 |
Rafael de Cabo, Ph.D., Investigator Aging, Metabolism, and Nutrition Unit Laboratory of Experimental Gerontology E-mail: deCaboRa@grc.nia.nih.gov Curriculum Vitae - (pdf) |
| Research Overview: The Aging, Metabolism, and Nutrition Unit (AMNU) applies whole body physiological and tissue-specific molecular approaches to investigate effects of nutritional interventions on basic mechanisms of aging and age-related diseases. Caloric restriction (CR), without malnutrition, is widely known to extend lifespan and retard a wide variety of aging processes in several short-lived species and is the primary paradigm employed by AMNU scientists. Research within this unit uses both rodent models of CR as well as an in vitro model for CR. |
| CR affects metabolic regulation to induce an overall phenotypic change leading to a decrease in cellular proliferation and growth rates. CR induces measurable changes on circulating levels of several hormones and growth factors that regulate cell growth and proliferation. Serum obtained from CR animals alters growth, proliferation and stress responses of cells in culture. We have demonstrated that it is possible to investigate certain aspects of CR using this in vitro approach. This approach lends itself to a more rapid investigation of possible mechanisms and, perhaps more importantly to the research, development and rapid evaluation of interventions that would be able to induce or promote a phenotype similar to that seen with CR, essentially a CR mimetic. |
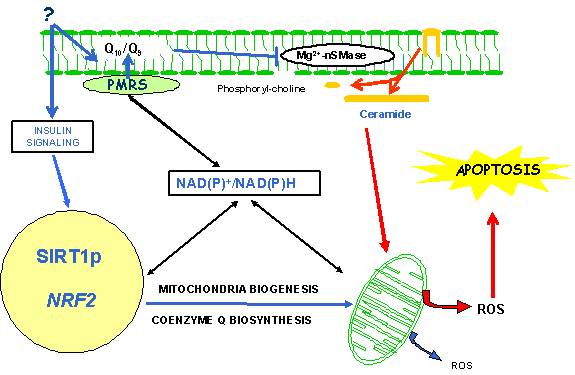
| CR extends lifespan in a variety of animal model systems and reduces oxidative stress during aging. At least in part, the reduction in oxidative stress may be explained by the fact that animals on CR reach a new bioenergetic equilibrium. Two major components in the bioenergetic pathway are the mitochondria electron transport chain and the plasma membrane (PM) redox system (PMRS). Ubiquinone is the central molecule of the PMRS and protects the membrane under different stress conditions. Aging induces general macromolecular damage that can be prevented and reversed by CR. Preliminary data suggest that several components of the PMRS are altered during aging and that several of these changes are modified by CR in rats and mice. Analysis of the bioenergetic balance between mitochondria and PM in rats and mice on CR can provide the information that might explain the enhanced resistance to oxidative stress that CR affords during aging. The role of the PMRS in the prevention of oxidative stress by CR during aging can provide the basis for the design of potential CR mimetics and nutritional interventions. |
| Background: Calorie restriction (CR), without malnutrition, is the only nongenetic intervention that has consistently been shown to increase the median and maximum life span in laboratory rodents. Lifespan extension produced by CR has also been reported in worms, flies, fish, spiders, water fleas, and other animals. In addition to increasing life span, CR in rodents also reduces the incidence and delays the onset of a number of age-associated diseases, cancer, nephropathy, cataracts, diabetes, hypertension, and hyperlipidemia as well as maintains function at more youthful levels. Animals on CR are also much more resistant to a wide variety of stressors and toxins. These results show that CR not only extends life span, but also retards the rate of biological aging. The mechanism(s) responsible for the retardation of aging with CR, however, are still open to debate. |
| In 1956, Denham Harman published a theory proposing that aging results from deleterious interactions of free radicals, produced as a byproduct of oxidative metabolism, with cell constituents. The free radical theory of aging continues to be among the most popular theories to explain aging. Therefore, several studies have investigated whether CR alters reactive oxygen species production or cellular oxidative damage. In rodent studies, CR has been shown to decrease oxidative damage to cellular proteins and lipids as well as increase defenses against oxidative stress. CR has also been shown to decrease the rates of mitochondrial ROS production. These findings support the idea that a reduction in oxidative stress may be a mechanism contributing to the retardation of aging with CR. |
| The mitochondrial and plasma membranes are the primary sites of ROS production and resulting oxidative damage). Therefore, it is attractive to hypothesize that a central function of CR may involve alteration of membranes in a manner that favors a decrease in ROS production and/or oxidative damage. |
