users reported that the manuals influenced them to make changes that they felt improved their practice.
Outside investigators can now gain access to the Project MATCH data set for independent analyses through the Project MATCH Website, which also provides information on the trial findings, background, and a bibliography of all publications and presentations resulting from Project MATCH. The database has been available to the community since Jan. 1998. Over 2700 contacts have been logged and about 40 researchers from 17 institutions have accessed the data. Related to the continued demand for the three treatment manuals and training on their use from providers, the Project MATCH Research groups is developing a standardized training program that can be used at conferences, in-service training, etc to provide state-of-the-art training for therapists.
Back to Top
B. PROJECT COMBINE: COMBINED BEHAVIORAL AND PHARMACOLOGIC TREATMENTS OF ALCOHOLISM
An RFA for a multisite clinical trial to find optimal combinations of behavioral and pharmacologic treatments for alcoholism was funded in the fall of 1997. The program builds on knowledge gained from Project MATCH on effective psychosocial therapies as well as the results of several years of pharmacologic studies, both from the TRB portfolio and from European studies. The pharmacologic agents are naltrexone and acamprosate. The two behavioral therapies are Combined Behavioral Therapy (CBI), a moderate intensity intervention combining elements from cognitive behavioral therapy, motivational enhancement therapy and Alcoholic Anonymous; and, Medication Management, a brief intervention designed to enhance compliance to medication and encourage drinking cessation. The main trial will be a randomized, placebo controlled study consisting of over 1300 patients in a 2 x 2 x 2 factorial design, plus an additional control cell with CBI only. Brief descriptions of the interventions follow.
Naltrexone, a nonspecific opioid antagonist, is the only non-aversive pharmacotherapy for alcoholism that is approved by the U.S. Food and Drug Administration. Acamprosate, the calcium salt of acetylhomotaurine, is used to treat alcoholism in Europe, but is not yet approved for use in the U.S. Several studies have shown that it is superior to placebo in promoting abstinence from alcohol. The mechanism of action of acamprosate may involve alteration of neuronal excitability at the N-Methyl-D-Aspartate (NMDA) receptor and other non-opioid effects.
Naltrexone and acamprosate have very different mechanisms of action and presumably target different aspects of drinking. As a result, the combined use of these two medications may yield a more effective treatment. Acamprosate may be particularly useful in helping participants avoid initial alcohol consumption and enhancing treatment retention by attenuating protracted alcohol withdrawal. Naltrexone may be particularly efficacious in reducing the likelihood of heavy drinking following a slip. Systematic, large sample comparisons of these drugs alone, in combination, and paired with well-documented psychotherapies have not been done.
COMBINE was undertaken with the expectation that alcoholism treatment can be enhanced by combining effective behavioral and pharmacological therapies. Two modalities of behavioral treatment were developed for the study, Combined Behavioral Intervention (CBI) and Medical Management (MM). CBI, a moderate intensity intervention, capitalizes on Project MATCH findings. It incorporates the putative strengths of each of the three Project MATCH treatments: Twelve-Step Facilitation, Motivational Enhancement Therapy and Cognitive Behavioral Therapy. Project MATCH findings showed marked improvement in drinking for the three MATCH treatments during the 12-month follow-up. This improvement was sustained at the subsequent 3-year follow-up. In addition, these interventions demonstrated relatively high retention rates and high levels of patient satisfaction, and were feasible to implement in a clinical trial. In clinical practice, therapists are likely to employ a range of techniques. Thus, it is desirable to examine the efficacy of a broad model of treatment that incorporates the putative active ingredients of each of the Project MATCH therapies.
Medical Management (MM) is a less intensive intervention designed to enhance medication compliance and support of sobriety, and is very suitable for ready incorporation into the daily routine of busy health care practitioners in primary care or managed care settings. It is intended to maximize exposure to the medications, facilitating evaluation of their effects. It represents a potentially cost-effective alternative to CBI. In developing the MM treatment, emphasis was placed on strategies that could be learned with minimal training and delivered rapidly with an orientation compatible with the delivery of other types of medical care. The MM treatment was developed from common themes in existing treatment manuals for brief therapy. The main focus is enhancement of medication compliance while also supporting the participant's efforts to change his or her drinking habits and abstain from alcohol. All participants in COMBINE (except those in the CBI alone control cell) will receive MM therapy. Since the study medications will be administered over a four-month period, both the low and moderate intensity treatments are provided over the same four-month duration.
Prior to the initiation of the Project COMBINE main trial in early FY 2000, two preliminary studies will be conducted. The first pilot study involves three participating sites and examines the behavioral and pharmacological toxicity, tolerance, and kinetic interactions between naltrexone and acamprosate (both alone and in combination) in treatment-seeking alcohol-dependent subjects. Defined medical endpoints will be used to ensure the health and safety of research volunteers and as clinical endpoints to determine the tolerance for high doses and various dose combinations.
The second preliminary study is a trial in which all sites will enroll patients and conduct all procedures and interventions (except for follow-ups) for approximately four months. This will enable field personnel to become familiar with all facets of this complex protocol, i.e. the integration of services required by the protocol, recruitment strategies, assessment procedures, etc. and provide an assessment of retention in treatment and compliance.
Back to Top
PHARMACOTHERAPY OF ALCOHOLISM
State of Knowledge (Henry R. Kranzler, M.D.)
During the past decade, renewed interest in medications to prevent relapse in alcoholics has yielded a number of promising candidates. Although two of these medications, naltrexone and acamprosate, are currently in clinical use in a number of countries, overall, their effectiveness appears to be limited (Kranzler et al., in press; Hersh et al., 1998; Roussaux et al., 1996; Gerra et al., 1992). Nalmefene, a compound with effects similar to naltrexone, as well as a sustained release formulation of naltrexone, may enhance the beneficial effects of opioid antagonist therapy (Mason et al., 1999). Disulfiram, the deterrent medication that was approved 50 years ago for the treatment of alcoholism, has not consistently been shown to be efficacious. However, since inadequate dosing and other modifiable factors may limit its deterrent effects, the recent identification of a more potent metabolite of disulfiram appears to warrant further evaluation. Studies of serotonergic agonist treatment of alcoholism have also yielded inconsistent results (Kranzler and Anton, 1994). However, there is evidence that certain subgroups of alcoholics may respond well to such medications, suggesting that careful treatment matching may enhance their efficacy (Pettinati et al., submitted).
Despite these promising developments, much remains to be learned about the pharmacotherapy of alcoholism. In this effort, the ongoing development and evaluation of novel medications should be given a high priority. However, answers to a number of basic questions are still unknown, including what is the optimal dosing strategy and duration of treatment for existing therapies. Similarly, combination therapy, involving either multiple medications or the combination of medication with specific psychotherapies, has not been well studied. The utility of specific pharmacotherapies in women, different ethnic/racial groups, adolescent and geriatric patients, and individuals with comorbid alcohol and drug use disorders (including nicotine dependence) is also largely unknown, as is the suitability of medication therapy for treatment of early problem drinkers.
The ultimate goal of these efforts should be the development of algorithms for the pharmacological treatment of heavy drinking, which incorporate the characteristics of the patient and of pharmacological and psychosocial treatments with demonstrated efficacy. Although a general framework for such an effort currently exists, much detail is needed before it will be of widespread clinical value.
Specific recommendations:
- Additional studies should be conducted on medication compliance and methods to enhance compliance with a variety of medications used to treat alcohol dependence.
- It is important to determine whether different subtypes of alcoholics (e.g., based on age of onset, or severity of alcohol dependence, or derived using a multivariate approach) respond differentially to specific medications.
NIAAA PORTFOLIO ON MEDICATIONS DEVELOPMENT
(Raye Z. Litten, Ph.D.; Joanne Fertig, Ph.D.; John P. Allen, Ph.D.)
During the past decade significant advances have been made in medications development for alcoholism treatment. Throughout the 1990s the Treatment Research Branch (TRB) has funded 48 pharmacotherapy human trials. The fruits of these trials have been most clearly demonstrated by FDA approval of naltrexone. This is the first medication approved for alcoholism treatment in 50 years since the introduction of disulfiram and the first medication approved to reduce the desire or urge for drinking. Progress has also been made in developing pharmacological guidelines for treating alcoholics suffering comorbid depression. Finally, the methodology for conducting clinical trials has markedly improved, particularly in the area of assessment, outcome, and statistical analyses.
At this point TRB is funding 26 pharmacological clinical trials and nine human laboratory studies for a total of $14.65 million (Table 1). The portfolio of pharmacotherapy projects has significantly expanded since 1990 when fewer than a half-dozen were funded. Grants are categorized according to funding mechanism and type of medication in Tables 1 and 2.
In spite of the progress, additional investigations and further elucidation of important clinical issues remain to be done. Exploration of new types of medications, development of effective dosing regimens, evaluation of short- versus long-term pharmacological treatments, and consideration of medications in combination remain high priorities. In addition, determination of the optimal combination of medication and behavioral-psychosocial interventions, establishment of effective treatment protocols for comorbid alcoholics, specification of clinical and patient characteristics that predict success with the medication, and strategies to ensure patient compliance are important goals for TRB. In the following sections and in Table 3,, the current portfolio is reviewed.
- Naltrexone
In December 1994, the FDA approved the opioid antagonist naltrexone as an adjunctive treatment for alcoholism. Since then, naltrexone has been approved in 28 countries. The efficacy portion of FDA approval was grounded in results of the two NIAAA-supported trials by Dr. Joseph Volpicelli (AA07517) and by Dr. Stephanie O'Malley (AA03510) and their colleagues. Both investigations revealed that alcohol dependent patients treated with naltrexone experienced fewer days drinking and were less likely to relapse to heavy drinking than were placebo-treated patients. In addition, in O'Malley's study the type of psychosocial intervention had an impact on treatment outcome in combination with naltrexone. Patients receiving naltrexone and a supportive therapy emphasizing total abstinence enjoyed a higher rate of complete sobriety. In contrast, those receiving naltrexone and a coping skills/relapse prevention therapy suffered fewer relapses to heavy drinking if, in fact, they did sample alcohol. Since these two landmark trials, new studies have been initiated to assess the full clinical potential as well as the limitations of naltrexone.
- Long-Term Studies
The length of treatment in Volpicelli's and O'Malley's studies was three months. However, no information exists on the effect of naltrexone over an extended period of time. To explore this, TRB is supporting three naltrexone studies ranging from nine to 15 months.
Dr. O'Malley (AA09538) has recently completed a nine-month study of naltrexone. In an initial 10-week open label study, alcoholic dependent subjects received naltrexone (50 mg/day) with either a coping skills therapy or primary care counseling. Treatment responders in the open trial (i.e. patients who had no more than two days of heavy drinking in the last four weeks and took the medication at least 60 percent of the time) were then randomized to either continue naltrexone or to take placebo for six additional months and continue their original psychotherapy. The results showed that those receiving coping skills therapy maintained treatment gains throughout the six-month period irrespective of naltrexone use. In contrast, placebo-treated patients receiving primary care counseling drank more often and had more days of heavy drinking than did patients continuing naltrexone treatment. These results indicate that naltrexone can maintain efficacy for up to nine months (in the primary care group) without serious side effects. Also, as shown in her earlier study, the type of behavioral-psychosocial intervention can influence the type of outcome with naltrexone. Dr. O'Malley is continuing to analyze the data for additional findings.
Dr. Volpicelli (AA07517) also completed a nine-month trial of naltrexone, differing in experimental design from that of Dr. O'Malley. Alcohol dependent subjects were randomized into three groups: the first received 100 mg/day naltrexone for nine months; the second, 100 mg/day naltrexone for three months followed by placebo for six months; and the last group took placebo daily for nine months. Preliminary analyses indicate that patients receiving naltrexone for nine months consumed alcohol at lower levels. Data analyses are ongoing.
Finally, Dr. Barbara Mason (AA10518) is conducting a naltrexone maintenance trial including an augmentation component for non-responders. This study has two phases: an open acute treatment phase (weeks 0-12) followed by a maintenance treatment phase (13-64 weeks). Alcohol dependent patients are administered naltrexone 50 mg daily and a weekly session of coping skills therapy for 12 weeks. The subjects are then classified into three groups: full responders (total abstinence), partial responders (equal or less than two heavy drinking days or 50 percent reduction from baseline in alcohol consumption over the last six weeks of acute treatment), and non-responders. The full and partial responders then enter the maintenance treatment phase and receive either 50 mg naltrexone or placebo daily and continue with coping skills therapy for 64 weeks. The non-responders enter the augmentation phase and receive either 2 gm acamprosate or placebo daily and continue with 50 mg/day naltrexone and coping skills therapy. This study will provide valuable knowledge on long-term treatment of naltrexone and determine if patients who maintained abstinence in the acute treatment phase will respond better to maintenance naltrexone treatment than those who only reduced their drinking. Also, information will be obtained on the combination of acamprosate, naltrexone, and coping skills therapy in influencing non-responders to become full or partial responders.
In summary, preliminary results indicate that long-term treatment of naltrexone even at 100 mg/day dosage, is efficacious and safe. Current studies are evaluating treatment factors such as the type of behavioral-psychosocial intervention (see below) and patient characteristics that suggest the long-term usage of naltrexone.
2. Naltrexone in Combination with Behavioral-Psychosocial Intervention(s)
Upon FDA approval, it was recommended that naltrexone be used in conjunction with behavioral-psychosocial therapy. Two important research questions arise from this recommendation. First, does the type of behavioral-psychosocial intervention influence treatment outcome with naltrexone? Second, what is the most effective behavioral-psychosocial intervention(s) to be used with naltrexone?
Dr. O'Malley's earlier naltrexone trials (AA03510 and AA09538) (see above) demonstrated that the type of behavioral-psychosocial intervention can influence the type of treatment outcome with naltrexone. TRB is funding several studies to investigate the optimal treatment combination of naltrexone and behavioral-psychosocial therapy.
Dr. Ray Anton (AA09568) completed a trial that combined naltrexone with a cognitive behavioral therapy in alcohol dependent subjects. Recently abstinent alcoholic outpatients were administered either naltrexone (50 mg) or placebo and were treated with 12 weekly sessions of manual guided cognitive behavioral therapy. The results demonstrated that naltrexone-treated subjects drank less, were less likely to relapse to heavy drinking, and persisted longer between relapses than the placebo group. Dr. Anton postulated that the therapeutic effects of naltrexone and the cognitive behavioral therapy might enhance each other, perhaps by improving cognitive resistance to continue to drink once alcohol is sampled.
However, not all the trials with naltrexone and psychotherapy have shown efficacy. Dr. Henry Kranzler (AA00239) completed a study comparing the efficacy of naltrexone with nefazodone, a serotonin reuptake inhibitor and a 5-HT2 antagonist, with alcohol dependent patients also received weekly relapse prevention therapy. In this 12-week study no significant differences in drinking outcomes were observed among the naltrexone, nefazodone, and placebo groups. Interestingly, naltrexone was associated with significantly more adverse events, though none were serious, which resulted in reduced medication compliance and a lower rate of treatment completion. Inadequate medication compliance has been shown to reduce the effectiveness of naltrexone (see the Medication Compliance section below).
