 |
||||
Ontogenetic and Seasonal Variation of Young Non-Native Fish Energy Densities in Lake Michigan
Tomas
Hook
Cooperative Institute for Limnology and Ecosystems Research (CILER
web site),
Collaborators
Steve Pothoven, Great
Lakes Environmental Research Laboratory
Executive Summary
Information on seasonal variation of young fish energy densities can aid quantitative predictions of fish growth, survival, recruitment, population production, and trophic interactions. Such information is necessary to apply bioenergetics models intended to predict either, consumption and growth of 1) piscivorous fish (for which young fish are prey) or 2) the young fish themselves. Alewives are a key component of the Lake Michigan ecosystem, serving both as the forage base for economically important salmonines and as voracious predators on zooplankton and early-life stages of fish. Despite their importance, recent data on young alewife energy densities are limited. Round gobies are relatively recent invaders in Lake Michigan, who have spread throughout the system and become ecologically important. Due to their novelty, there is no published information on seasonal variation of young goby energy densities in Lake Michigan. Through the proposed project, we will quantify individual young alewife and goby dry/wet weights and energy densities (from larval stage through over-winter survival to age-1), and evaluate the relative importance of ontogeny and time of year as determinants of individuals' energy densities.
Project Rationale
We will quantify seasonal and ontogenetic variation in energy densities of young alewives (Alosa pseudoharengus) and round gobies (Neogobius melanostomus) in Lake Michigan. Detailed measures of age-0 alewife and round goby energy densities are necessary to adequately understand and manage Lake Michigan's ecosystem. As described below, the specific management rationales for quantifying ontogenetic and seasonal variation of energy densities differ for young alewives and round gobies. However, for both alewives and round gobies such measures provide a unique opportunity to explore how young fish in a seasonal environment allocate energy for storage and structural growth.
Alewives
Understanding the dynamics of Lake Michigan's alewife population is fundamental
to effectively managing the entire Lake Michigan ecosystem. Alewives are
the primary prey resource of economically important salmonines (salmon
and trout) in Lake Michigan (Stewart et al. 1981; Jude et al. 1987; Eshenroder
et al. 1995; Madenjian et al. 2003). In addition, alewives compete with
and prey upon several planktivorous species (Smith 1970; Brandt et al.
1987, Mason and Brandt 1996) and selective foraging by alewives has the
potential to dramatically alter zooplankton communities (Brooks and Dodson
1965; Wells 1970; Scavia et al. 1986; O'Gorman et al. 1991; Evans 1992).
The capacity of the alewife population to both support salmonine stocks
and structure plankton communities is dependent not only on the biomass
of the alewife population, but also on its composition (i.e., sizes and
energy densities of individual alewives). For instance, an alewife population
composed primarily of small individuals of low energy density likely constitutes
a less favorable forage base for salmonines and will differentially influence
zooplankton dynamics than a similar size alewife population composed primarily
of large individuals of high energy density.
Bioenergetics models of Great Lakes fishes have been used extensively to relate prey supply with predatory demand (e.g., Hewett and Stewart 1989; Brandt et al. 1991; Stewart and Ibarra 1991; Rand et al. 1995). Successful application of bioenergetics models depends not only model parameterization, but also on high quality input data (including ambient temperatures, diet composition, predator and prey biomass, and predator and prey energy densities; see Bartell et al. 1986). Past bioenergetics model applications involving Lake Michigan alewives suggest that 1) there are upper bounds to the biomass and energy content of salmonine stocks which alewife populations can support (e.g., Stewart and Ibarra 1991) and 2) alewives are dominant planktivores and play a critical role in structuring biomass and composition of zooplankton communities (e.g., Stewart and Binkowski 1986; Hewett and Stewart 1989). These analyses, however, relied on input data collected prior to some dramatic changes to Lake Michigan's ecosystem (i.e., reductions in nutrient inputs, arrival of a suite of invasive species, and declines of some established species). Although the biomass of Lake Michigan's alewife population has varied since these ecosystem-level changes (Madenjian et al. 2002; Madenjian et al. 2005), alewives continue to constitute the primary prey of salmonines (Eshenroder et al. 1995; Madenjian et al. 2002; R. Elliot, U.S. Fish and Wildlife Service, Green Bay, WI 54311, unpublished data) and likely remain dominant planktivores. Thus, it is an important question how these ecosystem level changes have affected alewife energy densities.
