
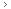 1663
1663
|
|

The Smartest Living NanomachineUnraveling the Secrets of Nature’s Protein Factory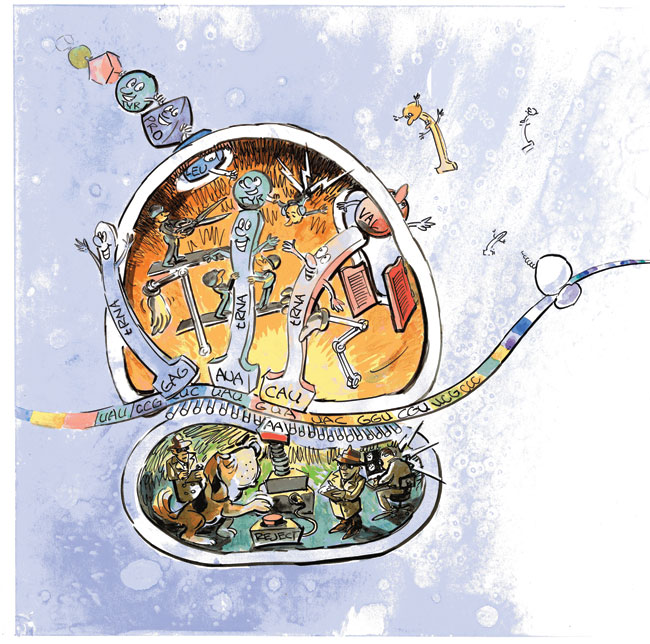
Quality control in the ribosome factory. A chemical middleman, a tRNA with a three-letter "anticodon" for its feet and an amino acid atop its head, attaches to the next-available codon on the mRNA conveyor. Down below, the security forces use the ribosomal "read head" (AA) to check that the anticodon fits and properly matches the codon. If it does, the protein assembly crew in the ribosome's upper level is told to detatch the amino acid and hook it up to the growing protein chain. How that message is communicated is reported in this article. Illustration by David R. Delano
From childhood on, we take for granted that the human body is a chemical factory that breaks down food and converts it into the molecules needed for life. But the body is also a container for a mind-blurring number of nanofactories called ribosomes, all working at full capacity around the clock to keep us alive. In each of the trillions of cells in the human body, a million ribosomes continuously churn out proteins: the tangled, ribbonlike chains of amino acids that run the chemistry of all living things. That's a quintillion protein factories rebuilding our entire bodies every 7 years. Ribosomes are found in practically identical form in every living cell on Earth, whether it be the single-celled archaea in the thermal vents of the ocean floor, the bacteria on the surface of the planet, or the cells in the human body. Because they have retained the same form throughout most of evolution, ribosomes are believed to be among the most-ancient molecular machines of life. Says Los Alamos theoretical biologist Kevin Sanbonmatsu, "The ribosome has been studied for almost half a century. But only now can we use supercomputers to investigate, in atomic detail, how this very-complex machine really works. It has been a holy grail for people who do biomolecular simulations, and now our team at Los Alamos is making it happen." Already Sanbonmatsu and Chang-Shung Tung, Theoretical Biology and Biophysics group leader, have created the first atomic-level computer model of a single ribosome. They used it to simulate the initial steps in protein synthesis, in which the correct amino acids are brought into the ribosome. Sanbonmatsu and his team, which includes Andrea Vaiana, Yanan Yu, and Scott Hennelly, are now ready to simulate the entire process. Their simulations require close collaboration with experimentalists Scott Blanchard of Cornell and Simpson Joseph of the University of California, San Diego. Two goals are driving their intensive work. One is a matter of basic science. Sanbonmatsu and his team are hoping their 3-D simulations, based on the fundamental forces among the ribosome's 250,000 atoms, can break through the conflicting interpretations of ribosome experiments by integrating the results into a coherent picture. Their simulations have already revealed the active molecular players that keep the ribosome's error rate down to no more than 1 amino acid in a sequence of 5,000. The second goal is a health matter. Bacterial ribosomes are the target of about 50 percent of the antibiotics used in U.S. hospitals. But bacteria such as the deadly MRSA superbug (methicillin-resistant Staphylococcus aureus) are developing resistance to these antibiotics and now infect nearly 5 percent of all U.S. hospital patients. By simulating the ribosome at the molecular level, researchers can gain information needed for designing new combinations of drugs that will interfere with MRSA's ribosome function. Meet the RibosomeOnly 25 nanometers (billionths of a meter) in diameter, the ribosome performs the most-complex information-processing task of any molecular machine. It reads protein recipes, which are written in the four-letter language of nucleic acids, and produces finished proteins, which are constructed from amino acids. 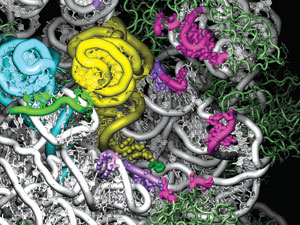
Ribosomal Sites for New Antibiotics