Dr. Peter Monti (AA07850) is conducting a naltrexone trial using coping skills training and cue exposure treatment (CSTCET). In this study alcoholic patients receive two weeks of either CSTCET or meditation/relaxation/education control treatment in an inpatient setting followed by either naltrexone (50 mg/day) or placebo administration for 12 weeks in an outpatient setting. This is the first study to combine cue exposure treatment with naltrexone.
Several ongoing trials are investigating the use of naltrexone with less versus more intense behavioral-psychosocial interventions. Dr. Anton (AA09568) is conducting a 12-week naltrexone trial comparing a cognitive behavioral therapy with less intense motivational enhancement therapy. Both therapies are delivered by trained therapists using Project MATCH manuals.
Dr. Volpicelli (AA07517) is conducting a six-month trial of naltrexone comparing three types of therapies: simple Medication Management (MM), MM plus compliance enhancement techniques, and MM plus cognitive behavioral therapy as performed in Project MATCH. The first two therapies can be administered in a primary care setting, while the latter is appropriate for a specialized alcohol treatment setting.
Finally, TRB has initiated a randomized double-blind, placebo-controlled 11-site trial called Project COMBINE (AA11721), utilizing the cooperative agreement mechanism. The objective of Project COMBINE is to assess the efficacy of various combinations of pharmacotherapy and behavioral interventions. The medications to be investigated include naltrexone, acamprosate, and the combination of naltrexone and acamprosate. Behavioral-psychosocial treatments consist of Medical Management and Combined Behavioral Intervention. Medical Management is a low intense intervention that could be conducted in a non-specialized alcohol treatment setting. This therapy includes elements of compliance enhancement, information about alcoholism and medications, encouragement to remain abstinent, and referral to peer support groups. Combined Behavioral Intervention is a much more intense therapy administered in a specialized alcohol treatment setting. Its components entail Medical Management, motivation enhancement, cognitive behavioral skills training, and facilitation of involvement in mutual-help groups. Basically, this intervention integrates key elements of treatment from the three Project MATCH therapies. The main trial is scheduled to begin in February 2000.
Current studies should provide important clinical information such as whether less intense and potentially less costly therapies are efficacious when combined with naltrexone. The effectiveness of more intensive behavioral therapies will also be evaluated and contrasted with less intensive therapies with and without the presence of naltrexone over various periods of treatment.
3. Optimal Dosage of Naltrexone
Most of the clinical trials have used 50 mg/day naltrexone based on the dosage used to treat opioid addiction. However, it is unclear if this dosage is optimal for alcoholism treatment. Dr. Betsy McCaul (AA09569) has completed the first dosage study of naltrexone in alcoholics with and without cocaine or cocaine/heroin dependence. Patients were administered either 50 mg or 100 mg of naltrexone or placebo daily for six months. Preliminary analyses revealed that patients receiving 100 mg of naltrexone consume fewer drinks than those on placebo during the first two months treatment. However, only a trend toward decreased drinking was observed in the 50 mg naltrexone group. Interestingly, the amount of drinking was negatively correlated with serum 6-beta-naltrexol, an active metabolite of naltrexone. Patients having 6-beta-naltrexol serum levels greater than 40 ng/ml did not relapse at all and most of these patients were in the 100 mg dosage group. In addition to analyzing the data of this trial, Dr. McCaul is also evaluating the results of a human laboratory study, again comparing 50 mg versus 100 mg dosage of naltrexone. This study focuses on the effects of naltrexone on the subjective, physiological, and psychomotor responses to alcohol ingestion.
Currently, Dr. Volpicelli (AA07517) is conducting a long-term study using 100 mg doses of naltrexone (see Long-Term Studies above). So far, no increases in side effects or adverse effects have been reported with the higher dose. As described above, Project COMBINE is also planning on using 100 mg doses of naltrexone.
4. Patient Compliance
Patient compliance appears to play an important role in treatment outcome for naltrexone. In a very recent trial Dr. Volpicelli (AA07517) demonstrated that patients who were highly compliant responded to naltrexone better than patients who were less compliant. Most of the new trials, including Project COMBINE, are developing a protocol to strengthen patient compliance. Simple approaches such as establishing reminders for the patient to take the medication, reviewing side effects at each medical visit, and being empathetic to the patient's situation are being incorporated.
Another strategy to better ensure medication compliance would be a sustained-release naltrexone depot. This formulation would release naltrexone into the bloodstream for up to 30 days. Dr. Kranzler (AA00239) is now conducting a trial of a long-acting, biodegradable, injectable depot of naltrexone in alcohol dependent patients. Subjects in this 12-week study randomly receive depot naltrexone and an oral placebo daily, an oral naltrexone daily and a depot placebo, or oral placebo daily and a depot placebo. Preliminary studies by Dr. Kranzler indicate that alcohol dependent patients can tolerate the naltrexone depot without serious side effects. Dr. Nuwayser (AA82007) has also begun a dose response study of depot naltrexone in alcoholics as part of an SBIR phase 1 study. Development of a successful naltrexone depot may increase the therapeutic potential of naltrexone, particularly with patients where compliance is an issue.
5. Predicting Treatment Outcomes with Naltrexone
Individual responsiveness to naltrexone varies significantly. Investigators in all the TRB funded trials are conducting secondary analyses to attempt to identify clinical and patient factors that predict successful outcome with naltrexone therapy. In particular, Dr. McCaul (AA11855) is correlating endogenous opioid activity and mu opioid receptor binding with naltrexone treatment outcome. In this study alcohol dependent patients participate in a two-week inpatient testing protocol. Included in the protocol are a naloxone challenge test as a marker of opioid system activity, measurement of unblocked mu opioid receptor binding at steady-state naltrexone levels using Positron Emission Tomography (PET), and measurements of serum 6-beta naltrexol levels, alcohol craving, alcohol withdrawal, and mood. During this period patients are randomized to receive once daily 100 mg naltrexone, twice daily 50 mg naltrexone, or placebo. After discharge, subjects then initiate a 12-week outpatient trial continuing their randomized study condition. The number of unblocked mu opioid receptors, endogenous opioid activity, serum 6-beta naltrexol, and alcohol craving will be correlated with drinking outcome in the outpatient study. This is the first study to use neuroimaging technique to predict clinical success to medication treatment.
6. Naltrexone Treatment for Different Populations
- Early Problem Drinkers
Many individuals drink heavily, but are not classified as alcohol dependent. Two TRB-supported trials are evaluating the efficacy of naltrexone in a subpopulation of heavy drinkers. Dr. Michael Bohn (AA09948) has conducted a six-week, placebo-controlled, double-blind trial of naltrexone in non-dependent heavy drinkers. The results of the trial are currently being analyzed. Preliminary findings showed naltrexone effective in reducing the frequency and amount drinking.
In Dr. Kranzler's study (AA11062), early problem drinkers are randomized to four treatment groups: daily naltrexone and placebo groups in which patients are administered either 50 mg naltrexone or placebo daily; targeted naltrexone and placebo groups in which patients are instructed to progressively reduce the number of naltrexone capsules (50 mg) or placebo per week. All patients receive coping skills training. The subjects assigned to the targeted naltrexone and placebo groups also receive additional counseling on use of medication as an aid to avoid excessive drinking. Dr. Kranzler has completed a pilot study and reported that early problem drinkers receiving the targeted naltrexone and coping skills training reduced their drinking. Completion of the main trial will undoubtedly provide important information on alternative treatment strategies for this population.
- Alcoholic Women with Eating Disorders
Alcohol dependent women entering treatment often show a unique clinical profile including a high rate of eating disturbances. Moreover, the efficacy of naltrexone for alcoholism treatment has not yet been clearly established in women. To address these concerns, Dr. O'Malley (AA10225) is conducting a 12-week trial of naltrexone in women with and without eating disorders. (Interestingly, naltrexone has also been used in the treatment of eating disturbances in nonalcoholic women.) In addition, a subsample of women taking antidepressants but are not suffering eating disturbances are also being recruited. In this study patients are administered 50 mg naltrexone or placebo daily along with a coping skills/relapse prevention group psychotherapy. The investigation is scheduled for completion next year.
- Alcoholics with a Cocaine/Heroin Dependence
Dr. Betsy McCaul (AA09569) finalized a six-month trial of naltrexone in alcoholics with and without cocaine or cocaine/heroin dependence. Patients received either 50 mg or 100 mg dosage of naltrexone daily or placebo. In addition, all participated in a four-week intensive day program consisting of cognitive-behavioral therapy with a traditional 12-step approach followed by group counseling and individual outpatient services for the remaining five months. The results of the trial should be forthcoming.
d. Alcoholics with a Nicotine Dependence
A large percentage of alcohol dependent patients are also dependent on nicotine. However, little is known about the interaction of alcohol and tobacco treatments and how this might influence resolution of each. (See Alcohol and Tobacco section for greater detail.) Several ongoing clinical trials are investigating the role of naltrexone on this significant public health concern.
Dr. Barbara Mason (AA11210) is conducting a 12-week trial in which alcohol and nicotine dependent subjects will be exposed to both alcohol and tobacco treatment. Patients either receive 50 mg/day naltrexone and a nicotine replacement patch or a placebo tablet and patch. Each one will receive weekly individual coping skills and smoking cessation behavioral therapy.
In the 12-week study of Dr. Joy Schmitz (AA11216), alcohol/nicotine patients are randomized to four treatment conditions: 1) 50 mg/day naltrexone and a nicotine replacement patch; 2) 50 mg/day naltrexone and a placebo patch; 3) a placebo tablet and a nicotine replacement patch; and 4) a placebo tablet and a placebo patch. All subjects also receive weekly cognitive-behavioral/relapse prevention therapy.
Finally, Dr. Andrea King (AA00276) is conducting a laboratory and clinical trial on the effects of naltrexone on smoking and drinking outcomes. Subjects who are both regular heavy smokers (20 cigarettes/day) and moderate to heavy drinkers will be recruited for both studies. Both naltrexone (50 mg) and transdermal nicotine patch (21 mg) will be tested in combination along with smoking cessation therapy.
e. Alaskan Natives
Alcoholism is a problem of significant proportions in Alaska and among Alaskan Natives in particular. Due to the remoteness of the state, provision of care is often difficult. Pharmacological agents may prove a practicable means to augment services in this region. Dr. O'Malley (AA12028) has just begun a 12-week clinical trial to determine whether naltrexone or the combination of naltrexone and the selective serotonin reuptake inhibitor (SSRI) sertraline reduces the risk of relapse in Alaskan Natives with alcohol dependence. This project will provide important information in developing state-of-the-art treatment strategies for alcoholism in this understudied population.
7. Naltrexone in Combination with Other Medications
Drinking behavior is not driven solely by a single transmitter system. The multiple bases involved include opioidergic, glutamatergic, GABAergic, serotonergic, and dopaminergic systems. Since naltrexone, an opioid antagonist, is not a "magic bullet" for alcoholism, a rational strategy to augment naltrexone's effect is to use it in consort with other types of pharmacological agents that act on different neuroreceptors. Combinatoral medication therapies are commonly employed in clinical management of other medical disorders, such as high blood pressure and cancer. We are currently funding several pharmacotherapy projects that employ this strategy.
Dr. Conor Farren (AA11222) is conducting a trial to investigate the combination of the SSRI sertraline with naltrexone. In this study alcohol dependent patients are administered naltrexone (50mg/day) for one week and then randomized to either naltrexone (50mg/day) and sertraline (50 mg/day increasing to 100 mg/day) or naltrexone (50mg/day) and placebo for 12 weeks. In addition, all subjects receive weekly relapse prevention group therapy. A pilot study has shown that the naltrexone and sertaline in conjunction is more effective than naltrexone alone in reducing the frequency and amount of drinking.
Dr. Helen Pettinati (AA09544) has started a 12-week, investigation of naltrexone and sertraline in patients who are alcohol dependent and suffer from major depression. Subjects are randomized to four treatment cells: 1) 200 mg/day sertraline and naltrexone placebo; 2) 100 mg/day naltrexone and sertraline placebo; 3) 100 mg/day naltrexone and 200 mg/day sertraline; and 4) naltrexone and sertraline placebos. In addition, all patients receive a weekly cognitive behavioral therapy that has been adapted to include a medication compliance enhancement component.
Dr. Barbara Mason (AA10518) is evaluating joint medication therapy to augment the treatment intensity for non-responders. This investigation is part of a maintenance trial of naltrexone. All alcohol dependent patients are given 50 mg/day naltrexone and weekly coping skills therapy for 12 weeks. The non-responders (i.e. those with more than two heavy days of drinking during last six weeks of treatment) then enter the augmentation phase. Each patient receives either 2.0 gm/day acamprosate or placebo and continues with 50 mg/day naltrexone and coping skill therapy. This study will determine if combining acamprosate with naltrexone and coping skills therapy can convert non-responders to full or partial responders.
Dr. Mason (AA10518) is conducting a supplemental study of combined naltrexone and sertraline in alcohol dependent women with a comorbid depression. In this study subjects are administered sertraline (up to 200 mg/day) and either naltrexone (50 mg/day) or placebo. After four weeks of treatment those who respond clinically continue the medication treatment up to one year. Preliminary results indicate that the combination of naltrexone and sertraline is more effective than sertraline alone in reducing the frequency and amount of drinking. Interestingly, the dose of sertraline in the combination group needed to be increased by 20 mg daily to achieve a comparable reduction in the severity of depressive symptoms.
Project COMBINE (AA11721) is also planning to use a combination of naltrexone and acamprosate in their 11-site trial. A pilot study is underway to evaluate the safety of combining these two medications at various doses.
Finally, several studies (Mason, AA11210; Schmitz, AA11216; King, AA00276) are investigating the combination of naltrexone and a transdermal nicotine replacement in patients suffering from alcohol use disorder and nicotine dependence (see Tobacco and Alcohol chapter).
8. Mechanism of Action
Several studies are exploring the mechanism of naltrexone accounting for reductions in drinking behavior. Understanding this may be important in developing the rationale for use of it in clinical practice as well as better understanding the biomedical mechanisms driving excessive drinking. In addition, understanding the physiological dynamics of naltrexone's action might provide insight into the development of new types of medications.
Dr. O'Malley (AA00171) has developed a laboratory self-administration paradigm using non-treatment seeking alcohol dependent subjects. After six days on randomized medication regimen (50 mg/day naltrexone or placebo) subjects are given a priming dose of alcohol to establish blood levels of .03 g %. After 40 minutes patients have the opportunity to consume up to eight drinks, at a rate of no more than four per hour, for two hours. During the priming and self-administration phases various measurements are collected on alcohol craving, subjective effects, and neuroendocrine functioning. Dr. O'Malley has now analyzed the findings and is in the process of publishing the findings. The results seem to suggest that naltrexone reduces the craving for alcohol both in the priming and self-administration conditions. Also the naltrexone-treated subjects consumed less alcohol and obtained lower blood alcohol levels. Finally, naltrexone also increased cortisol level. Suppressed HPA activity had been reported in alcoholics and perhaps naltrexone may act to normalize its function. Intriguingly, this type of paradigm might also have utility as a human screening model for testing promising medications.