| Molecular Mechanisms of CR-- An in vitro Model of CR: Our laboratory has shown that certain features of CR, including reduced proliferation and enhanced stress responsiveness, can be reproduced in vitro by culturing cells with serum obtained from rodents that were fed CR diets. Our findings suggest that several effects of CR may be mediated through changes in the composition of the serum induced by this dietary manipulation. Our data further suggest that, at least at the cellular level, enhanced stress responsiveness does not require long-term exposure to CR diets. Some of the cytoprotective effects of CR serum occur rapidly and are freely reversible, since it is possible to induce CR-like responses in hepatocytes from AL rats by culturing them for 48 h in CR serum, while use of AL serum blunts the CR phenotype of hepatocytes isolated from CR rats. These observations agree with findings with recent studies in mice showing that CR-induced changes in protein oxidation can be reversed by placing CR animals on an AL feeding regimen. Our results further indicate that culturing cells with sera from rhesus monkeys that were fed either AL or CR diets induced comparable effects on cell proliferation and stress responsiveness as those seen using rat sera. Indeed, most physiological and disease-related parameters examined in rhesus monkeys on CR are similar to those reported in rodents. Our findings thus suggest that responses to this intervention occur at a fundamental level of cell biology that is relevant to all species. |
| There are several possible explanations for the observed effects of culturing cells in CR serum. It is well known that many hormones and nutrients whose levels are altered during CR including insulin, IGF-1, glucose, fatty acids among others, modulate gene expression and signaling pathways involved in stress responses. Moreover, signaling events triggered by insulin-like signals are emerging as important regulators of life span in several animal models, including nematodes, fruit flies and mice. As support for this emerging in vitro model, it is of particular interest that we have identified insulin and IGF-1 as key serum components responsible for CR effects. |
| Taken together, our results show that the reduced growth and enhanced stress responsiveness known to occur in animals on CR following heat stress can be reproduced in vitro by the addition of serum from CR animals to cell cultures. Our findings provide support for a possible neuroendocrine-based mechanism of CR and provide a model for the screening and investigation of CR mimetics. |
| Aging, Oxidative Stress and the Plasma Membrane: Because of its unique location and composition, the PM is involved in the cellular response to oxidative stress and can regulate the physiology of the cell by controlling the relationship of the cell to its environment. Since the PM is the initial site of attack of extracellular oxidants, those redox systems that can regenerate antioxidants at this location are of vital importance because they could protect the intracellular components from oxidant damage. One means by which cell responds to oxidative stress is to increase the transfer of electrons across the PM to reduce external oxidants with cytosolic reducing equivalents, mainly NAD(P)H and ascorbate. Reduced CoQ is a powerful lipophilic antioxidant which protects lipids from oxidative damage, and maintains other antioxidants in their reduced active forms. Also, the regeneration of extracellular ascorbate is an efficient mechanism to preserve this molecule in a location where it can recycle a-tocopherol and thus prevent lipid peroxidation. Under conditions promoting oxidative stress, the CoQ-dependent antioxidant system can be upregulated in rat liver PM due to increases in CoQ9 and CoQ10 and cytochrome b5 reductase levels, and the translocation of soluble DT-diaphorase to PM. Because the regeneration of CoQH2 is linked to the major metabolic routes via the oxidation of pyridine nucleotides, CoQ could be considered as a central molecule for the maintenance of an antioxidant system which protects membranes from lipid peroxidation that can damage the PM and lead to cell death. |
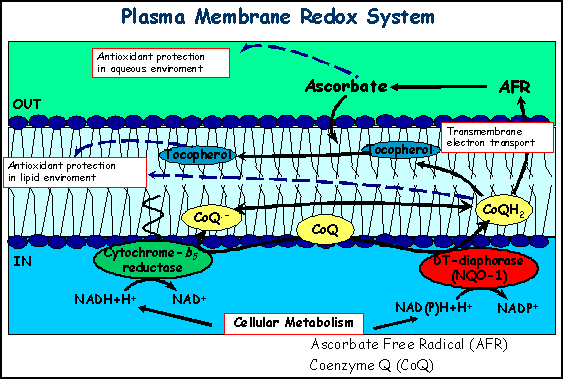 |
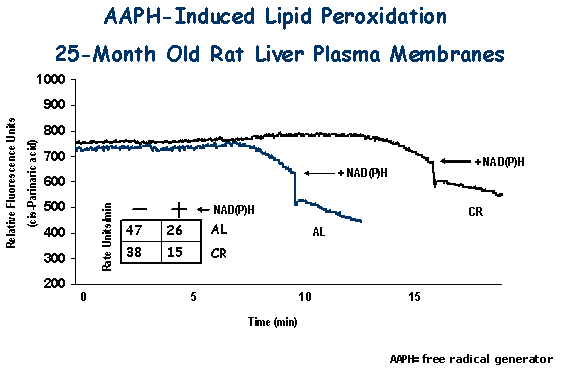