Sanbonmatsu and his team are using their simulations to discover potential sites for new antibiotics that will interfere with the ribosomes of disease-causing bacteria. This frame from the simulation looks into the tRNA entry channel in the ribosome's large subunit. The amino-acid ends of the yellow and blue tRNAs have fully entered the ribosome. The pink and purple regions indicate 68 ribosomal bases that interact with the tRNAs during entry. Eighteen of those are identical in every organism ever sequenced, making them promising binding sites for new antibiotics that would block tRNA passage and thereby halt a bacterium's protein synthesis. The tool it uses in its work is RNA (ribonucleic acid), a molecular cousin to DNA. Two types of RNA are involved. The first is messenger RNA (mRNA), which carries a copy of the recipe for making a specific protein. (The original information is stored in the cell's DNA.) The information is encoded in the sequence of bases (nucleotide bases, named for their nucleic acids) that hang like charms from the chainlike RNA. All RNA molecules are composed of four different nucleotide bases, designated by the letters A, C, G, and U. In the mRNA strand, any group of three consecutive bases, for example, AUG or CCA, is called a codon. Each codon codes for an amino acid—AUG codes for methionine, and CCA codes for proline. The mRNA strand is therefore a chain of codons specifying the order in which amino acids are to be linked to form a specific protein. The ribosome is itself an assemblage of RNA and protein. It has large and small subunits that cooperate during construction of a protein. The mRNA moves through the small subunit like a molecular conveyor belt, presenting each codon in turn. The small subunit decodes each codon, and the large subunit responds by adding the prescribed amino acid to the protein chain. The process ends when a STOP codon at the end of the mRNA strand causes the ribosome to release the completed chain, which then folds into the tangled shape of a finished protein. The amino acids for this construction project are delivered by the second type of RNA, transfer RNA (tRNA). Molecules of tRNA float to the ribosome's small subunit through the cellular fluid, each carrying an amino acid on one end. At its other end, each tRNA has an "anticodon"—a triplet of bases that will bond strongly to only the mRNA codon that specifies the amino acid carried by the tRNA. When a tRNA arrives at the small subunit, its anticodon tries to bond to the next-available codon on the mRNA strand. If the fit is strong, the tRNA is accepted into the ribosome through a process called accommodation. A weak fit indicates that the tRNA is carrying the wrong amino acid, in which case the tRNA is rejected to float back into the cellular fluid. That the ribosome accommodates only the right tRNAs and rejects the wrong ones has been known for quite a while. But the selection mechanism—the "how"—was unknown until Sanbonmatsu figured it out. Building a 3-D ModelSanbonmatsu came to his ribosome studies indirectly. Originally a theoretical plasma physicist, he left that field of study in 2000 to pursue an interest in the origin of life, an interest that became more urgent the more he read. "Popular science books," he says, "would begin with explanations of the building blocks of life—DNA and proteins—but when they came to the ribosome, they would punt and say either God made the ribosome or aliens must have brought it to Earth." The ribosome was a mystery. Researchers knew it was composed of RNA and protein and that it used mRNA and tRNA to translate the genetic code into proteins. But nothing was known about the details. What were the forces at work inside the ribosome? The route to an answer opened up in 2000 when researchers around the world solved the 3-D atomic-level structure of each of the ribosome's two subunits. Sanbonmatsu and Tung then fit the two very asymmetrical subunits into a single mathematical representation and built a computer code to represent how all the constituent atoms and molecules could interact and move over time: a molecular-dynamics code. Combined, the two subunits formed a very challenging structure, dominated by a rat's nest of RNA loops. Sanbonmatsu and Tung could see no obvious way for a tRNA to be accommodated into the interior. "Our simulation work was to find the tRNA's entry route and to explore how the right tRNAs are accepted into the ribosome and the wrong ones rejected," explains Sanbonmatsu. The first simulation was the largest biomolecular-dynamics simulation ever done, encompassing more than 2.5 million atoms. (About 10 percent of the atoms were in the ribosome, the tRNAs, and the mRNA strand. The rest were in the water and ions permeating the whole molecular complex.) Sanbonmatsu was warned that the simulation was too ambitious for a newcomer to the field, but it was successful and won him the PECASE Award (the Presidential Early Career Award for Scientists and Engineers). The Path to AccommodationDuring accommodation, the tRNA moves between two positions that are known from experiments. In the initial partially bound position, the tRNA's anticodon is bonded strongly to the correct mRNA codon in the small subunit. The tRNA is bent, as if it were spring-loaded, and the end with the amino acid is entirely outside the ribosome. In the final fully bound position, the tRNA has straightened, and the entire molecule, including the amino acid, has been accepted inside the ribosome.  