Dr. Dena Davidson (AA00273) will determine the effects of naltrexone in altering alcohol-induced subjective stimulation, alcohol craving, and alcohol intake. In this crossover, human laboratory study, social drinkers receive either 50 mg/day or 100 mg/day dosage of naltrexone or placebo for seven days. They then consume alcohol ad lib at a local cocktail bar/restaurant setting. Various measurements are assessed including alcohol intake, alcohol craving, the stimulant and sedative effects of alcohol, and blood alcohol levels.
Finally, Dr. Margaret Rukstalis (AA00251) will evaluate the effects of naltrexone to alcohol responses as it relates to gender, menstrual cycle, and family history of alcohol dependence. Male and female social drinkers are stratified according to gender and family history of alcoholism. The female subjects are tested during luteal and follicular phases. Subjects are administered either 25 mg or 100 mg naltrexone or placebo followed by 0.8 g of alcohol per kg of body weight or placebo. Various measurements are performed including alcohol craving, blood alcohol levels, stimulant and sedative effects, mood, and several hormones (estradiol, progesterone, testosterone, and beta endorphin). Knowledge from this study should elucidate sex differences in alcoholism and how these may affect individual responsiveness to naltrexone.
Back to Top
B. Nalmefene
Since naltrexone has shown efficacy for alcoholism treatment, it is fundamental to determine if other opioid antagonists are also effective (or perhaps more effective) than naltrexone. Several investigators have expressed interest in nalmefene. This opioid antagonist appears to offer several pharmacological benefits over naltrexone. For example, in contrast to naltrexone, nalmefene presents no risk of liver toxicity. In addition, its oral bioavailability is higher, its antagonist action longer, and its affinity for the opioid subtype receptors greater.
Dr. Barbara Mason (AA09560) has completed a 12 week-trial of nalmefene. The results showed that nalmefene significantly curbed relapses to heavy drinking. Patients treated with nalmefene were 2.4 times less likely to relapse than those treated with placebo. No major adverse effects were observed. This is the first trial to demonstrate that an opioid antagonist other than naltrexone has been shown to be efficacious in reducing drinking in alcoholics.
Recently, Dr. Elie Nuwayser (AA82006) has begun a study to develop an injectable, sustained-released depot for nalmefene in an SBIR phase 1 study. After its development the depot will be evaluated for safety and efficacy. Advancement of a nalmefene depot would be potentially valuable to patients for whom compliance is problematic.
C. Acamprosate
Acamprosate has been studied extensively in Europe and is currently approved for alcoholism treatment in 29 countries. Interestingly, its mechanism of action remains unclear although several studies suggest it may modulate the activity of the glutamate system, unlike the action of naltrexone.
Dr. O'Malley (AA00171) is investigating the effect of acamprosate on drinking behavior. In her paradigm nontreatment seeking alcohol dependent subjects are randomly assigned to 1.5 g/day or 3.0 g/day acamprosate or placebo for 12 days. The first seven days is an outpatient drug stabilization period during which drinking can, of course, occur. On day 8 patients enter inpatient treatment and are withdrawn from alcohol for three days. On day 11 they are given a priming dose of alcohol (0.3 g %) and after 40 minutes the alcohol self-administration phase begins in which they can consume up to eight drinks over two hour. Patients are given $24 to purchase drink at $3 each. Intake, craving, blood alcohol levels, severity of withdrawal symptoms as well as subjective, cognitive, and neuroendocrine effects are measured. This study addresses several vital research questions. For example, does acamprosate attenuate the severity of acute alcohol withdrawal symptoms? Does it decrease response to alcohol? And finally, does acamprosate dose differentially reduce alcohol self-administration and craving, at least, when patients are paying for their own drinks? Since Dr. O'Malley has also studied naltrexone in a similar paradigm, this presents an excellent opportunity to compare the mechanisms of acamprosate and naltrexone responsible for reductions in drinking behavior.
As described earlier in the Naltrexone section, Project COMBINE (AA11721) plans to investigate acamprosate alone and in combination with naltrexone in alcohol dependent patients.
Finally, Dr. Suzette Evans (AA12599) is conducting a series of human laboratory paradigms with acamprosate and memantine. This study is described later in the Memantine section.
D. Ondansetron
The serotonergic system has been implicated in alcohol drinking behavior. In particular, the serotonin3 (5-HT3) receptor has been shown to regulate the release of dopamine in the mesolimbic area, particularly in the nucleus accumbens. It has been postulated that alcoholism is associated with reduced serotonergic function resulting in an upregulation of 5-HT3 receptors. Blocking these receptors with pharmacological agents can result in a decrease in alcohol-induced dopamine release and thus decrease alcohol-drinking behavior. Several animal studies have associated a reduction in alcohol intake with various 5-HT3 antagonists. Dr. Bankole Johnson (AA10522) is studying this effect in alcohol dependent humans in a 12-week trial of the 5-HT3-antagonist ondansetron. Patients are stratified into early onset alcoholics (EOA) and late onset alcoholics (LOA). (EOAs develop problem drinking during youth, experience severe behavioral problems, and have high familial incidence of alcoholism. LOAs develop drinking problems in adulthood and often as a consequence of psychosocial stressors.) Subjects are administered either 1, 4, or 16 ug ondansetron/kg body weight b.i.d. or placebo and receive a weekly cognitive behavioral therapy based on the treatment manual of Project MATCH. Initial findings show that EOAs treated with ondansetron drink on fewer days and consume less on even those days than do controls. The subjects receiving the 4 ug/ kg dose of ondansetron have the maximal effect. In contrast, there are no differences in drinking outcome between the LOA ondansetron and placebo groups. Tests to measure serotonergic function are well underway and the results will be considered in light of treatment outcome. It is hypothesized that depressed serotonergic function is characteristic of EOA subjects and accounts for the differential effect of ondansetron therapy.
- Sertraline
1. Non-Depressed Alcoholic Patients
Selective serotonin reuptake inhibitors (SSRIs) have shown considerable promise in reducing alcohol intake in animal models. However, their efficacy in humans has not been fully established. TRB has funded several well-designed trials to better understand the potential of these agents. Some of these trials have now been completed. In randomized double-blind, placebo-controlled trials of Kranzler (AA00143), Sullivan (AA09564), and Nurnberg (AA09549) fluoxetine and sertraline did not appear beneficial in reducing consumption by non-depressed alcohol dependent patients.
Dr. Pettinati (AA09544) has recently completed a 14-week trial of sertraline in depressed and non-expressed alcoholics. Patients received either 200 mg/day sertraline or placebo and weekly twelve-step facilitation therapy using the Project MATCH manual. In contrast to the findings of the studies above, the non-depressed patients treated with sertraline drank less often and consumed less alcohol than did the non-depressed placebo group. Interesting, the depressed sertraline group experienced no significant improvement in drinking outcome or depression over the comparable placebo group. When the subjects were re-classified as higher risk/severity type A alcoholics and lower risk/severity type B alcoholics, the data analyses presented unexpected findings. The type A subjects treated with sertraline reported fewer days drinking, were more likely to maintain abstinence, and were less likely to relapse to heavy drinking than the type A placebo group. In contrast, the sertraline-treated type B subjects actually drank more often than the placebo type B group. These results were similar to findings by Kranzler (AA0143). This was somewhat surprising since type B alcoholics were thought to be serotonergic deficient. Nonetheless, these studies emphasize the complexity of serotonergic function and their relation to alcohol drinking behavior.
2. Depressed Alcoholic Patients
Development of effective treatments for alcoholics with a comorbid psychiatric disorder is important for several reasons. First, these patients are more likely to seek treatment than non-comorbid alcoholics. In addition, they generally have poorer prognosis, higher risk for suicide, and are more likely to dropout from treatment. Research on pharmacological treatment of this population is in early stages. Many fundamental questions need to be addressed. For example, does treatment of either alcoholism or psychiatric syndrome improve outcome for the other disorder? Should pharamacologic treatment of alcoholics suffering psychiatric comorbidity differ from that offered to non-comorbid alcoholics? Does gender relate to choice of treatment for comorbid alcoholics? How should treatment for alcohol problems and psychiatric conditions be sequenced? Finally, should the chronology of onset of the psychiatric disorder influence outcome for either condition?
Several TRB-supported pharmacotherapy trials have been completed in depressed alcoholics. Mason (AA08111) and McGrath (AA08030) found that the tricyclic antidepressants imipramine and desipramine were effective in reducing the severity of depressive symptoms and, to a small extent, decreasing drinking in mild to moderate depressed outpatient alcoholics. Using similar populations of depressed alcoholics, McGrath (AA09539) and Pettinati (AA09544) reported that the SSRIs fluoxetine and sertraline were the comparable to placebo in improving depression and reducing drinking. In contrast, Cornelius and colleagues (AA09127) found fluoxetine effective in reducing depressive symptoms and alcohol intake in a severe inpatient population of alcoholics who suffered from major depression and were often suicidal. Finally, Dr. Darlene Moak (AA10476) is conducting a trial with depressed alcoholics testing the efficacy of sertraline. This 12-week study is scheduled for completion within the year. In this project subjects are administered either 200 mg/day sertraline or placebo and receive cognitive behavioral therapy based on the Project MATCH manual.
As is apparent from the preceding synopsis, no clear view on the value of SSRI in treating depressed alcoholics is yet available. It appears that antidepressants can improve depression and in at least limited conditions may also help to reduce drinking. Nonetheless, efficacy may be dependent on the type of medication used as well as on the type of patient. A reasonable strategy is to treat separately the alcoholism and the depression. Drs. Helen Pettinati (AA09544) and Barbara Mason (AA10518) are conducting trials to test this possibility. (See Naltrexone in Combination with Other Medications section.) In these two studies patients suffering from alcohol dependence and concurrent major depression are administered both naltrexone and sertraline. Results of these trials will likely have important implications in the treatment of this population of alcoholics.
Back to Top
F. Valproate
Bipolar Alcoholic Patients
Alcohol use disorder commonly occurs among patients suffering from severe mental illnesses. For example, over 42 percent of individuals with bipolar disorder satisfy lifetime criteria for alcohol abuse and dependence. This population is most difficult to treat, presenting a serious clinical challenge. Unfortunately, to date, little research has been performed on effective treatment interventions. Dr. Ihsan Salloum (AA10523) is performing the first pharmacological trial in this population. In this six-month study patients with diagnoses of alcohol dependence and co-occurring manic episode of bipolar disorder receive either valproate titrated to achieve a serum concentration of 50 to 100 mg/L or placebo and also are given the standard treatment intervention. This consists of lithium titrated to reach serum concentrations of 0.8 to 1.5 mmol/L and individual and family counseling interventions dealing with alcoholism and bipolar disease. This research represents the first step in developing effective treatment interventions for this important subpopulation of alcoholism.
G. Clozapine
Schizophrenic Alcoholic Patients
Alcohol use disorder is the most common comorbid condition in patients suffering from schizophrenia. Excessive drinking contributes significantly to overall morbidity in this population. Recent preliminary findings demonstrate that clozapine, an atypical antipsychotic, significantly reduces alcohol intake in dependent schizophrenic patients. In contrast, typical antipsychotic medications are much less effective. Dr. Alan Green (AA11904) has initiated a 12-week trial to evaluate the efficacy of clozapine with alcoholic, schizophrenic patients. Patients are administered either clozapine (titrated to 500 mg/day) or a typical antipsychotic medication being taken by the patient to date. In addition, all subjects receive a standardized psychosocial intervention consisting of substance abuse counseling, group therapy for dual diagnosis, and referral to self-help groups. It is hypothesized that clozapine acts directly to decrease alcohol drinking behavior through its interactions with the dopaminergic, adrenergic, and serotonergic systems. This timely study will advance our current knowledge of treatment in this field.
- Gabapentin
Alcoholics with Comorbid Insomnia
Sleep disturbances are common among alcohol dependent patients. This comorbidity is costly and is associated adverse consequences including daytime performance impairment, increased motor vehicle accidents, increased utilization of hospital and other health care services, and premature mortality. Unfortunately, little research exists regarding the treatment of this comorbidity. Dr. Kirk Brower (AA00304) has begun a trial of gabapentin, an anticonvulsant, in alcohol dependent patients suffering from comorbid insomnia. In a preliminary open trial gabapentin ameliorated the symptoms of insomnia in alcoholic patients. In this six-week study subjects are administered either gabapentin (titrated to 1500 mg/day) or placebo and receive the manual-guided Compliance Enhancement Therapy. This study is the first to test a pharmacological treatment in this population and will provide a foundation for future studies.
I. Methylphenidate
Children with Prenatal Alcohol Exposure
The effects of heavy prenatal alcohol exposure on the developing fetus are devastating because it results in significant CNS abnormalities and behavioral impairments. Unfortunately, little research exists on effective treatment interventions in this population. Dr. Sarah Mattson (AA12596) is investigating the effects of methylphenidate, a stimulant commonly used in the treatment of attention deficit-hyperactivity disorder (ADHD), in children with documented histories of prenatal alcohol exposure. Children between the ages of seven and 12 years with three or more symptoms of the ADHD receive either a predetermined dose methylphenidate or placebo for seven days in a randomized, double-blind, crossover design. Response to methylphenidate is determined by the Paired-Associated Learning Test, a paradigm used to assess learning and memory. In addition, other tests are performed dealing with neuropsychological, psychiatric, and physical (e.g., facial dysmorphology) variables. This information will be used to help predict which patients respond best to methylphenidate treatment.
J. Bupropion
Recovering Alcoholics with a Comorbid Nicotine Dependence
Dr. Richard Hurt (AA11219) is conducting a 52-week, double-blind, placebo-controlled trial of bupropion in recovering alcoholics (at least one year of continuous abstinence) who are current smokers (greater than 20 cigarettes/day). This study is described in the Alcohol and Tobacco chapter.
K. Transdermal Nicotine Replacement
Subjects with Co-Occurring Alcohol/Nicotine Disorders
The following investigators are conducting studies with nicotine replacement patches to evaluate the effects of nicotine treatment in alcohol treatment outcome (see Alcohol and Tobacco chapter): Dr. David Abrams (AA11211), Dr. Ned Cooney (AA11197), Dr. Andrea King (AA00276), Dr. Anne Joseph (AA11124), Dr. Barbara Mason (AA11210), and Dr. Joy Schmitz (AA11216).
L. Mecamylamine
Dr. Jeb Rose (AA11128) is conducting a laboratory study of mecamylamine, a nicotinic antagonist, and a clinical trial on the efficacy of combined nicotine/mecamylamine treatment with nicotine dependent drinkers (see Alcohol and Tobacco chapter).
M. Naloxone
Dr. Suchitra Krishnan-Sarin (AA11139) is conducting a human laboratory study to evaluate the ability of an opioid antagonist naloxone to precipitate withdrawal symptoms in male and female alcohol and nicotine dependent patients (see Alcohol and Tobacco chapter). This study will provide information on the involvement of the opioidergic system in alcohol and nicotine dependence.