Adult alewife energy densities clearly vary seasonally (see Figure 1), with dramatic increases in individual energy densities during late summer and fall (a period of increased feeding) and dramatic decreases in energy densities over winter (i.e., a period of resource scarcity; Flath and Diana 1985; Stewart and Binkowski 1986; Rand et al. 1994). In order to evaluate how ecosystem-level changes may have affected these seasonal cycles in adult alewife energy densities, Pothoven, Madenjian and Dettmers have recently measured energy densities of adult alewives collected during 2003 and 2004. Their preliminary results suggest that while adult alewife energy densities continue to vary seasonally, mean adult alewife energy densities have decreased dramatically over the past 25 years (Figure 1). These changes are consistent with a recent decrease in mean age ≤1 alewife condition (Fulton's K; Madenjian et al. 2003).
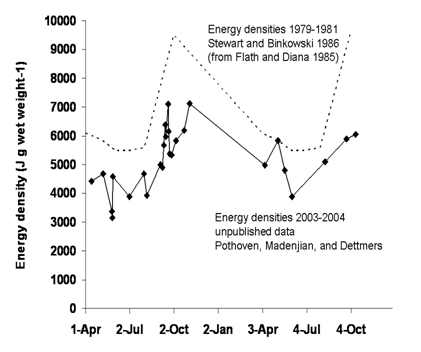
Figure 1. Energy densities of adult alewives in Lake Michigan during 1979-1981 (Flath and Diana 1985; Stewart and Binkowski 1986) and 2003-2004 (preliminary, unpublished data from Pothoven, Madenjian, and Dettmers).
Comparison between Pothoven et al.'s preliminary results and data from Flath and Diana (1985) clearly demonstrate a decrease over time in mean adult alewife energy densities (Figure 1). However, these results can not be extended to young, age-0 alewives because past studies suggest that age-0 alewives have lower energy densities than older individuals (Flath and Diana 1985; Stewart and Binkowski 1986). In order to measure alewife energy densities, Pothoven et al. collected alewives with bottom trawls. These researchers did not age individuals, but grouped alewives into two categories based on length (small, <120-mm and large, >120-mm), and subsequently homogenized individuals and collectively measured mean energy density for multiple individuals collected at the same time. Based on observed growth rates, it is likely that the small category included both age-0 and age-1 alewives, while the large category included age-1 and older individuals (Madenjian et al. 2003). During trawling collections, Pothoven et al. primarily captured large alewives (>120-mm, age-1 and older). However, on 11 occasions during 2003 and 2004 Pothoven et al. captured sufficient small alewives to measure energy densities. Given hatching date distributions and observed growth rates (Höök 2005), it is unlikely that age-0 alewives could be captured in bottom trawls before mid-August. Six of the eleven measurements Pothoven et al. obtained for small alewives were based on collections made prior to 6-August, and thus likely represent age-1 alewife energy densities. The other five measurements were based on collections during September and October and likely included a combination of age-0 and age-1 alewives.