A simulation of tRNA accommodation, the step by which amino acids are brought into the ribosome. Left: The blue tRNA is inside the ribosome, and the yellow tRNA, with a tiny green amino acid on one end, is partially inside the ribosome, with its body in a bent (spring-loaded) position. (In order to clearly display the tRNA positions, the top half of the ribosome is not shown in this figure.) Both tRNAs have their anticodon ends bound to the long mRNA strand (green) that winds through the ribosome's small subunit (purple). Center:The yellow tRNA begins to straighten out as its amino acid end starts entering the ribosome's large subunit (white). Right: The amino acid end of the yellow tRNA has moved past the ribosomal gate (red) and has disappeared inside the large subunit.
Sanbonmatsu's simulation computed the forces among the atoms to determine the path of least resistance—the minimum-energy path—from the initial, partially bound position to the final, fully bound one. Viewing the results was the hardest part. Sanbonmatsu had to sift through many terabytes of data to make a 3-D movie of the tRNA traversing the entrance path. He then inspected the movie from every angle, zooming in on the active regions. After months of staring at each frame, literally getting to know every atom near the path of the tRNA, he found the heretofore-invisible entry channel. It was a pathway where only 68 of the ribosome's 5,000 nucleotide bases interacted with the tRNA as it entered the ribosome. The only real resistance along the accommodation pathway came from a kind of gate made of ribosomal RNA that seemed to block the way. In the simulation, the end of the tRNA with the amino acid was deflected backwards to get around that barrier (shown in red in the simulation of tRNA accommodation shown above) and reach the final position inside the ribosome's large subunit. Like a master sleuth, Sanbonmatsu saw the gate's presence as a major clue to how the ribosome rejects the wrong tRNAs. The Mechanics of RejectionSanbonmatsu conjectured that a tRNA that carries the complementary anticodon for the mRNA codon must somehow be securely bound at the decoding position in the small subunit. He conjectured further that once bound, the tRNA would use its mechanical strength, or rigidity, to get past the gate. In other words, if the tRNA is held fixed at its anticodon end, then as its body unflexes, it will be able to swing rigidly into the ribosome, bringing its amino acid with it. Conversely, if the tRNA is incorrectly matched, Sanbonmatsu thought the binding of the anticodon must be much less secure. As a result, the release from its spring-loaded position would dislodge the tRNA from its footing, and no entry would be made. This conjecture implied that the tRNA is an active player, transmitting between the ribosomal subunits the information that the correct amino acid is being delivered. Previously, people thought that information was sent through an elaborate ribosomal mechanism, not through the tRNA. Sanbonmatsu then ran another simulation, showing in great detail how the anticodon end of the tRNA interacts with the small subunit's molecular "read head," a small RNA loop that chemically "proofreads" the attempted anticodon-codon match. If the match is good, nine chemical interactions anchor the tRNA to the mRNA read-head complex. If the match is poor, only seven chemical interactions take place, so the connection is weaker, drastically reducing the tRNA's footing and its chances of getting through the gate. Thus, tRNA is revealed as playing an active role in ensuring correct translation of the genetic message, which suggests that it may well have existed long before the ribosome in evolutionary time and facilitated protein synthesis in prelife systems.  Sanbonmatsu stands inside his ribosome simulation, the largest biomolecular-dynamics simulation to date.