N. Memantine
Glutamatergic activity, particularly at the N-methyl-D-aspartate (NMDA) site, most likely contributes to many of the pathophysiological effects of alcohol including intoxication, withdrawal, seizures, delirium tremens, and fetal alcohol syndrome. Recently, NMDA antagonists have been demonstrated to curb alcohol self-administration in animal models. Dr. Suzette Evans (AA12599) is testing memantine, a non-competitive NMDA antagonist, in a series of human laboratory paradigms as well as in a pilot clinical trial. The results will be compared with effects of acamprosate whose site of action is also at the NMDA receptor, possibly at the polyamine site. The different laboratory paradigms consist of the following: 1) effects of memantine and acamprosate on alcohol intoxication; 2) effects of the medication maintenance on alcohol self-administration and reactivity to alcohol-related cues; and 3) discriminative stimulus effects of alcohol under memantine and acamprosate maintenance. Finally, a small 12-week, placebo-controlled trial of mementine and acamprosate will be conducted to establish the validity of the laboratory studies and determine the effect size of memantine. This is the first attempt to systematically evaluate the therapeutic potential of glutamergic agents in humans.
O. Serotonergic, Noradrenergic, HPA, and NPY Agents
A successful medications program is dependent on a strong infrastructure system for drug development. Essential to this is the presence of predictive, reliable screening models for new medications. Testing only the medications that has a reasonable chance for FDA approval is essential in phases 2 and 3 of human clinical trials because of the length and expense of these projects. To strengthen this area, Drs. Barbara Mason and George Koob (AA12602) are developing animal and human screening models of protracted abstinence. Various promising agents will be tested including FG 5974, a 5-HT1A agonist/5-HT2A agonist; WAY-100635, a selective 5-HT1A antagonist; venlafaxine, a 5-HT/norepinephrine (NE) reuptake inhibitor; reboxetine, a NE reuptake inhibitor; antalarmine and D-Phe CRF 12-41, corticotrophin-releasing factor (CRF) antagonists; mifepristone and RU-38486, cortisol antagonists; and neuropeptide Y (NPY), p[Leu31, Pro 34] NPY, and NPY 13-36, all NPY agonists. This study will contribute to development of screening models, an essential component for a successful medications development program.
Back to Top
P. Kudzu
Herbal therapy has become a popular trend in the United States, although there have been few rigorous trials to evaluate their efficacy and safety. The kudzu plant (Pueraria lobata) was used by the Chinese 1400 years ago to treat alcohol intoxication. In recent times, several studies have demonstrated the effectiveness of crude extract of kudzu root and its isolated isoflavone components, NPI-031G (puerarin), NPI-031D (daidzin), and NPI-031E (daidzin), to reduce alcohol intake in animal models. Dr. Scott Lukas (AA10536) is now conducting a human laboratory study to measure the effects of kudzu on the pharmacokinetics of alcohol ingestion and on alcohol-induced physiological, subjective, and psychomotor responses. Preliminary results showed that pretreatment with kudzu reduces plasma levels of alcohol in light drinkers, but not in heavy drinkers. In addition, alcohol-induced subjective effects, such as feeling "high" and "drunk," are reduced with kudzu-treated light drinkers, but again not in kudzu-treated heavy drinkers. Finally, no adverse side effects were reported with the extract.
Dr. David Lee (AA82008) has completed an SBIR phase 1 study to isolate and characterize kudzu's active component NPI-031G (puerarin). After purifying over 250 g of NPI-031G under Good Manufacturing Practice (GMP) conditions, efficacy and toxicity experiments were conducted. NPI-031G reduced alcohol intake in rats at an optimal dose of 200 mg/kg. The compound attenuated drinking to the same extent as naltrexone in the alcohol-preferring (P) and high alcohol drinking (HAD) rats and was three times more potent than acamprosate in the Fawn-Hooded (FH) rats. Chronic dosage of NPI-031G produced a favorable toxicity profile, revealing no histopathological signs of liver damage. The group is planning to submit a phase 2 study for further toxicity, pharmacokinetic, and efficacy experiments, the results of which will be used to support an Investigational New Drug (IND) application for human testing.
Q. Summary and Future Possibilities
This paper has synopsized important advances made in medication development for alcoholism treatment. In particular, naltrexone and acamprosate have now been approved in many countries for alcoholism treatment. Studies are currently being conducted to determine the therapeutic potential and limitations of each alone and in combination. New knowledge is also being gained in the pharmacologic treatment of comorbid alcoholics. Although these new medications are helpful, they are clearly not a "magic bullet." The search for new medications continues to be a high priority of TRB. This initiative includes investigating medications that bind to subtype receptors in opioidergic, serotonergic, dopaminergic, GABAergic, and glutamatergic systems as well as developing drugs that alter various hormonal systems such as the hypothalamic-pituitary-adrenal axis. However, to maintain a successful drug discovery program for alcoholism treatment, research efforts must be applied beyond the neurotransmitter systems and their receptors to exploring new targets such as intracellular sites. For example, new medications might be developed to activate or block key regulatory sites of second messenger pathways, pathways altered by acute and chronic alcohol consumption. Another potential target for medications is the activation or suppression of genes that are associated with alcohol drinking behavior. As our science enters the next millenium, many exciting possibilities exist. As rigorous, competent researchers explore these possibilities, better treatment outcome for alcoholism should result, relieving the grievous suffering for alcohol dependent patients and their families.
Back to Top
Table 1
Pharmacotherapy Portfolio: Mechanism of Support
|
Type of Grant
|
No. of Grants |
$ Total Amount/Year |
|
R01 |
23 |
8,418,209 |
|
R29 |
3 |
318,693 |
|
U10 |
1 |
5,000,000 |
|
K01 |
1 |
121,266 |
|
K02 |
2 |
199,512 |
|
K08 |
1 |
124,576 |
|
K23 |
1 |
158,261 |
|
K24 |
1 |
108,972 |
|
N43 |
2 |
200,000 |
|
|
|
|
|
TOTAL |
35 |
14,649,489 |
Back to Top
Table 2
Pharmacotherapy Portfolio: Type of Medication
|
Type of Medication
|
No. of Grants |
$ Total Amount/Year |
|
Naltrexone |
18 |
10,157267 |
|
Nalmefene |
1 |
100,000 |
|
Acamprosate* |
1 |
105,705 |
|
Ondansetron |
1 |
634,168 |
|
Sertraline** |
1 |
94,296 |
|
Valproate |
1 |
111,584 |
|
Clozapine |
1 |
272,678 |
|
Gabapentin |
1 |
108,972 |
|
Methylphenidate |
1 |
140,410 |
|
Bupropion |
1 |
263,850 |
|
Nicotine Replacement*** |
4 |
1,486,137 |
|
Naloxone |
1 |
238,102 |
|
Memantine |
1 |
290,646 |
|
Serotonergic, Noradrenergic, HPA and NPY Agents |
1 |
439,957 |
|
Kudzu |
1 |
205,717 |
|
TOTAL |
35 |
14,649,489 |
*Project COMBINE and Mason's augmentation study of combined acamprosate and naltrexone are counted under Naltrexone
**Three studies of combined sertraline and naltrexone are counted under Naltrexone
***Three studies of combined naltrexone and nicotine replacement are counted under Naltrexone
#Pharmacotherapy grants investigating alcohol/nicotine disorders have been subtracted from the total
Table 3
Outline of Current Pharmacotherapy Portfolio
A. Naltrexone
1. Long-term Studies
- Nine-months
(Volpicelli - AA07517)
- 15-months
(Mason - AA10518)
2. Naltrexone in Combination with Behavioral-Psychosocial Intervention (s)
- Coping Skills Training and Cue Exposure vs.
Meditation/Relaxation/Education Treatment
(Monti - AA07850)
- Cognitive Behavioral vs. Motivational Enhancement Therapy
(Anton - AA09568)
- Medication Management (MM) vs. MM plus Compliance
Enhancement vs. MM plus Cognitive Behavioral Therapy
(Volpicelli - AA07517)
- Combined Behavioral Intervention vs. Medical Management
(Project COMBINE - AA11721)
3. Optimal Dosage of Naltrexone
- 50 mg vs. 100 mg
(McCaul - AA09569)
4. Patient Compliance
- Sustained-Release Naltrexone Depot
(Kranzler - AA00239)
(Nuwayser - AA82007)
5. Predicting Outcome with Naltrexone
- Opioid Activity and Receptor Binding
(McCaul - AA11855)
6. Naltrexone Treatment for Different Populations
- Early Problem Drinkers
(Kranzler - AA11062)
- Alcoholic Women with Eating Disorders
(O'Malley - AA10225)
- Alcoholics with a Cocaine/Heroin Dependence
(McCaul - AA09569)
- Alcoholics with a Nicotine Dependence
(Mason - AA11210)
(Schmitz - AA11216
(King - AA00276)
- Alaskan Natives
(O'Malley - AA12028)
7. Naltrexone in Combination with Other Medications
- Naltrexone and Sertraline
(Farren - AA11222)
(Pettinati - AA09544)
Mason - AA10518)
- Naltrexone and Acamprosate
(Project COMBINE - AA11721)
(Mason - AA10518)
8. Mechanism of Action
(Davidson - AA00273)
(Rukstalis - AA00251)
B. Nalmefene
Sustained-Release Nalmefene Depot
(Nuwayser - AA82006)
C. Acamprosate
- Clinical Trial
(Project COMBINE - AA11721)
- Mechanism of Action
(O'Malley - AA00171)
D. Ondansetron
Early Onset Alcoholics versus Late Onset Alcoholics
(Johnson - AA10522)
E. Sertraline
- Non-Depressed Alcoholic Patients
(Pettinati - AA09544)
- Depressed Alcoholic Patients
(Pettinati - AA09544)
(Moak - AA10476)
(Mason - AA10518)
F. Valproate
Bipolar Alcoholic Patients
(Salloum - AA10523)
G. Clozapine
Schizophrenic Alcoholic Patients
(Green - AA11904)
H. Gabapentin
Alcoholics with Comorbid Insomnia
(Brower - AA00304)
I. Methylphenidate
Children with Prenatal Alcohol Exposure
(Mattson - AA12596)
J. Bupropion
Recovering Alcoholics with Comorbid Nicotine Dependence
(Hurt - AA11219)
K. Transdermal Nicotine Replacement
Subjects with Co-Occurring Alcohol/Nicotine Disorders
(Abrams - AA11211)
(Cooney - AA11971)
(King - AA00276)
(Joseph - AA11124)
(Mason - AA11210)
(Schmitz - AA11216)
L. Mecamylamine
Human Laboratory Paradigm and Clinical Trial of Combined Mecamylamine and Nicotine
(Rose AA11128)
M. Naloxone
Opioidergic Involvement in Alcohol and Nicotine Dependence
(Krishnan-Sarin - AA11139)
N. Memantine
Human Laboratory Paradigms and Small Clinical Trial
(Evans - AA12599)
O. Serotonergic, Noradrenergic, HPA, and NPY Agents
Animal and Human Screening Models of Protracted Abstinence
(Mason - AA12602)
P. Kudzu
Human Laboratory Study
(Lukas - AA10536)
Back to Top
SELECTED TREATMENT TOPICS
TREATMENT OF ADOLESCENT ALCOHOL PROBLEMS
State of Knowledge (Sandra A. Brown, Ph.D.)
Alcohol use has increased in adolescents in recent years. Currently, one-third of seniors report having been drunk in the past 30 days and two-thirds 12th graders who report consuming alcohol indicate they have experienced at least one alcohol-related problem (O'Malley et al., 1998). Two of the most common alcohol-related problems reported by high school students are that alcohol caused them to behave in ways that they later regretted (approximately one-half of drinkers) and that alcohol use had interfered with the ability to think clearly (one-third of drinkers).
Epidemiological studies indicated that the diagnosis of alcohol use disorders increases from 3.5% at ages 14 to 16 to 14.6% by ages 17 to 20. Epidemiological (Lewensohn et al., 1996) and clinical studies (Langenbucher and Martin, 1996) have found that several dependence symptoms (tolerance, drinking larger amounts or for longer periods of time than intended, and reduced activities in favor of alcohol use) to be more common than abuse symptoms for adolescents. Withdrawal symptoms of adolescents vary significantly from adults and appear to be more predictive of long-term neurocognitive deficits than other drinking indices (Tapert and Brown, 1999). Specification of optimal diagnostic criteria across the adolescent age range has only recently been evaluated and has yet to be thoroughly examined in relation to stage of puberty development, gender and ethnic differences, topography of drinking patterns, prognostic significance for treatment outcome, and factors influencing treatment outcome (Martin and Winters, 1998).
Over 125,000 youth (less than 21 years) are treated in alcohol and drug treatment facilities in the U.S. annually, with approximately 7% of those treated under 18 years of age (Alcohol and Health, 1997). It appears that there is a substantial discrepancy between adolescent need for treatment services and service provision, and that youth more commonly receive treatment for alcohol problems outside specialized alcohol treatment programs. Although rates vary across service settings, more males than females enter adolescent alcohol and drug treatment programs. As with adults, it appears that a portion of adolescents with alcohol problems successfully stop or reduce their alcohol use without involvement in formal treatment.
Family-based interventions have been found to be as effective for adolescents with alcohol and drug problems as for adults (Stanton and Shadish, 1997). Multisystemic Therapy has been shown to be successful in reducing substance use and criminal behavior over four years for adolescent substance abusing delinquents. Over 90% of adolescent alcohol and drug treatment programs include Alcoholics Anonymous (AA), and it has been noted that treatment for alcohol-related problems is more successful for those adolescent who have regularly attended 12-Step, AA meetings (Brown, 1993). There are a number of new approaches being developed for the treatment of adolescent alcohol-related problems, including behavioral therapies, brief interventions, diversion programs, and pharmacotherapy. Epidemiological investigations suggest a "maturing out" for a portion of adolescents, and one prospective study reported that approximately 50% of a high-risk sample of adolescents (N = 122) aged 13-18 years followed over six years developed alcohol abuse, and that half of these youth resolved hazardous drinking and related problems for at least a two-year duration without formal treatment (Wagner et al., 1999).
In general, most recent studies have found that length of time in treatment and age (older) to be associated with better alcohol and drug outcomes, and poorer outcomes to be predicted by fewer perceived problems, more severe psychiatric symptoms, and early onset of disorder. Predictors of alcohol and drug use outcome of inpatient and outpatient adolescent programs tend to be similar. Brown et al. (1989) reported that exposure to alcohol in the immediate environment, peer influences, coping skills, behavioral intention/motivation for abstinence, self-esteem, and neurocognitive abilities to be associated with relapse. Alcohol abusing/dependent adolescents without other major forms of psychotherapy relapse almost exclusively in unsupervised social situations with few or no abstention models (Brown et al., 1989). Treatment fostered coping skills were found to be more predictive of outcome when intelligence, attention, or problem solving abilities were compromised (Tapert et al., 1999).
Clinical samples of adolescent alcohol abusers are remarkably heterogeneous with regard to concomitant substances of abuse and psychiatric comorbidity. The most common co-occurring psychopathology among alcohol and drug use disorder adolescents are internalizing disorders (anxiety and depression), conduct disorders, and attention deficits/hyperactivity disorder (Grilo et al., 1995). Gender differences exist both in use patterns, presenting symptoms, and needs for adolescent clients with alcohol problems. Epidemiological evidence indicates that substantially higher proportions of youth report multiple problems with alcohol than receive treatment (Wagner et al., 1999).
Specific recommendations:
- Encourage behavior change process studies of clinical and community samples to guide development of new approaches to treatment for adolescents.
- More efficacy studies for comorbid mental health disorders and alcohol use disorders should be conducted in adolescents.