For a number of reasons, there is a need to extend Pothoven et al.'s data set to include age-0 alewife energy densities. First, age-0 alewives are an important component of the Lake Michigan ecosystem. They constitute a seasonally significant portion of salmonine diets (Jude et al. 1987; Stewart and Ibarra 1991; R. Elliot, U.S. Fish and Wildlife Service, Green Bay, WI 54311, unpublished data). In addition, due to their numerical abundances age-0 alewives are prominent planktivores. In fact, Hewett and Stewart (1989) estimated that age-0 alewives account for 50% of total annual zooplankton consumption by the Lake Michigan alewife population. Second, given their ecological importance it is imperative to assess if mean age-0 alewife energy densities have decreased over time in a manner similar to adult alewife energy densities. Third, even if mean age-0 alewife energy densities have changed little over time it is now appropriate to fill in some glaring data gaps. That is, there is only a single published measure of age-0 alewife energy density (5,020 J g wet weight-1, for individuals with mean length of 50-mm collected during October 1979-1981, Stewart and Binkowski 1986 from Flath and Diana 1985; see Figure 2). In simulating growth and consumption of Lake Michigan alewives, other authors have made assumptions regarding age-0 alewife energy densities. For instance, based on measures of age-1 alewives Stewart and Binkowski (1986) assumed that on 1-July, 31-mm age-0 alewives have an energy density of 4,185 J g wet weight-1. Similarly, based on Pacific herring data, Hewett and Stewart (1989) assumed that age-0 alewife energy density increased linearly from 2,511 J g wet weight-1 at hatching to 4,185 J g wet weight-1 31 days post-hatch (Figure 2). Fourth, although data are limited for alewives, measures of numerous fish species demonstrate that during early life energy densities increase rapidly with ontogeny (for examples see Hanson et al. 1997). Thus, detailed size-specific measures are necessary to adequately describe how energy densities vary during early life.

Figure 2. Previously measured and assumed energy densities of age-0 alewives in Lake Michigan
In order to consider the implications of long-term reductions in alewife energy densities on salmonine consumption, we here present results from a suite of bioenergetics simulations using the Wisconsin Fish Bioenergetics 3.0 computer package (Hanson et al. 1997). First, we considered the prey biomass an individual chinook salmon (Oncorhynchus tshawytscha; a representative salmonine) would have to consume in order to match observed growth rates. Using bioenergetics parameters from Stewart and Ibarra 1991, we simulated the growth of average chinook salmon during a 180 day growing season (day 1 is 1-June). Based on available temperatures in Lake Michigan during the growing period (Höök et al. 2004) and chinook salmon temperature preferenda (Stewart and Ibarra 1991), we assumed that the simulated salmon continuously occupied 11°C waters. Further, we assumed that chinook salmon diet varied seasonally, consisting of a) non-alewife prey, b) age ≥1 and c) age-0 alewives (Figure 3; Jude et al. 1987; Stewart and Ibarra 1991), with prey energy densities varying seasonally (Stewart and Binkowski 1986; see Figure 3). Based on these input variables, an individual age-1 (lake age) chinook salmon would have to consume 5.8-kg of prey in order to match observed growth (from 0.5 to 2.5 kg; Höök et al. 2003), and an age-2 (lake age) chinook salmon would have to consume 15.4-kg of prey in order to match observed growth (from 2.5 to 6.5 kg; Höök et al. 2003). If the energy densities of adult alewives decreased by 25% (i.e., as suggested by Pothoven et al.'s preliminary data; see Figure 1), then age-1 and age-2 salmon would have to increase consumption by 21% (7.0-kg total) and 22% (18.8-kg total), respectively, in order to maintain observed growth rates. Further, if age-0 alewife energy densities also decreased by 25% (which seems feasible), then age-1 and age-2 salmon would have to increase consumption by 33% (7.7-kg total) and 35% (20.8-kg total), respectively, in order to maintain observed growth rates. In short, potential long-term changes in age-0 alewife energy densities coupled with the observed decrease in adult alewife energy densities (Pothoven et al. unpublished data) may have greatly diminished the capacity of Lake Michigan's alewife population to support the current biomass of salmonine stocks. This has clear implications for future salmonine stocking rates.
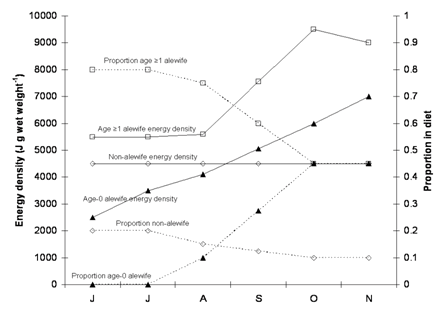
Figure 3. Input data for baseline Chinook bioenergetics model described above, including seasonal prey energy densities (open symbols) and diet composition (closed symbols) for age ?1 alewives (squares), age-0 alewives (triangles), and non-alewife prey (circles).