| Changes elicited by CR in the plasma membrane (PM) antioxidant system have been studied recently. Age-related declines in the ratio of CoQ10/CoQ9 and a-tocopherol levels at the PM were attenuated by CR compared to animals fed ad libitum. CoQ-dependent transPM electron transport and translocation of NQO1 to the PM were also dramatically induced by CR. As a result, PM from CR animals displayed significantly enhanced protection against oxidative damage. Thus, modifications induced by CR in the PM redox system could support not only a higher oxidation of NAD(P)H, but also a higher antioxidant capacity, preventing the accumulation of damage to macromolecules during aging. |
 |
| Apoptotic Cell Death during CR: In response to damage or stress, cells attempt to repair and defend themselves, but if unsuccessful, they often undergo programmed cell death, or apoptosis. Numerous studies show that aging is associated with increased rates of stress-induced apoptosis, and the cumulative effects of cell loss have been implicated in various diseases including neurodegeneration, retinal degeneration, cardiovascular disease, and frailty. |
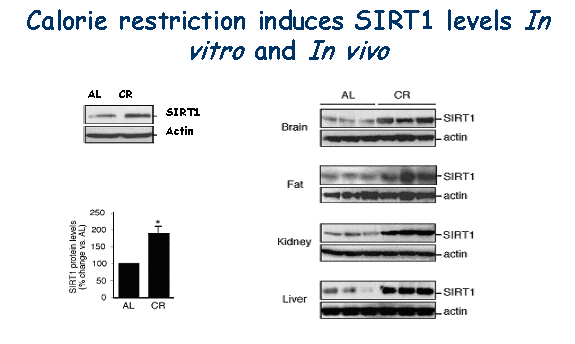
| In mammals, CR delays the onset of numerous age-associated diseases including cancer, atherosclerosis, and diabetes, and can greatly increase life-span. The molecular mechanisms underlying this effect are not known. In yeast, CR extends life-span by increasing the activity of the Sir2 protein, a member of the nicotinamide adenine dinucleotide (NAD+)-dependent deacetylases family. In lower organisms, lifespan can be extended by the presence of extra copies of the SIR2/Sir-2.1 gene, or by a group of small-molecules that activate the sirtuins. In mammals, it is becoming increasingly apparent that SIRT1 is a key regulator of cell defense and survival in response to stress. |
| A major cause of aging is thought to result from the cumulative effects of cell loss over time. We have recently shown that expression of mammalian Sir2 (SIRT1) is induced in CR rats as well as in human cells that are treated with serum from these animals. Insulin and insulin-like growth factor 1 (IGF-1) attenuated this response. SIRT1 inhibits, through K70, Bax mediated stress-induced apoptotic cell death. Thus, CR could extend life-span by inducing SIRT1 expression and promoting the long-term survival of irreplaceable cells. |
 |
 |
| Nutritional Interventions for Healthy Aging: Nutritional interventions can retard and even reverse age-related declines in health and function including cognitive and motor performance in rats. Our laboratory and others have shown that as an animal age, cells are increasingly vulnerable to oxidative stress, particularly in the brain. Dietary supplementation with food extracts high in antioxidants can decrease this vulnerability to oxidative stress as assessed in vivo and in vitro by examining reductions in damage and oxidative stress markers. |
| CR Mimetics: Given the well-established effects of CR in rodents and the promising findings from the initial primate studies, it is likely that CR will have similar beneficial effects in humans. Despite the obvious potential benefits, it is not likely that most people will undertake the disciplined dietary practices necessary to achieve the required reduction in calories. Thus, it is of interest to investigate other interventions that achieve similar results without reducing food intake. Researchers in AMNU and the Cellular and Molecular Neurosciences Section have found that supplementation with a non-metabolizable analog of glucose may "mimic" certain CR effects. For example, short -term (6-month) feeding of 2-deoxy-D-glucose to rodents lowered body weight, temperature, and fasting insulin levels without significant reduction in food intake (Lane MA, Ingram DK, Roth GS. 2-deoxy-d-glucose feeding in rats mimics physiological effects of calorie restriction. J. Anti-Aging Med. 1(4): 327-337, 1999). Also, neuronal toxicity studies suggest that this analogue may afford protection similar to that achieved with CR (Duan W, Mattson MP. Dietary restriction and 2-deoxyglucose administration improve behavioral outcome and reduce degeneration of dopaminergic neurons in models of Parkinson's disease. J. Neurosci. Res. 57(2): 185-206, 1999; Lee J, Bruce-Keller AJ, Kruman U, Chan S, Mattson MP. 2-deoxy-D-glucose protects hippocampal neurons against excitotoxic and oxidative injury: involvement of stress proteins. J. Neurosci. Res. 57:48-61, 1999). A long-term survival study is currently underway in F344 rats. In addition to survival analyses, molecular, physiological, and behavioral studies are planned. Future studies will extend some of this work to nonhuman primates and will include additional "anti-metabolic" compounds thought to have similar effects. |
| | LEG Home Page | |
| IRP Home What's New Contact Us Accessibility Disclaimer Privacy Site Search Site Map NIA Home |
 |
 |
 |