TREATMENT FOR ALCOHOL-RELATED PROBLEMS
IN SPECIAL POPULATIONS
State of Knowledge (Edith S. Gomberg, Ph.D.)
It is recognized that within a specific special population there is significant heterogeneity arising from differences in country of origin, culture, age, education, income, degree of assimilation, social class, health status, religion, etc.
Women. When male and female alcoholics are compared, the following differences emerge: (1) women report more positive family history; (2) men begin drinking at a younger age, and onset of alcoholism is earlier; (3) women more frequently report a spouse/lover who is a heavy drinker; (4) men are more likely to drink in public places; (5) men are more likely to present an early history of impulsive, acting out behavior; (6) women report more marital disruption; and (7) women more frequently report dual diagnoses. Although women have more negative social consequences and more barriers to entering treatment, resulting in underrepresentation, they do not have a poorer response to treatment (Vannicelli, 1986).
Elderly. Increasing life expectancy will result in more elderly individuals with alcohol use disorders. Special populations will increase disproportionately, with more women than men and larger numbers of minorities. Although alcohol abuse and dependency may occur less frequently among older adults, moderate social usage of alcohol is greater than originally estimated (Adams et al., 1993). The second highest rate of hospitalization for individuals 65 and older is for alcohol-related disorders (Adams et al., 1993). Older age is not associated with poor treatment outcome, particularly in recent onset alcoholics, and age-specific treatment is encouraged (Atkinson, 1995). Improved outcome is associated with (1) age-specific group treatment with supportive approach, avoiding confrontation; (2) focus on negative emotional states and overcoming losses; (3) rebuild social support network, teaching appropriate skills; (4) employ staff, experienced and interested in working with elderly; (5) develop linkage with aging services, medical services, and institutional settings; and (6) alter pace/content for elderly (Dupree and Schonfeld, 1998).
Minorities. Literature on treatment for alcohol use disorders with minorities is sparse. Highest percentage of heavy alcohol use is by Mexican-Americans, other Hispanics, African Americans, and Native Americans (Caucasians ranked second). Alcohol dependence is highest for Native Americans, followed by Mexican-Americans, Puerto Ricans, and African Americans (SAMHSA, 1998).
Native Americans. There is much variability in alcohol use among tribes, with some tribes banning the use of intoxicating beverages. Alcohol abuse/dependency, however, is still a major medical/social problem for many tribes. Treatment outcome studies show poor results, presumably because of the cultural bias of existing interventions (Weibel-Orlando, 1985). Programs most successful are those that integrate a variety of spiritual elements and activities into their treatment strategies.
African-Americans. Ambivalence about alcohol in the U.S. is particularly high among blacks. Drinking patterns among blacks are dynamic and probably changing (Herd, 1989); in 1984, black and white men had similar drinking patterns while half the black women and only a third of white women were abstainers. Black adolescents drank less than white adolescents, but there was more heavy/problematic use among middle-aged blacks. Black culture is "familistic" (Gaines, 1985), and it is difficult for them to seek treatment from "strangers"; there is lack of trust and even danger. Black society is a collection of subgroups and subcultures, and this should be considered in research on treatment needs.
Asian Americans. Lower rates of alcohol use and alcoholism are observed in Asian Americans. Heterogeneity exists, with Chinese and Japanese having a higher proportion of drinkers than abstainers while the reverse is true for Koreans and Filipinos (Kitano and Chi, 1989). Very few Japanese Americans participate in alcohol treatment programs because drinking is often associated with social problems and is considered to be a private matter (Kitano et al., 1985).
Hispanic Americans. This heterogeneous group consists of Mexicans, Puerto Ricans, Dominicans, Guatemalans, etc., and each subgroup brings its own attitudes and customs relative to consuming alcoholic beverages (Randolph et al., 1998). Mexican Americans drink more than other subgroups and report more alcohol-related problems (Caetano, 1989). Quantity/frequency of drinking varies within the Hispanic American population by age, income, and education. There appears to be a maintenance of alcohol-related problems by men in their twenties and thirties that does not decline until their forties (Caetano, 1989). Hispanics rarely seek treatment in formal clinical settings.
Specific recommendations:
- There should be increased emphasis on alcohol and treatment research across the life span. Collaboration with other agencies, e.g., National Institute on Aging, is recommended.
- Research should be conducted into treatment for alcohol-related problems, integrating standard treatment modalities with minority groups' own cultural preferences.
Back to Top
TREATMENT FOR ALCOHOL AND SMOKING
State of Knowledge (Richard D. Hurt, M.D.)
Tobacco-related diseases account for 19% of all deaths in the United States, while alcoholism and other non-nicotine drug dependence account for another 6% (McGinnis and Foerge, 1993). The prevalence of smoking among alcoholics and substance abusers is two to three times that of the general population, and they are generally more addicted to nicotine than nonalcoholic smokers (Kozlowski et al., 1993). In alcoholics admitted to an inpatient addictions program, there was almost a three-fold increased risk of death in these patients compared to the general population (Hurt et al., 1996). Tobacco-related diseases accounted for 50.9% of all deaths while alcohol-related conditions accounted for 34.1% (Hurt et al., 1996).
Over three-quarters of alcoholic smokers entering an outpatient alcoholism treatment program may be willing to consider stopping smoking during or after alcoholism treatment (Ellingstad et al., 1999). Interest in stopping smoking is also high in alcoholism treatment professionals and patients in inpatient or residential alcoholism treatment programs. Among inpatient alcoholics receiving some type of behavioral intervention for smoking cessation, one-year stop rates ranged from 0% to 11% (Hurt et al., 1994). Therefore, low intensity interventions for nicotine dependence for patients undergoing alcoholism treatment are unlikely to yield high rates of abstinence from smoking (Bobo et al., 1998). There has been virtually no activity in treating nicotine dependence in adolescents, Native Americans or African Americans with alcoholism.
Though nicotine replacement therapy (gum, patches, nasal spray, and inhaler) has been available for many years, very few trials have been performed in alcoholics. Nicotine gum studies revealed that those with a history of alcoholism were less likely to be abstinent from smoking at one-year compared to those with no such history (7% vs. 19%) (Hughes, 1993). Abstinent alcoholics receiving a standard dose nicotine patch therapy achieved an initial stop rate of 46% following nicotine patch therapy, this dropped to 0% at one-year follow-up (Hurt et al., 1995). In both of these studies, recovering alcoholics had higher baseline (while smoking) blood cotinine levels and higher Fagerström Tolerance Questionnaire scores, indicating a higher level of nicotine dependence when compared to nonsmokers. Surprisingly, in smokers receiving nicotine patch therapy, abstinent alcoholics had more difficulty in stopping smoking than current alcoholics or non-alcoholics, with 6-month cessation rates of 15%, 25% and 28%, respectively (Hays et al., in press). Though nicotine replacement may be effective in alcoholics, underdosing with standard doses is likely to occur. High dose nicotine patch therapy appears to be safe, especially in heavy smokers, but improvement in cessation rates and withdrawal symptom relief have been mixed.
Bupropion has been reported to show efficacy for smoking cessation in a placebo-controlled, dose response trial (Hayford et al., 1999). In subjects with a past history of major depression or alcoholism, there was a significant dose response effect on smoking cessation that was independent of a history of depression or alcoholism (Hayford et al., 1999). Two recent reports show that nortriptyline has efficacy in helping smokers stop smoking, but it has not been tested in alcoholic smokers (Hall et al., 1998; Prochazka et al., 1998).
A growing number of studies indicate that smoking cessation does not adversely effect alcohol abstinence (Bobo et al., 1998), and some, but not all, studies have found that smoking cessation is associated with better alcohol use outcomes. Perhaps one of the more contentious issues over the past decade has been incorporating nicotine dependence treatment into the treatment of other addictions. There has been progress as many treatment units across the country have become smoke-free and have incorporated nicotine dependence treatment into the treatment milieu (Rustin, 1999). Staff attitudes surrounding this issue can be changed though it takes leadership on the part of the program administrators and the development of a plan well in advance of initiating a policy change to become a smoke-free unit and treating nicotine dependence like alcohol and other drugs of dependence.
Specific recommendations:
- Treatment for nicotine dependence should be incorporated into alcoholism treatment at all levels and for all age groups.
- It is important to refine and tailor behavioral therapy and pharmacotherapy, including relapse prevention, for treating active and recovering alcoholics.
NIAAA PORTFOLIO ON THE ROLE OF TOBACCO DEPENDENCE
IN THE TREATMENT OF ALCOHOLISM
(Joanne Fertig, Ph.D.)
During the past decade many lines of converging data have suggested that the links between alcohol and tobacco consumption are multiple and complex. Epidemiologic studies have established their association in terms of level of use, abuse and dependence. Heavy drinkers tend to be heavy smokers and vice versa. Developmental links also exist, with the two substances initiated in relative proximity and often in a particular sequence. Experimental studies have shown that consumption of alcohol provokes increased smoking while animal studies have pointed to activation of common neurobiologic mechanisms that mediate reward. Twin studies suggest genetic involvement in the covariation between use of alcohol and use of tobacco. Moreover, clinical studies have documented that as many as 90% of alcohol dependent individuals smoke more than thirty cigarettes per day greatly increasing the synergistic health risks of cancer and cardiovascular disease. In addition, as these studies have shown, alcoholics who smoke heavily have a much more difficult time with smoking cessation than do nonalcoholic smokers and are more likely to die of tobacco related diseases than from their alcoholism.
Although the links between alcohol and tobacco are well documented, satisfactory explanations for their association are few and before 1994 adequately validated treatment indications for alcoholic smokers were virtually nonexistent. To better understand the linkages and enhance the efficacy of treatment for nicotine addicted, alcohol dependent patients, NIAAA's Treatment Research Branch (TRB) implemented a series of initiatives to advance this field. Beginning in 1994 the Treatment Research Branch convened a series of interdisciplinary working groups to identify the salient issues and researchable topics in this area. Plans developed by these working groups culminated in an international meeting "Alcohol and Tobacco: From Basic Science to Policy." Ninety scientists and clinicians attended the meeting representing a wide variety of disciplines with expertise in nicotine and alcohol research. Their presentations reflected both the gaps in our knowledge and suggestions for new research strategies to systematically answer questions about the alcohol tobacco interaction. The presentations and provocative discussions from that meeting formed the basis for an NIAAA monograph published November 1995. The major research recommendations that emerged from the November conference were reflected in the suggested areas for research delineated in the RFA on the Role of Tobacco Dependence in Alcoholism Treatment issued as a TRB program announcement in 1997.
Current Portfolio
In response to the RFA and subsequent program announcement, eighteen alcohol and tobacco grants have been funded. The total FY99 cost of grants in the alcohol and tobacco portfolio is $3.8 million (Table 1). For FY99 14 grants are currently funded accounting for 26% of TRB's FY99 RPG budget. Eight of these grants are clinical studies/trials (total cost $2.8 million) while six are human laboratory studies that evaluate manipulatable variables such as pharmacologic challenges, cue reactivity and affect modulation (total cost $1 million). Two of these laboratory studies also have smaller clinical components.
Ten of the grants in the portfolio are RO1s, three are R29s and one is a KO8. It is interesting that more than 50% of the investigators currently funded in this portfolio are either first time investigators or first time grantees in the alcohol area. The primary source of new investigators has been researchers who traditionally have sought funding from NIDA and bring with them a wealth of smoking related expertise. These researchers have also brought a different methodological perspective in the behavioral pharmacology tradition. Before the smoking and alcohol initiatives human behavioral pharmacology studies were virtually non existent in our portfolio. This cross-fertilization of different methodological perspectives and disciplines has broadened and enriched this portfolio.
The research portfolio on the role of tobacco dependence in alcoholism treatment includes populations of alcoholics in active treatment, alcoholics who are twelve months abstinent as well as heavy social drinkers who do not meet dependence criteria. Transdermal nicotine replacement patches are being used in all the clinical studies. Alcohol and smoking outcomes are monitored in all clinical studies.
Clinical Studies
Of the ten currently funded clinical trials (or laboratory studies with clinical components) five are investigating the use of pharmacological adjuncts for smoking cessation to enhance treatment outcome. The compounds being studied are naltrexone, an opioid antagonist, buproprion, a monocyclic antidepressant with noradrenergic, serotonergic and dopaminergic activity, and mecamylamine, a nicotinic antagonist. Of the remaining clinical studies one is testing a mood management strategy for abstinent alcoholics with a history of major depression, a particularly treatment refractory population. The second grant seeks to determine if the sequencing of alcoholism and smoking cessation treatment should be simultaneous or sequential. Two clinical studies are evaluating the incremental efficacy of several smoking cessation treatments for patients in inpatient alcoholism treatment and a group entering outpatient treatment. The final clinical study examines the impact on alcohol and smoking treatment outcome following a brief course of smoking cessation advice or an intensive smoking cessation program. This clinical trial will be followed by two weeks of computerized self-monitoring allowing real time measurement of cravings and urges for alcohol and cigarettes in the natural environment.
Laboratory Studies
Projects primarily characterized as laboratory studies in the alcohol and tobacco portfolio are currently investigating the mechanisms underlying conjoint alcohol and tobacco use. One study is attempting to delineate the subjective, cardiovascular and behavioral interactions between nicotine and alcohol by using the nicotinic antagonist, mecamylamine as a pharmacologic probe. Another laboratory study seeks to establish a role for the endogenous opioid system in nicotine and alcohol dependence by evaluating the ability of an opioid antagonist, naloxone, to precipitate withdrawal symptoms similar to opiate withdrawal. Another laboratory study in this group is investigating the effects of the opioid antagonist naltrexone on smoking behavior and craving for cigarettes in a controlled laboratory session. The final study in this group is one that evaluates the putative neuroendocrine and platelet markers of early onset alcoholism to determine whether they are influenced by cigarette smoking.
The two additional grants in the portfolio are using nonpharmacologic methods to attempt to elucidate mechanisms of joint alcohol and tobacco use. In one study the investigators will manipulate cue reactivity parameters to determine whether, and the extent to which, smoking cues/nicotine withdrawal promote cravings for alcohol. The second grant will test the mechanisms underlying the separate and combined effects of cigarette smoking and alcohol consumption both on anxiety in experimentally stressed participants and on attention-processing capacity. The study will test two competing cognitively based theories that alcohol and nicotine are used concurrently (1) for their additive, attention mediated effects on stress reduction, and/or (2) because nicotine enhances attention capacity and compensates for processing capacity reductions induced by alcohol.
Preliminary Findings of Grants Funded in the Alcohol and Tobacco Portfolio
Fourteen of the eighteen grants funded to investigate the role of tobacco dependence in the treatment of alcoholism are still in progress and consequently have not been analyzed, however several investigators have reported preliminary results and these are discussed below. Although a plethora of epidemiological, clinical and laboratory evidence has shown a high correlation between cigarette smoking and alcohol use, the mechanisms underlying this association are not clear.
