|
The Public Health Security and Bioterrorism Preparedness and Response Act of 2002 (Bioterrorism Act), section 305, added section 415 to the Federal Food, Drug, and Cosmetic Act, which requires domestic and foreign facilities to register with FDA by December 12, 2003, if they manufacture, process, pack, or hold food for human or animal consumption in the U.S. The purpose of registration is to provide FDA with sufficient and reliable information about food and feed facilities. When used with the administrative detention, recordkeeping, and prior notice provisions in sections 303, 306, and 307, respectively, of the Bioterrorism Act, registration helps provide FDA with information on the origin and distribution of food and feed products and thereby aid in the detection and quick response to actual or potential threats to the U.S. food supply. Registration information also helps FDA to notify facilities that may be affected by the actual or potential threat.
In November, 2004, FDA issued an updated Compliance Policy Guide (CPG) to replace the previous version issued in December, 2003. The updated CPG provides guidance on the agency's strategy for enforcing and achieving compliance with the registration interim final rule. The CPG states that if FDA, or a state agency acting on its behalf, determines that a domestic facility required to register has not registered, or failed to update mandatory elements of the registration, the investigator should note this failure in the establishment inspection report and bring such failure to the attention of the District Compliance Branch. The CPG further states that the field investigator should advise the facility's management of the requirement and provide the facility with a copy of FDA's booklet (dated November 2003), "What You Need to Know About Registration of Food Facilities," which includes the website for electronic registration (www.access.fda.gov) and the address of the FDA office from which a paper copy of the registration form (Form 3537) may be obtained.
The updated CPG states that after November 8, 2004, FDA will enforce the registration provision as appropriate in each situation regarding domestic facilities. Circumstances which could merit regulatory action include a continuing failure to register, and a threat on the food supply that poses a threat of serious adverse health consequences or death to humans or animals. In addition, FDA may also consider the failure to register as an additional charge in an enforcement action that is based on other statutory violations.
For foreign facilities, FDA generally intends to enforce the registration requirement in accordance with the policies set out in CPG 110.310, "Prior Notice of Imported Food Under the Public Health Security and Bioterrorism Preparedness Response Act of 2002" (revised November, 2005).
In the preamble to the Registration Final Rule (68 FR 58894 (Oct. 10, 2003), as affirmed by 70 FR 57505 (Oct. 3, 2005)), FDA estimates that the total number of food facilities that must register with FDA is approximately 420,000, approximately half of which are domestic. As of July 10, 2008, FDA had received 353,949 registrations, of which 207,724 are foreign facilities and 146,225 are domestic facilities. The following graph shows the rate of these registrations by date:
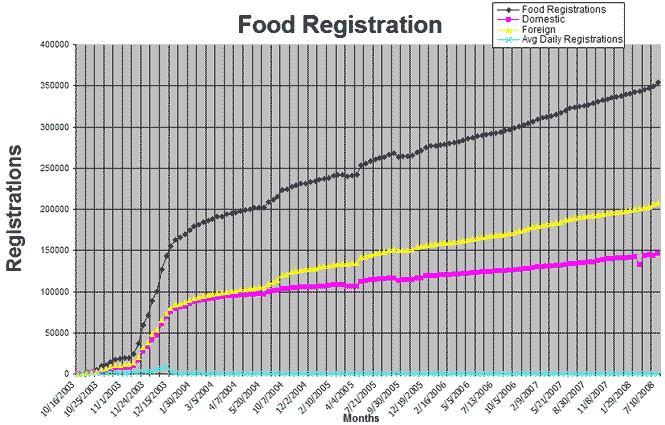
FDA has received an estimated 6,842 more registrations since we issued the previous compliance summary information guide on April 7, 2008. FDA will continue to ensure that all facilities required to register have done so.