Theory Attracts a FollowingSanbonmatsu's simulations have set a precedent in ribosomal research. Not only are his findings gaining traction, but other theorists and experimentalists are also choosing simulation to both interpret and plan experiments. Meanwhile, Sanbonmatsu and his small team are readying a new simulation code for Roadrunner, the new supercomputer at Los Alamos that has taken the lead as the most-powerful supercomputer in the world. Roadrunner will enable Sanbonmatsu's team to mimic the ribosome's massive coordination of its moving parts in a step called translocation. This amazing process resets the ribosomal machinery after the addition of each new amino acid to the protein chain. The ribosome's two subunits somehow swivel relative to each other in a ratchet-like motion that moves the mRNA conveyor belt forward by exactly one codon. According to Sanbonmatsu, the motion may be powered in part by spring-loaded tRNAs. To check out such speculation and guide the simulations, the team will work with its Laboratory colleagues to experimentally track these complicated movements. Sanbonmatsu announces his plans with a cool matter-of-factness, but beneath the calm is the unmistakable air of intense excitement. Many mysteries are waiting to be solved. —Necia Grant Cooper |

Key words- ribosome, proteins, archaea, protein synthesis, DNA, RNA, tRNA, mRNA, bimolecular simulations, bacterial ribosomes, antibiotics 
From DNA to Protein
DNA. The DNA of an organism is a sequence of four chemical units called nucleotide bases and designated by A, G, C, and T. Because the base C is complementary (binds strongly) to G, and A is complementary to T, a single strand of DNA will bind to another strand that has the complementary bases, forming the famous double helix. From DNA to RNA. When a protein is to be made, the double helix unwinds, and the DNA sequence (the gene) for that protein is copied into a strand of RNA, a close molecular cousin of DNA. (In RNA, the base U takes the place of T. Thus, A is complementary to U, while C remains complementary to G.) The RNA copy of the gene is called messenger RNA, mRNA, and is shown in green in the figures. From RNA to Protein. To start protein synthesis, the mRNA wraps around the neck of the small subunit of the ribosome, and the (blue) transfer RNA (tRNA) binds its anticodon to the START codon, AUG, which marks the beginning of the mRNA's protein-coding sequence. Simultaneously, the large subunit of the ribosome descends on top of the small subunit. 
When a second (orange) tRNA arrives, its anticodon ninds to the next mRNA codon. The ribosome's RNA "read head" checks whether the anticodon and codon are fully complementary, that is, every A is binding to U, and every C is binding to G. 
The tRNA is accepted (accommodated) inside the ribosome's large subunit. Its amino acids binds chemically to the first amino acid, and the growth of the protein begins. 
The small subunit pivots (not shown) over the large subunit, and the tRNA and mRNA move to the left by exactly one codon to reset the machine. The growing protein is pushed along the exit channel.
|