Nicotinic Influences on Alcohol Use and Dependence (AA11128)
A recently presented study investigated the subjective and behavioral interactions between nicotine, alcohol and the nicotinic antagonist mecamylamine. Forty-eight smokers who regularly consume alcoholic beverages participated in four laboratory sessions presenting a 2 (controlled dose of nicotine vs. de-nicotinized smoke) x 2 (oral mecamylamine hydrochloride 10 mg vs. placebo) x 2 (alcohol 0.5 g/kg vs. placebo) design, with alcohol as a between-subjects factor. Dependent measures included blood alcohol concentrations (BAC), as assessed by breathalyzer, subjective mood effects and ad lib smoking during a 2 h period. Peak BAC averaged 0.03 g/100 ml in the alcohol condition.
The investigators found that alcohol significantly potentiated nicotine reward as indexed by subjective questionnaires. This interaction was specific to nicotine as opposed to smoking in general, in that the response to nicotine-containing cigarettes was potentiated significantly more than the response to de-nicotinized cigarettes. Alcohol also potentiated liking and satisfaction ratings of nicotine containing vs. de-nicotinized cigarettes.
The mecamylamine manipulation revealed another intriguing interaction. As in previous studies of mecamylamine-induced compensatory smoking, the P.I. found that subjects increased ad lib smoking of nicotine-containing cigarettes in the mecamylamine vs. placebo conditions, but only if alcohol was not administered. In contrast, in the alcohol condition, mecamylamine produced no compensatory increase in smoking. Recent in vitro studies on the effects of alcohol on nicotinic receptor function may help account for these findings. For example, alcohol has been shown to stabilize the open-channel configuration of nicotinic receptors, which might account for the potentiation of nicotine effects, Additionally, preliminary data has shown that alcohol increases the affinity of mecamylamine for nicotinic receptors possibly explaining why subjects did not show compensatory changes in smoking behavior in the mecamylamine + alcohol condition, if the degree of nicotinic blockade was so complete that compensatory smoking was discouraged. These results not only help explain the behavioral link between alcohol consumption and cigarette smoking, but help point the way toward medication development to improve smoking cessation outcome, by more effectively blocking the reinforcing effects of nicotine that maintain tobacco addiction. Conversely, elucidation of common mechanisms of alcohol and nicotine effects could lead to alternative treatments for alcohol dependence.
Nicotine and Alcohol Dependence: Opioid Involvement (AA11139)
Starting from the assumption that nicotine and alcohol abstinence syndromes consist of signs and symptoms similar to those seen during abstinence from opiates, investigators employed the naloxone challenge test to establish a role for the endogenous opiates in nicotine and alcohol dependent subjects. Initial analyses of the non-alcoholic nicotine dependent subjects found that after being challenged with 0, .8, 1.6, or 3.2 mg/70 kg of naloxone, nicotine dependent subjects evidenced naloxone dose-dependent increases in withdrawal signs and symptoms. Lower doses of naloxone also produced increases in urges to smoke and demonstrated an attenuated cortisol release in response to challenge with naloxone.
In a preliminary analysis of six alcohol dependent smokers, three alcohol dependent non-smokers, seventeen non-alcohol dependent smokers and seventeen non-alcohol dependent, non-smoking controls the P.I. found that naloxone produced dose-related increases in measures of withdrawal as measured by the Clinical Institute Narcotic Assessment Scale and decreases in skin temperature in smokers compared with non-smokers. Additional analyses indicate that frequency and intensity of withdrawal symptoms varied across the three groups with non-alcoholic smokers experiencing more withdrawal than alcoholic smokers and both of these groups experiencing more withdrawal than control subjects. Thus while alcoholic smokers experienced more withdrawal symptoms when compared with controls, their symptoms were less than those seen in smokers who were not alcohol dependent. These data, while preliminary, suggest that endogenous opioid tone may be differentially altered by nicotine/alcohol consumption.
Clinically Relevant Markers of Alcoholic Subgroups (AA09735)
In an attempt to determine whether some of the trait markers of alcoholism might really be state markers of cigarette smoking. The investigators evaluated two putative trait markers of early-onset alcoholism 1) platelet monoamine oxidase B activity, and 2) prolactin response to a fenfluramine challenge, in subtypes of alcoholics and controls. Current smokers had decreased platelet MAO-B activity compared with non-smokers regardless of alcohol status. Smokers also had a blunted prolactin response to fenfluramine compared to nonsmokers regardless of whether or not they were alcoholic. These preliminary results demonstrate that some markers thought to be trait markers of early-onset alcoholism may in fact be state markers of cigarette smoking. Lowered platelet MAO-B activity and blunting of the response to fenfluramine challenge have traditionally been considered as indirect evidence of serotonergic dysfunction in alcoholic subtypes. However, few studies have controlled for cigarette smoking in alcoholics. Data from this study suggest that pharmacodynamic and pharmacokinetics factors related to cigarette smoking may be critical variables in the interpretation of serotonergic dysfunction in alcoholics.
Smoking, Alcoholics Reactivity, and Treatment Motivation (AA08734)
Although the prevalence of smoking among alcoholics seeking treatment ranges up to 97% little is known about the role tobacco may play in alcohol treatment recovery. Monti and colleagues (1995) randomly assigned male alcoholics in treatment to visual and olfactory exposure alcohol cues or control cues and then allowed them to smoke while continuing visual exposure to the same cues. Exposure to alcohol cues resulted in significantly greater self-reported urges to drink and smoke but had no significant effect on the topography of smoking behavior. When the investigators partialed out the variance due to urge to smoke, greater urge to drink correlated positively with number of cigarette puffs, providing support for a priming hypothesis of tobacco's role on alcoholism recovery.
Smoking Cessation in Recovering Alcoholics (AA9480)
In a recent study smokers with a past, but not current, history of alcohol dependence found nicotine to be a significantly more potent reinforcer than smokers without a past history of alcohol dependence. These results provide a potential behavioral mechanism to explain why smokers with a history of alcohol dependence are more nicotine dependent (smoke more cigarettes, smoke more heavily, are less likely to be successful at quitting) than non alcoholic smokers and provides a plausible explanation for why smokers with a history of alcoholism have been refractory to smoking cessation attempts. The findings also suggest that alcoholic smokers may be more successful at smoking cessation if they given higher doses of nicotine replacement medications.
Effectiveness of Mood Management Therapy for Alcohol Dependent Smokers with History of Depression (AA 11890)
A preliminary study provides the first evidence of a unique intervention to increase smoking abstinence in a group of treatment refractory alcoholics. Capitalizing on the fact that negative mood is the major precipitant of both smoking and alcohol relapse, the P.I. has developed a new and innovative treatment. By focusing on the management of negative mood, the investigators achieved smoking abstinence rates of 69% at the end of the treatment compared to 31% for those treated with only behavioral counseling. Of significant note is that these differences were sustained at the twelve month follow up with 46% of those treated with the negative mood management intervention still abstinent from smoking compared to only 12% of those treated with the standard behavioral counseling. Given the treatment refractory nature of this highly comorbid population and the fact that more alcoholics die from tobacco related diseases then from their alcoholism, replication of this pilot study will have major implications for the treatment community.
Future Directions
In 1994, despite what was known about alcohol and tobacco comorbidity, there was only one grant in the TRB portfolio investigating the mechanisms underlying concurrent alcohol and tobacco use. Since 1994 Institute resources committed to this area of research have increased dramatically and now constitute 26% of the Treatment Research Branch portfolio. Although most grants are still in progress and their completion will hopefully yield key information, there is still ample room for growth in this portfolio. One of the clearest gaps is the lack of research focusing on treatment interventions for the adolescent population during the period of initiation and early maintenance of use of both substances. Historically this may be due to the belief that adolescents did not suffer withdrawal and so would not meet dependence criteria. Intervention studies for smoking and alcohol in the youth population were conceptualized as primary or secondary prevention efforts and were developed within the framework of traditional school/community prevention models. Additionally, alcohol and tobacco adolescent intervention research has suffered from the prevailing dogma that cessation of smoking during the course of alcohol treatment would threaten sobriety. Recently some attempts have been made to study the effectiveness of brief intervention strategies for alcohol and tobacco with adolescents in the primary care arena. To date, however, no controlled treatment trials for alcohol and smoking cessation in comorbid adolescents have been funded by TRB. To encourage intervention studies for alcohol and tobacco abusing/dependent adolescents an RFA would be desirable. Such a solicitation should be narrowly focused and have as its goal developing recommendations for the concurrent treatment for both substances in an adolescent population.
Other gaps that research might address include:
Studies are needed to investigate the role of comorbidity or some other 'third factor' such as depression, in the initiation, maintenance and relapse of conjoint alcohol and tobacco use. Behavioral genetic analyses of twin registry data have suggested that heritability of alcohol and tobacco use/dependence may be strongly linked to depression. Although these findings are preliminary, the association may be especially compelling in females. Additionally, nicotine has been theorized to act as an antidepressant and the development of depressed affect during smoking cessation is a predictor of relapse to both substances.
Studies are needed that identify the optimal sequencing of alcohol and tobacco cessation in treatment programs. This is a critical question and one that is unlikely to be definitively settled by the single study currently in progress.
A growing number of studies indicate that smoking cessation does not adversely effect alcohol abstinence, and, may in fact improve outcomes for alcohol dependent individuals. Despite this fact the dogma persists among patients and addiction treatment staff that stopping smoking is more difficult and may actually be harmful. Studies are needed to develop effective methods of educating clinical/treatment staff about the critical importance of a smoking and alcohol cessation model and clear guidelines need to be established for their implementation.
Studies are also needed which test a broader range/dose of medication adjuncts. In addition to testing single medications, combinations of medications targeting multiple neurotransmitter systems need to be evaluated. The model here might be similar to that used in treating hypertension or asthma where different pharmaceutical strategies are implemented in relation to disease severity and stage. Additionally, dose finding/dose ranging studies need to be conducted so that clear recommendations about optimal dosing regiments are available to clinicians. Further human laboratory studies employing the methodologies of behavioral pharmacology are needed to develop and test models of alcohol and tobacco interaction.
Back to Top
Table 1
|
Alcohol and Tobacco Grants
|
|
By Category |
Number |
FY99 Total Dollars (millions) |
|
Pharmacologic |
6 |
$ 1.7 |
|
Non-Phamacologic |
8 |
2.1 |
|
Grant Type |
|
|
|
Clinical |
8 |
$ 2.8 |
|
Laboratory |
6 |
1.0 |
|
Mechanism |
|
|
|
R01 |
10 |
$3.4 |
|
R29 |
3 |
.28 |
|
K08 |
1 |
.12 |
ALCOHOL AND COMORBIDITY
State of Knowledge (Jack R. Cornelius, M.D., M.P.H.)
Comorbid psychiatric disorders and substance abuse disorders are common among alcoholics (Regier et al., 1990; Kessler et al., 1994) and often predict a shorter time to relapse to alcoholic behavior (Greenfield et al., 1998). Despite the prevalence and adverse effects of comorbidity, few controlled treatment studies had been conducted involving dual-diagnosis samples (Litten and Allen, 1999), with most studies restricted to alcoholics with comorbid major depression or anxiety disorders (Litten and Allen, 1995). The results of these studies suggest efficacy for selective serotonin reuptake inhibitors and tricyclic antidepressants for treating alcoholics with major depression and buspirone for treating alcoholics with anxiety disorders (Mason et al., 1996; Cornelius et al., 1997; Kranzler et al., 1994). Controlled treatment studies involving alcoholics with other comorbid disorders are almost totally lacking, resulting in no empirically proven treatment for alcoholics with other comorbid disorders.
Few of the treatment studies funded by NIAAA have involved alcoholics with comorbid disorders (Litten and Allen, 1998). Controlled studies involving comorbid disorders in special populations are almost entirely lacking, such as those involving adolescent or geriatric patients (Vitiello and Jensen, 1997). Little work has been done to clarify the optimal dose, duration of treatment, or sequence of treatment for various comorbid conditions in alcoholics (Litten and Allen, 1999). Longer-term studies, combination medication studies, and studies evaluating the optimal combination of pharmacotherapy and psychotherapy are scarce (Project MATCH Research Group, 1998; Cornelius et al., in press; Salloum et al., 1998; O'Malley et al., 1996). Moreover, little research has been conducted to evaluate predictors of treatment response, subtypes of dual disorders, and matching of dual-disorder patients to optimal treatment (Litten and Allen, 1998). Effectiveness trials (as opposed to efficacy trials) are scarce, as are studies conducted in conjunction with basic sciences studies in order to clarify the biological mechanisms underlying both disorders and underlying treatment response (Brady et al., 1999). Few studies have been conducted to clarify how treatment of either the alcoholism or the psychiatric disorder of dual-disorder patients affects the outcome of the other disorder (Litten and Allen, 1999).
Specific recommendations:
- Double-blind, placebo-controlled pharmacotherapy studies in alcoholics with specific comorbid disorders, e.g., dysthymia, post-traumatic stress disorder, biopolar I and II disorders, schizophrenia and other psychotic disorders, substance abuse/dependence, attention deficit hyperactivity disorder, trimorbid samples, suicidal risk, and nondependent alcohol-abusing patients with comorbid conditions.
- Pharmacotherapy studies in adolescent and geriatric alcoholics with comorbid disorders.
SELF-HELP GROUPS
State of Knowledge (Keith Humphreys, Ph.D.)
Self-help organizations (e.g., Alcoholics Anonymous, Moderation Management) compose the most commonly accessed sector of the de facto system of care for alcohol problems in the U. S. (Weisner et al., 1995). Moreover, concepts and approaches from 12-step self-help organizations have strongly influenced a significant number of professional treatment programs. Nevertheless, the number of longitudinal, comparative outcome studies on alcohol-related self-help groups and self-help-influenced treatments is not large, which impacts on both the certainty and scope of conclusions that can be drawn about these interventions.
Research to date suggests that involvement with community-based Alcoholics Anonymous is associated with the following results.
- Generally produces significant reductions in substance abuse and psychiatric problems, with effect sizes from "small" to "medium" (Tonigan et al., 1996). However, most of the AA research underlying these conclusions was correlational.
- Under conditions of close monitoring and substantial coercion, AA combined with inpatient treatment leads to fewer relapses than AA alone (Walsh et al., 1991), and, being ordered to AA does not reduce likelihood of future arrest for alcohol-related offenses (Ditman et al., 1967).
- AA involvement predicts reduced alcohol-related health care expenditures (Walsh et al., 1991; Humphreys and Moos, 1996).
- Enhanced coping and commitment to abstinence, increased self-efficacy and appraisal of substances as harmful, and improved friendship networks are part of the causal process through which AA involvement affects drinking behavior (Humphreys et al., 1999).
Involvement with 12-step oriented professional treatment is associated with the following results.
- Professional interventions can substantially increase patients' attendance at 12-step self-help group meetings (Tonigan et al., in press).
- Twelve-step oriented treatments generally produce outcomes comparable to those of cognitive-behavioral treatments, and have a slight advantage for abstinence outcomes (MATCH, 1998; Moos et al., 1999).
- Self-help oriented treatments are probably more cost-effective than otherwise similar non-self-help oriented treatments (Humphreys and Moos, 1999).
- Cognitive-behavioral and 12-step treatments share some change processes. At the same time, combining cognitive-behavioral treatments with AA/NA affiliation may be less helpful to patients than combining 12-step treatment with AA/NA affiliation, perhaps because consistency of overarching philosophy facilitates patients' learning of new ways of thinking and behaving (Humphreys et al., 1999).