The table below shows a country-by-country breakdown of the number of registrations per country (listed in alphabetical order) as of July 10, 2008:
| Country Name | Number of Registrations |
|---|---|
| AFGHANISTAN | 21 |
| ALBANIA | 79 |
| ALGERIA | 34 |
| ANTIGUA AND BARBUDA | 19 |
| ARGENTINA | 3376 |
| ARMENIA | 138 |
| ARUBA | 19 |
| AUSTRALIA | 4018 |
| AUSTRIA | 912 |
| AZERBAIJAN | 68 |
| BAHAMAS | 74 |
| BAHRAIN | 13 |
| BANGLADESH | 292 |
| BARBADOS | 70 |
| BELARUS | 76 |
| BELGIUM | 1136 |
| BELIZE | 69 |
| BERMUDA | 15 |
| BOLIVIA | 310 |
| BOSNIA AND HERZEGOWINA | 90 |
| BRAZIL | 4156 |
| BULGARIA | 523 |
| BURKINA FASO | 17 |
| BURUNDI | 19 |
| CAMBODIA | 26 |
| CAMEROON | 66 |
| CANADA | 13981 |
| CAPE VERDE | 18 |
| CAYMAN ISLANDS | 17 |
| CHILE | 3847 |
| CHINA | 19720 |
| COLOMBIA | 3013 |
| COMOROS | 13 |
| CONGO | 10 |
| CONGO (THE DEMOCRATIC REPUBLIC OF) | 18 |
| COOK ISLANDS | 10 |
| COSTA RICA | 1470 |
| CROATIA | 143 |
| CYPRUS | 110 |
| CZECH REPUBLIC | 317 |
| DENMARK | 564 |
| DOMINICA | 35 |
| DOMINICAN REPUBLIC | 903 |
| ECUADOR | 4083 |
| EGYPT | 481 |
| EL SALVADOR | 845 |
| ESTONIA | 92 |
| ETHIOPIA | 345 |
| FAROE ISLANDS | 30 |
| FIJI | 198 |
| FINLAND | 223 |
| FRANCE | 12892 |
| FRENCH POLYNESIA | 62 |
| GAMBIA | 14 |
| GEORGIA | 146 |
| GERMANY | 3767 |
| GHANA | 426 |
| GREECE | 1059 |
| GREENLAND | 30 |
| GRENADA | 34 |
| GUADELOUPE | 19 |
| GUATEMALA | 1266 |
| GUINEA | 167 |
| GUYANA | 312 |
| HAITI | 113 |
| HONDURAS | 1003 |
| HONG KONG, CHINA | 2338 |
| HUNGARY | 527 |
| ICELAND | 314 |
| INDIA | 4854 |
| INDONESIA | 1837 |
| IRAN (ISLAMIC REPUBLIC OF) | 334 |
| IRELAND | 442 |
| ISRAEL | 1307 |
| ITALY | 13539 |
| IVORY COAST | 291 |
| JAMAICA | 511 |
| JAPAN | 22482 |
| JORDAN | 155 |
| KAZAKHSTAN | 20 |
| KENYA | 343 |
| KOREA, DEMOCRATIC PEOPLES REPUBLIC | 33 |
| KOREA, REPUBLIC OF | 5587 |
| KUWAIT | 56 |
| LAO PEOPLES DEMOCRATIC REPUBLIC | 27 |
| LATVIA | 130 |
| LEBANON | 310 |
| LIBERIA | 48 |
| LITHUANIA | 224 |
| LUXEMBOURG | 44 |
| MACAU, CHINA | 21 |
| MACEDONIA | 125 |
| MADAGASCAR | 120 |
| MALAWI | 55 |
| MALAYSIA | 1561 |
| MALDIVES | 47 |
| MALI | 19 |
| MALTA | 23 |
| MARTINIQUE | 21 |
| MAURITANIA | 28 |
| MAURITIUS | 60 |
| MEXICO | 15716 |
| MOLDOVA | 106 |
| MONACO | 16 |
| MONGOLIA | 13 |
| MOROCCO | 396 |
| MOZAMBIQUE | 69 |
| NAMIBIA | 59 |
| NEPAL | 43 |
| NETHERLANDS | 1502 |
| NETHERLANDS ANTILLES | 32 |
| NEW CALEDONIA | 13 |
| NEW ZEALAND | 2376 |
| NICARAGUA | 601 |
| NIGERIA | 342 |
| NORWAY | 710 |
| OMAN | 34 |
| PAKISTAN | 508 |
| PANAMA | 640 |
| PAPUA NEW GUINEA | 125 |
| PARAGUAY | 168 |
| PERU | 2817 |
| PHILIPPINES | 1478 |
| POLAND | 1422 |
| PORTUGAL | 1135 |
| REUNION | 23 |
| ROMANIA | 185 |
| RUSSIAN FEDERATION | 1721 |
| RWANDA | 59 |
| SAINT KITTS AND NEVIS | 18 |
| SAINT LUCIA | 29 |
| SAINT VINCENT AND THE GRENADINES | 38 |
| SAMOA | 27 |
| SAUDI ARABIA | 128 |
| SENEGAL | 100 |
| SERBIA | 53 |
| SEYCHELLES | 11 |
| SIERRA LEONE | 30 |
| SINGAPORE | 714 |
| SLOVAKIA (Slovak Republic) | 97 |
| SLOVENIA | 138 |
| SOLOMON ISLANDS | 17 |
| SOUTH AFRICA | 1668 |
| SPAIN | 5597 |
| SRI LANKA | 662 |
| SUDAN | 18 |
| SURINAME | 30 |
| SWAZILAND | 10 |
| SWEDEN | 385 |
| SWITZERLAND | 979 |
| SYRIAN ARAB REPUBLIC | 213 |
| TAIWAN (CHINA) | 2836 |
| TANZANIA | 116 |
| THAILAND | 2900 |
| TOGO | 29 |
| TONGA | 55 |
| TRINIDAD AND TOBAGO | 256 |
| TUNISIA | 153 |
| TURKEY | 1462 |