- Identifying patient-treatment matches for 12-step and other treatments is very difficult even in large, well-designed studies. Hence, patient-treatment-matching effects may be too small or unstable to be of substantial use in real-world clinical practice.
Specific recommendations:
- Determine the long- and short-term clinical outcomes and health-care cost consequences of brief interventions (e.g., 1-2 sessions) designed to facilitate affiliation with AA.
- Determine the maximally effective combination of outpatient treatment and self-help group involvement. For example, is 12-step professional counseling combined with AA attendance more or less effective than (a) cognitive-behavioral therapy combined with AA attendance, or (b) cognitive-behavioral therapy combined with a cognitive-behavioral self-help group (e.g., Moderation Management, SMART recovery)?
NATURAL RESOLUTION OF ALCOHOL-RELATED PROBLEMS
State of Knowledge (Jalie A. Tucker, Ph.D., M.P.H.)
Research on natural resolution (renamed from spontaneous remission in recognition of its common and predictable nature) increases understanding of the drinking behavior change process and enables development of interventions that support resolution to the under-served majority of problem drinkers who do not seek help.
Most studies are either: (1) large sample surveys of the incidence and prevalence of natural resolution; or (2) smaller sample studies that investigated more intensively the circumstances and processes associated with natural resolution (Blomqvist, 1996).
Surveys have yielded six robust findings:
- seeking help for drinking problems is uncommon; <20% of those in need do so (Marlatt et al., 1997);
- most resolutions occur without interventions (Sobell et al., 1996);
- among resolved problem drinkers, abstinent and moderation outcomes are proportionately higher in treated and untreated samples, respectively (Sobell et al., 1996);
- treatment samples tend to have more serious psychosocial and health problems than natural resolution samples, but natural resolutions occur even among individuals with alcohol dependence (Dawson, 1996);
- alcohol use and associated problems decrease with age, independent of help-seeking (Fillmore, 1988);
- natural resolution process is influenced by surrounding environmental contexts (Robins, 1993).
Process-oriented studies have provided more detail about the contexts that surround natural resolution, behavior processes involved, and extent to which these features are shared with intervention-assisted resolutions. This research has shown:
- natural resolutions often are preceded by increased negative events and followed by increased positive events during the first year or so of maintenance (Blomqvist, 1999);
- interventions often help consolidate, rather than initiate, changes in drinking and make positive contributions to the change process during maintenance (Tucker, 1995);
- drinking pathways to natural resolution are variable, including (a) abruptly initiated, continuous abstinence; (b) abstinence followed by resumption of moderate drinking; and (c) gradual reductions in problem drinking followed by sustained moderate drinking (Sobell et al., 1992);
- problem drinkers who resolved naturally generally recognized they had problems, but concerns about interventions deterred help-seeking (Marlatt et al., 1997).
Specific recommendations:
- Emphasize studies on environmental contexts supporting positive behavior change and the variable processes involved in resolutions achieved with and without interventions.
- Increase the number of studies of help-seeking patterns and processes for alcohol problems and of how influences on help-seeking may differ from influences on behavior change.
Back to Top
REFERENCES
Adams WL, Yuan Z, Barboriak JJ, Rimm AA: Alcohol related hospitalization of elderly people. JAMA 1993;270: 1222-1225.
Alcohol and Health: Ninth Special Report to the U.S. Congress by NIAAA, 1997.
Allen JP, Litten RZ, Anton R: Measures of alcohol consumption in perspective. In: Litten RZ, Allen JP (eds.), Measuring Alcohol Consumption: Psychosocial and Biochemical Methods. Totowa, NJ, Humana, 1992, pp. 205-226.
Atkinson RM: Treatment programs for aging alcoholics. In: Beresford TP, Gomberg ESL (eds.), Alcohol and Aging. New York, Oxford University Press, 1995, pp. 186-210.
Blomqvist J: Recovery with and without treatment: A comparison of resolutions of alcohol and drug problems. Presented at the International Conference on Natural History of Addictions: Recovery from Alcohol, Tobacco, and Other Drug Problems without Treatment. Les Diablerats, Switzerland, 1999.
Blomqvist J: Paths to recovery from substance misuse: Change of lifestyle and the role of treatment. Substance Use Misuse 1996;31: 1807-1852.
Bobo JK, McIlvain HE, Lando HA, et. al.: Effect of smoking cessation counseling on recovery from alcoholism: Findings from a randomized community intervention trial. Addiction 1998;93: 877-887.
Brady KT, Halligan P, Malcolm RJ: Dual diagnosis. In: Galanter M, Kleber HD (eds.), Textbook of Substance Abuse Treatment, Second Edition. Washington, DC, American Psychiatric Press, 1999, pp. 475-483.
Brown SA: Recovery patterns in adolescent substance abuse. In: Baer JS (ed.), Addictive Behaviors Across the Life Span: Prevention, Treatment, and Policy Issues. Sage Publ., Newbury Park, CA, 1993, pp. 161-183.
Brown SA, Vik PW, Creamer VA: Characteristics of relapse following adolescent substance abuse treatment. Addict Beh 1989;14: 291-300.
Caetano R: Drinking patterns and alcohol problems in a national sample of U.S. Hispanics. In: Alcohol Use among U.S. Ethnic Minorities. NIAAA, 1989, pp. 147-162.
Chung T, Colby S, Barnett NP, et. al.: Screening adolescents for problem drinking: Performance of brief screens against DSM-IV alcohol diagnoses. J Stud Alcohol, in press.
Connors GJ, Allen JP, Cooney NL, et. al.: Assessment issues and strategies in alcoholism treatment matching research. J Stud Alcohol (Suppl) 1994;12: 92-100.
Cornelius JR, Salloum IM, Ehler JG, et. al.: Fluoxetine in depressed alcoholics: A double-blind, placebo-controlled trial. Arch Gen Psychiatry 1997;54: 700-705.
Crits-Christoph P: Met-analysis of therapist effects in psychotherapy outcome studies. Psychotherapy Res 1991;1: 81-91.
Dawson DA: Correlates of past-year status among treated and untreated persons with former alcohol dependence: United States, 1992. Alcohol: Clin Exp Res 1996;20: 771-779.
Ditman KS, Crawford GG, Forgy EW, et. al.: A controlled experiment on the use of court probation for drunk arrests. Am J Psychiatry 1967;124: 64-67.
Dupree LW, Schonfeld L: Older alcohol abusers: Recurring treatment issues. In: Alcohol Problems and Aging. NIAAA, 1998, pp. 339-358.
Ellingstad TP, Sobell LC, Sobell MB, et. al.: Alcohol abusers who want to quit smoking: Implications for clinical treatment. Drug Alcohol Dep 1999;54: 259-265.
Elman BD, Dowd ET: Correlates of burnout in inpatient substance abuse treatment therapists. J Addict Offend Counsel 1997;17: 56-65.
Epstein EE, McCrady BS: Behavioral couples treatment of alcohol and drug use disorders: Current status and innovations. Clin Psychol Rev 1998;18: 689-711.
Fillmore KM: Alcohol Use across the Life Course: A Critical Review of 70 Years of International Longitudinal Research. Addiction Research Foundation, Toronto, Ontario, 1988.
Fleming MF, Mundt MP, French MT et. al.: Benefit-cost analysis of brief physician advice with problem drinkers in primary care settings. Med Care 1999, in press.:
Fleming MF, Barry KL, Manwell LB, et. al.: Brief physician advice for problem alcohol drinkers: A randomized controlled trial in community-based primary care practices. JAMA 1997;277: 1039-1045.
Gaines AD: Alcohol cultural conceptions and social behavior among urban blacks. In: Bennet LA, Ames GM (eds.), The American Experience with Alcohol. New York, Plenum Press, 1985, pp. 171-197.
Galanter M: Network therapy for addictions: A model for office practice. Am J Psychiatry 1993;150: 28-36.
Gentilello LM, Rivara FP, Donovan DM, et. al.: Alcohol interventions in a trauma center as a means of reducing the risk of injury recurrence. Ann Surg 1999;230.
Gerra G, Caccavari R, Delsignore R, et. al.: Effects of fluoxetine and Ca-acetyl-homotaurinate on alcohol intake in familial and nonfamilial alcohol patients. Cur Therapeutic Res 1992;52: 291-295.
Greenfield SF, Weiss RD, Muenz LR, et. al.: The effect of depression on return to drinking. Arch Gen Psychiatry 1998;55: 259-265.
Grilo CM, Becker DF, Walker ML, et. al.: Psychiatric comorbidity in adolescent inpatients with substance use disorders. J Am Acad Child Adolesc Psychiatry 1995;34: 1085-1091.
Hall SM, Reus VI, Munoz RF, et. al.: Nortriptyline and cognitive-behavioral therapy in the treatment of cigarette smoking. Arch Gen Psychiatry 1998;55: 683-690.
Hays JT, Offord KP, Croghan IT, et. al.: Smoking cessation rates in active and recovering alcoholics treated for nicotine dependence. Annals Beh Med, in press.
Herd D: The epidemiology of drinking patterns and alcohol-related problems among U.S. Blacks. In: Alcohol Use among U.S. Ethnic Minorities. NIAAA, 1989, pp. 3-50.
Hersh D, Van Kirk JR, Kranzler HR: Naltrexone treatment of comorbid alcohol and cocaine use disorders. Psychopharmacology 1998;139: 44-52.
Hughes JR: Treatment of smoking cessation in smokers with past alcohol/drug problems. J Sub Abuse Treat 1993;10: 181-187.
Humphreys K: Clinicians' referral and matching of substance abuse patients to self-help groups after treatment. Psychiatric Services 1997;48: 1445-1449.
Humphreys K, Klaw E, Moggi F: Natural recovery by problem drinkers in a self-help group. Presentation at a meeting of the Kettill Bruun Society for Alcohol Epidemiology, Les Diablerets, Switzerland, 1999.
Humphreys K, Mankowski E, Moos RH, Finney JW: Do enhanced friendship networks and active coping mediate the effect of self-help groups on substance use? Annals Beh Med 1999;21: 54-60.
Humphreys K, Moos R: Reduced substance abuse-related health care costs among voluntary participants in Alcoholics Anonymous. Psychiatric Services 1996;47: 709-713.
Humphreys K, Moos R: Can encouraging substance abuse inpatients to participate in self-help groups reduce the demand for outpatient aftercare: A quasi-experimental study. 1999, submitted.
Humphreys K, Moos RH, Cohen C: Social and community resources and long-term recovery from treated and untreated alcoholism. J Stud Alcohol 1997;58: 231-238.
Hurt RD, Dale LC, Offord KP, et. al.: Nicotine patch therapy for smoking cessation in recovering alcoholics. Addiction 1995;90: 1541-1546.
Hurt RD, Eberman KM, Croghan IT: Nicotine dependence treatment during inpatient treatment for other addictions: A prospective intervention trial. Alcohol: Clin Exptl Res 1994:18: 867-872.
Hurt RD, Offord KP, Croghan IT, et. al.: Mortality following inpatient addictions treatment: Role of tobacco use in a community-based cohort. JAMA 1996;275: 1097-1103.
Israel Y, Hollander O, Sanchez-Craig M: Screening for problem drinking and counseling by the primary care physician-nurse team. Alcohol Clin Exp Res 1996;20: 1443-1450.
Kessler RC, McGonagle KA, Zhao S, et. al.: Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States: Results from the National Comorbidity Survey. Arch Gen Psychiatry 1994;51: 8-19.
Kitano HHL, Chi I: Asian Americans and alcohol: The Chinese, Japanese, Koreans and Filipinos in Los Angeles. In: Alcohol Use among U.S. Ethnic Minorities. NIAAA, 1989, pp. 375-382.
Kitano HHL, Hatanaka H, Young W, Sue S: Japanese-American drinking patterns. In: Bennett LA, Ames GM (eds.), The American Experience with Alcohol. New York, Plenum Press, 1985, pp. 335-358.
Kozlowski LT, Henningfield JE, Keenan RM, et. al.: Patterns of alcohol, cigarette, and caffeine and other drug use in two drug abusing populations. J Sub Abuse Treat 1993;10: 171-179.
Kranzler HR, Anton RF: Implications of recent neuropsychopharmacologic research for understanding the etiology and development of alcoholism. J Consult Clin Psychol 1994;62: 1116-1126.
Kranzler HR, Burleson JA, Del Boca FK, et. al.: Buspirone treatment of anxious alcoholics. Arch Gen Psychiatry 1994;51: 720-731.
Kranzler HR, Modesto-Lowe V, Van Kirk J: Placebo-controlled trial of naltrexone vs. nefazodone for treatment of alcohol dependence. Neuropsychopharmacology, in press.
Langenbucher JW, Martin CS: Alcohol abuse: Adding content to category. Alcohol: Clin Exptl Res 1996;20(Suppl): 270A-275A.
Lewinsohn PM, Rohde P, Seeley JR: Alcohol consumption in high school adolescents: Frequency of use and dimensional structure of associated problems. Addiction 1996;91: 375-390.
Litt MD, Babor F, DelBoca FK, et. al.: Types of alcoholics, II: Application of empirically derived typology to treatment matching. Arch Gen Psychiatry 1992;49: 609-614.
Litten RZ, Allen JP: Pharmacotherapy for alcoholics with collateral depression or anxiety: An update of research findings. Exp Clin Psychopharmacology 1995;3: 87-93.
Litten RZ, Allen JP: Pharmacologic treatment of alcoholics with collateral depression: Issues and future directions. Psychopharmacology Bull 1998;34: 107-110.
Litten RZ, Allen JP: Medications for alcohol, illicit drug, and tobacco dependence: An update of research findings. J Sub Abuse Treatment 1999;16: 105-112.
Longabaugh R, Clifford PR, Wirtz PW: Behavioral relationship enhancement for alcoholics: Boundaries of effectiveness. Annual Meeting American Psychological Association, New York, NY, 1995.
Marlatt GA, Tucker JA, Donovan DM, Vuchinich RE: Help-seeking by substance abusers: The role of harm reduction and behavioral-economic approaches to facilitate treatment entry and retention. In: Onken LS, Blaine JD, Boren JJ (eds.), Beyond the Therapeutic Alliance: Keeping the Drug-Dependent Individual in Treatment. NIDA Monograph No. 165, pp. 44-84, 1997.
Martin CS, Winters KC: Diagnosis and assessment of alcohol use disorders among adolescents. Alcohol Health Res World 1998;22: 95-105.
Mason BJ, Salvato FR, Williams LD, et. al.: A double-blind, placebo-controlled study of oral nalmefene for alcohol dependence. Arch Gen Psychiatry 1999;56: 719-724.
Mason BJ, Kocsis JH, Ritvo CE, Cutler RB: A double-blind, placebo-controlled trial of desipramine for primary alcohol dependence stratified on the presence or absence of major depression. JAMA 1996;275: 761-767.
McCrady BS: Outcomes of family-involved alcoholism treatment. In: Galanter M (ed.), Recent Developments in Alcoholism, Vol. 7. New York, Plenum Press, 1989, pp. 165-182.
McCrady BS, Epstein EE: Marital therapy in the treatment of alcoholism. In: Gurman AS, Jacobson N (eds.), Clinical Handbook of Marital Therapy. New York, Guilford Press, 1995, pp. 369-393.