| TURKS AND CAICOS ISLANDS | 13 |
| UGANDA | 99 |
| UKRAINE | 746 |
| UNITED ARAB EMIRATES | 309 |
| UNITED KINGDOM | 3368 |
| UNITED STATES | 146225 |
| URUGUAY | 410 |
| UZBEKISTAN | 36 |
| VANUATU | 15 |
| VENEZUELA | 471 |
| VIET NAM | 5358 |
| VIRGIN ISLANDS (BRITISH) | 31 |
| YEMEN | 36 |
| YUGOSLAVIA | 198 |
| ZAMBIA | 14 |
| ZIMBABWE | 41 |
| Total Registrations | 353949[1] |
The table below shows a state-by-state breakdown of the number of registrations (listed in alphabetical order) as of July 10, 2008:
| State Name | State Id | Number of Registrations |
|---|---|---|
| ALABAMA | AL | 1722 |
| ALASKA | AK | 557 |
| AMERICAN SAMOA | AS | 11 |
| ARIZONA | AZ | 2053 |
| ARKANSAS | AR | 1429 |
| CALIFORNIA | CA | 21002 |
| COLORADO | CO | 2104 |
| CONNECTICUT | CT | 1154 |
| DELAWARE | DE | 307 |
| DISTRICT OF COLUMBIA | DC | 231 |
| FLORIDA | FL | 8783 |
| GEORGIA | GA | 3636 |
| GUAM | GU | 56 |
| HAWAII | HI | 978 |
| IDAHO | ID | 1095 |
| ILLINOIS | IL | 6143 |
| INDIANA | IN | 2302 |
| IOWA | IA | 4277 |
| KANSAS | KS | 2037 |
| KENTUCKY | KY | 1935 |
| LOUISIANA | LA | 1856 |
| MAINE | ME | 1112 |
| MARYLAND | MD | 2099 |
| MASSACHUSETTS | MA | 3002 |
| MICHIGAN | MI | 3335 |
| MINNESOTA | MN | 3076 |
| MISSISSIPPI | MS | 1120 |
| MISSOURI | MO | 2765 |
| MONTANA | MT | 841 |
| NEBRASKA | NE | 1630 |
| NEVADA | NV | 745 |
| NEW HAMPSHIRE | NH | 574 |
| NEW JERSEY | NJ | 5284 |
| NEW MEXICO | NM | 838 |
| NEW YORK | NY | 9286 |
| NORTH CAROLINA | NC | 2984 |
| NORTH DAKOTA | ND | 986 |
| NORTHERN MARIANA ISLANDS | MP | 16 |
| OHIO | OH | 4297 |
| OKLAHOMA | OK | 1544 |
| OREGON | OR | 2719 |
| PENNSYLVANIA | PA | 5086 |
| PUERTO RICO | PR | 989 |
| RHODE ISLAND | RI | 490 |
| SOUTH CAROLINA | SC | 1484 |
| SOUTH DAKOTA | SD | 746 |
| TENNESSEE | TN | 2269 |
| TEXAS | TX | 9450 |
| UTAH | UT | 942 |
| VERMONT | VT | 569 |
| VIRGINIA | VA | 2853 |
| VIRGIN ISLANDS | VI | 48 |
| WASHINGTON | WA | 4874 |
| WEST VIRGINIA | WV | 622 |
| WISCONSIN | WI | 3513 |
| WYOMING | WY | 361 |
| Total Registrations | 146225[2] | |
[1] Under the Bioterrorism Act, the following are not subject to disclosure under 5 U.S.C. 552 (the Freedom of Information Act): FDA's list of specific registered facilities, documents submitted under the registration requirement, and any information derived from such list or registration documents that would disclose the identity or location of a specific registered facility. Thus, to protect the identity of registered facilities, FDA has eliminated from the above list countries from which FDA has received less than ten registrations. However, the number of total registrations reflects all registrations FDA has received.
[2] Under the Bioterrorism Act, the following are not subject to disclosure under 5 U.S.C. 552 (the Freedom of Information Act): FDA's list of specific registered facilities, documents submitted under the registration requirement, and any information derived from such list or registration documents that would disclose the identity or location of a specific registered facility. Thus, to protect the identity of registered facilities, FDA has eliminated from the above list states from which FDA has received less than ten registrations. However, the number of total registrations reflects all registrations FDA has received.
November 2003: Booklet (SECG): What You Need to Know About Registration of Food Facilities