McGinnis JM, Foerge WH: Actual causes of death in the United States. JAMA 1993;270: 2207.
McLellan AT, Woody GE, Luborsky L, Goehl l: Is the counselor an "active ingredient" in substance abuse rehabilitation? An examination of treatment success among four counselors. J Nerv Men Dis 1988;176: 423-430.
Miller WR, et. al.: What works? A methodological analysis of the alcohol treatment outcome literature. In: Hester RK, Miller WR (eds.), Handbook of Alcoholism Treatment Approaches: Effective Alternatives. pp. 12-44, 1995.
Miller, WR, Meyers RJ, Tonigan JS: Engaging the unmotivated in treatment for alcohol problems: A comparison of three strategies for intervention through family members. J Consult Clin Psychol 1999;67: 688-697.
Monti PM, Colby SM, Barnett NP, et. al.: Brief intervention for harm reduction with alcohol-positive older adolescents in a hospital emergency department. J Consult Clin Psychol 1999;67: 989-994.
Monti PM, Rohsenow DJ, Colby SM, Abrams DB: Smoking among alcoholics during and after treatment: Implications for Models, Treatment Strategies, and Policy. In: Fertig JB, Allen JP (Eds.), Alcohol and Tobacco: From Basic Science to Clinical Practice. NIAAA Research Monograph No. 30, pp. 187-206, 1995.
Monti PM, Rohsenow DJ, Hutchison KE: Toward bridging the gap between biological, psychobiological and psychosocial models of craving. Addiction, in press.
Moos RH, Finney JW, Ouimette PC, Suchinsky RT: A comparative evaluation of substance abuse treatment: I. Treatment orientation, amount of care, and 1-year outcomes. Alcohol: Clin Exp Res 1999;23: 529-536.
Najavits LM, Weiss RD: Variations in therapist effectiveness in the treatment of patients with substance use disorders: An empirical review. Addiction 1994;89: 679-688.
Nirenberg TD, Maisto SA: The relationship between assessment and alcohol treatment. Int J Addict 1990;25: 1275-1285.
O'Malley P, Johnston LD, Bachman JG: Alcohol use among adolescents. Alcohol Health Res World 1998;22: 85-93.
O'Malley SS, Carroll KM: Psychotherapeutic considerations in pharmacological trials. Alcohol: Clin Exp Res 1996;20: 17A-22A.
Pettinati HM, Volpicelli JR, Luck G, et. al.: Sertraline treatment of alcoholism: Interactive effects of medication and alcohol subtype. Submitted.
Prochazka AV, Weaver MJ, Keller RT, et. al.: A randomized trial of nortriptyline for smoking cessation. Arch Int Med 1998;158: 2035-2039.
Project MATCH Research Group: Matching alcoholism treatment to client heterogeneity: Project MATCH three-year drinking outcomes. Alcohol: Clin Exp Res 1998;22: 1300-1311.
Randolph WM, Stroup-Benham C, Black SA, Markides KS: Alcohol use among Cuban-Americans, Mexican-Americans and Puerto Ricans. Alcohol Health Res World 1998;22: 265-269.
Regier DA, Farmer ME, Rae DE, et. al.: Comorbidity of mental disorders with alcohol and other drug abuse: Results from the Epidemiologic Catchment Area (ECA) study. JAMA 1990;264: 2511-2518.
Robins LN: Vietnam veterans' rapid recovery from heroin addiction: A fluke or normal expectation? Addiction 1993;88: 1041-1054.
Roth A, Fonagy P: Alcohol dependency and abuse. In: Roth A, Fonagy P (eds.). What Works for Whom? A Critical Review of Psychotherapy Research. New York, Guilford Press, pp. 216-233, 1996.
Roussaux JP, Hers D, Ferauge M: Does acamprosate diminish the appetite for alcohol in weaned alcoholics. J Pharmacie Belgique 1996;51: 65-68.
Rustin TA: Incorporating nicotine dependence into addiction treatment. J Addict Dis 1998;17: 83-108.
Salloum IM, Cornelius JR, Thase ME, et. al.: Naltrexone utility in depressed alcoholics. Psychopharmacology Bull 1998;34: 111-115.
SAMHSA: Prevalence of Substance Use among Racial and Ethnic Subgroups in the United States, 1991-1993. 1998.
Sobell LC, Cunningham JA, Sobell MB: Recovery from alcohol problems with and without treatment: Prevalence in two population surveys. Am J Public Health 1996;86: 966-972.
Sobell LC, Sobell MB, Toneatto T: Recovery from alcohol problems without treatment. In: Heather N, Miller WR, Greeley J (eds.), Self-Control and Addictive Behaviors. New York, Maxwell MacMillan, 1992, pp. 192-242.
Stanton MD, Shadish WR: Outcome, attrition, and family-couples treatment for drug abuse: A meta-analysis and review of the controlled, comparative studies. Psychol Bull 1997;122: 170-191.
Sterling RC, Gottheil E, Weinstein SP, Serota R: Therapist/patient race and sex matching: Treatment retention and 9-month follow-up outcome. Addiction 1998;93: 1043-1050.
Tapert SF, Brown SA: Neuropsychological correlates of adolescent substance abuse: Four year outcomes. J Int Neuropsychol Soc 1999;5: 475-487.
Tonigan JS, Connors GJ, Miller WR: Participation and involvement in Alcoholics Anonymous. In: Babor T, DelBoca F (eds.), Treatment Matching in Alcoholism, in press.
Tonigan JS, Toscova R, Miller WR: Meta-analysis of the literature on Alcoholics Anonymous: Sample and study characteristics moderate findings. J Stud Alcohol 1996;57: 65-72.
Tucker JA: Predictors of help-seeking and the temporal relationship of help to recovery among treated and untreated recovered problem drinkers. Addiction 1995;90: 805-809.
Vannicelli M: Treatment considerations. In: Women and Alcohol: Health-Related Issues. NIAAA, 1986, pp. 130-153.
Vitiello B, Jensen PS: Medication development and testing in children and adolescents. Arch Gen Psychiatry 1997;54: 871-876.
Wagner EF, Brown SA, Monti P, et. al.: Innovations in adolescent substance abuse intervention. Alcohol: Clin Exp Res 1999;23: 236-249.
Walsh D, Hingson R, Merrigan D, et. al.: A randomized trial of treatment options for alcohol-abusing workers. New Eng J Med 1991;325: 775-782.
Weibel-Orlando J: Indians, ethnicity and alcohol: Contrasting perceptions of the ethnic self and alcohol use. In: Bennett LA, Ames GM (eds.), The American Experience with Alcohol: Contrasting Cultural Perspectives. New York, Plenum Press, 1985, pp. 201-226.
Weisner C, Greenfield T, Room R: Trends in the treatment of alcohol problems in the U.S. General Population, 1979 through 1990. Am J Public Health 1995;85: 55-60.
Wilk AI, Jensen NM, Havighurst TC: Meta-analysis of randomized control trials addressing brief interventions in heavy alcohol drinkers. J Gen Int Med 1997;12: 274-283.
World Health Organization Brief Intervention Study Group: A cross-national trial of brief interventions with heavy drinkers. Am J Pub Health 1996;86: 948-955.
Back to Top
APPENDIX A
Subcommittee for Review of Treatment Portfolio
Co-Chairs
Mary E. McCaul, Ph.D.
Department of Psychiatry and
Behavioral Sciences
Johns Hopkins University School of Medicine
911 N. Broadway
Baltimore, MD 21205
Peter Monti, Ph.D.
Center for Alcohol/Addiction Studies
Brown University - Box G-BH
Providence, Rhode Island 02912
Experts in Alcohol-Related Areas
Marc Galanter, M.D.
Department of Psychiatry
New York University School of Medicine
550 First Avenue
New York, NY 10016
Barbara J. Mason, Ph.D.
Division of Substance Abuse
1400 N.W. 10th Ave., Suite 307 (D79)
Department of Psychiatry
University of Miami School of Medicine
Miami, FL 33136
William R. Miller, Ph.D.
Department of Psychology
Logan Hall
University of New Mexico
Albuquerque, NM 87131-1161
Experts in Non-Alcohol-Related Areas
David H. Barlow, Ph.D.
Department of Psychology
Boston University
648 Beacon Street
Boston, MA 02215
Stephen T. Higgins, Ph.D.
University of Vermont
Human Behavioral Pharmacology Lab
Ira Allen School
38 Fletcher Place
Burlington, VT 05401-1419
M. Tracie Shea, Ph.D.
Department of Psychiatry and Human Behavior
Brown University School of Medicine
Butler Hospital/Duncan Building
700 Butler Drive
Box G-BH
Providence, RI 02906
Back to Top
APPENDIX B
Experts in Treatment
Sandra A. Brown, Ph.D.
Psychology Department 0109
University of California, San Diego
San Diego, CA 92093-0109
Jack R. Cornelius, M.D., M.P.H.
Western Psychiatric Institute and Clinic
University of Pittsburgh
3811 O'Hara Street, Room 1091
Pittsburgh, PA 15213-2593
Carlo DiClemente, Ph.D.
UMBC - Department of Psychology
1000 Hilltop Circle
Baltimore, MD 21250
Michael F. Fleming, M.D., M.P.H.
Department of Family Medicine
University of Wisconsin-Madison Medical School
777 S. Mills Street
Madison, WI 53715-1896
Edith Gomberg, Ph.D.
Department of Psychiatry
University of Michigan School of Medicine
400 E. Eisenhower Parkway, Suite 2A
University of Michigan
Ann Arbor, MI 48108
Keith Humphreys, Ph.D.
VA Palo Alto Health Care System
Menlo Park Division 152
795 Willow Road
Menlo Park, CA 94025
Richard D. Hurt, M.D.
Mayo Nicotine Dependence Center
Baldwin Building, Mayo Clinic
200 First Street, SW
Rochester, MN 55905
Ronald Kadden, Ph.D.
Department of Psychiatry
University of Connecticut Health Center
Farmington, CT 06030-2103
Henry Kranzler, M.D.
Department of Psychiatry
University of Connecticut School of Medicine
263 Farmington Ave., MC-2103
Farmington, CT 06030-2103
Richard Longabaugh, Ed.D.
Center for Alcohol and Addiction Studies
Box G-BH
Brown University
Providence, RI 02912
Lisa M. Najavits, Ph.D.
Department of Psychology
Harvard Medical School
McLean Hospital
115 Mill Street
Belmont, MA 02478
Robert L. Stout, Ph.D.
Butler Hospital
345 Blackstone Blvd.
Providence, RI 02906
Jalie A. Tucker, Ph.D., M.P.H.
Department of Psychology
226 Thach Hall
Auburn University
Auburn, AL 36849
Back to Top
APPENDIX C
NIAAA Program Staff
John Allen, Ph.D.
Treatment Research Branch, NIAAA
6000 Executive Blvd., Suite 505 Bethesda, MD 20892-7003
Joanne Fertig, Ph.D.
Treatment Research Branch, NIAAA
6000 Executive Blvd., Suite 505
Bethesda, MD 20892-7003
Richard K. Fuller, M.D.
Division of Clinical and Prevention Research, NIAAA
6000 Executive Blvd., Suite 505
Bethesda, MD 20892-7003
Raye Litten, III, Ph.D.
Treatment Research Branch, NIAAA
6000 Executive Blvd., Suite 505
Bethesda, MD 20892-7003
Cherry Lowman, Ph.D.
Treatment Research Branch, NIAAA
6000 Executive Blvd., Suite 505
Bethesda, MD 20892-7003
Margaret Mattson, Ph.D.
Treatment Research Branch, NIAAA
6000 Executive Blvd., Suite 505
Bethesda, MD 20892-7003
APPENDIX D
NIAAA Staff and Guests
Henri Begleiter, Ph.D., M.D.
Department of Psychiatry
Box 1203
State University of New York
Health Science Center at Brooklyn
450 Clarkson Avenue
Brooklyn, New York 11203
Captain Darryl Bertolucci
Epidemiology Branch, NIAAA
6000 Executive Blvd., Suite 515
Bethesda, MD 20892-7003
Susan Cahill
Planning and Financial Management Branch, NIAAA
6000 Executive Blvd., Suite 412
Bethesda, MD 20892-7003
Faye Calhoun, D.P.A.
Office of Collaborative Research, NIAAA
6000 Executive Blvd., Suite 400
Bethesda, MD 20892-7003
Mary Dufour, M.D., M.P.H.
Deputy Director, NIAAA
6000 Executive Blvd., Suite 400
Bethesda, MD 20892-7003
Michael J. Eckardt, Ph.D.
Office of Scientific Affairs, NIAAA
6000 Executive Blvd., Suite 409
Bethesda, MD 20892-7003
Enoch Gordis, M.D.
Director, NIAAA
6000 Executive Blvd., Suite 400
Bethesda, MD 20892-7003
Harold D. Holder, Ph.D.
Director, Prevention Research Center
Pacific Institute for Research and Evaluation
2150 Shattuck Avenue, Suite 900
Berkeley, California 94704
Nancy Hondros
Planning and Financial Management Branch, NIAAA
6000 Executive Blvd., Suite 412
Bethesda, MD 20892-7003
Robert Huebner, Ph.D.
Division of Clinical and Prevention Research, NIAAA
6000 Executive Blvd., Suite 505
Bethesda, MD 20892-7003
William M. Lands, Ph.D.
Office of the Director, NIAAA
6000 Executive Blvd., Suite 400
Bethesda, MD 20892-7003
Ting-Kai Li, M.D.
Department of Medicine
Indiana University School of Medicine
Emerson Hall 421
545 Barnhill Drive
Indianapolis, IN 46202-5124
Stephen Long
Office of Planning and Resource Management, NIAAA
6000 Executive Blvd., Suite 400
Bethesda, MD 20892-7003
Matt McGue, Ph.D.
Department of Psychology
Elliot Hall, Room N-218
75 East River Road
University of Minnesota
Minneapolis, MN 55455
Suzanne Medgyesi-Mitschang, Ph.D.
Office of the Director, NIAAA
6000 Executive Blvd., Suite 405
Bethesda, MD 20892-7003
Carrie L. Randall, Ph.D.
Department of Psychiatry and Behavioral Science
Medical University of South Carolina
171 Ashley Avenue
Charleston, SC 29425
Carmen Richardson
Planning and Financial Management Branch, NIAAA
6000 Executive Blvd., Suite 412
Bethesda, MD 20892-7003
Ronald Suddendorf, Ph.D.
Office of Scientific Affairs, NIAAA
6000 Executive Blvd., Suite 409
Bethesda, MD 20892-7003
Ernestine Vanderveen, Ph.D.
Division of Basic Research, NIAAA
6000 Executive Blvd., Suite 402
Bethesda, MD 20892-7003
Kenneth Warren, Ph.D.
Office of Scientific Affairs, NIAAA
6000 Executive Blvd., Suite 409
Bethesda, MD 20892-7003
Migs Woodside
35436 Indian Camp Trail
Scottsdale, AZ 85262
Sam Zakhari, Ph.D.
Division of Basic Research, NIAAA
6000 Executive Blvd., Suite 402
Bethesda, MD 20892-7003
Posted: July 19, 2000