Round Gobies Round gobies are relatively recent invaders of the Great Lakes. Since their detection during 1990 (Jude et al. 1992), they have rapidly expanded throughout the region (Charlebois et al. 2001; Clapp et al. 2001; Corkum et al. 2004). Gobies have become locally dominant and ecologically important, serving as important prey (Steinhart et al. 2004), predators (Djuricich and Janssen 2001), and competitors (Janssen and Jude 2001; Balshine et al. 2005). A recently developed bioenergetics model (Lee and Johnson 2005) now allows for several potential bioenergetics-based analyses of the importance of round gobies as consumers and prey. However, in order to undertake such analyses detailed ontogentic and season-specific measures of energy density are necessary. There is limited peer-reviewed and published information on round goby energy densities in the Great Lakes (and existing unpublished data do not sufficiently capture ontogenetic and seasonal variation). Thus, the need to fill in this glaring data gap easily justifies quantification of round goby energy densities.
Information on the dynamics of YOY round goby energy densities can also help elucidate a potential mechanism leading to goby year-class strength variation. Due to their significance as forage for piscivores and consumers of benthic macro-invertebrates, it has become increasingly important to predict goby year-class strength. For many fish populations one can explore factors affecting year-class strength variation by analyzing long-term time series of stock-recruitment data. However, due to their recent invasion such analyses are not possible for round gobies in the Great Lakes. Instead, mechanistic explorations of potential determinants of year-class strength are more tractable. By quantifying goby energy densities, we will explore if over-winter mortality (winter severity) is a potential mechanism of year-class strength variation.
2006 Plans
- Quantify ontogenetic and seasonal patterns of young alewife and round goby energy densities.
- Compare data on alewife with limited existing data to evaluate long-term changes in young alewife condition.
- Document changes in energy densities and potential mortalities over winter, thereby gaining understanding of a potential determinant of alewife and round goby year-class strength (i.e., selective over-winter mortality).
Methods
In the laboratory, we will thaw individual fish and measure their total lengths and wet mass. Then, we will remove gut contents (see below) and oven-dry fish at 60-65°C. We will record an individual's final dry mass when its drying mass does not change between subsequent days. We will then grind dry fish carcasses and measure energy density using a Parr 1261 isoperibol bomb calorimeter. We anticipate that a minimum of 0.33-g of dry fish material is necessary to obtain a realistic measure of energy density. Assuming that dry mass is 20% of wet mass, this corresponds with a 1.7-g alewife (~45-mm). Thus, for age-0 alewives ≥ 45-mm we can likely measure energy densities of individual fish. However, for smaller fish we will combine dry material from >1 similar-sized fish collected at the same time.
REFERENCES
Balshine, S., A. Verma, V. Chant, and T. Theysmeyer. 2005. Competitive interactions between round gobies and logperch. Journal of Great Lakes Research 31: 68-77.
Bartell, S.M., J.E. Breck, R.H. Gardner, and A.L. Brenkert. 1986. Individual parameter perturbation and error analysis of fish bioenergetics models. Canadian Journal of Fisheries and Aquatic Sciences 43: 160-168.
Bergstedt, R.A. and R. O'Gorman. 1989. Distribution of alewives in southeastern Lake Ontario in autumn and winter: a clue to winter mortalities. Transactions of the American Fisheries Society 118:687-692.
Biro, P.A., A. E. Morton, J.R. Post, and E.A. Parkinson. 2004. Over-winter lipid depletion and mortality of age-0 rainbow trout (Oncorhynchus mykiss). Canadian Journal of Fisheries and Aquatic Sciences 61: 1513-1519.
Brandt, S.B., D.M. Mason, D.B. MacNeill, T. Coates, and J.E. Gannon. 1987. Predation by alewives on larvae of yellow perch in Lake Ontario. Transactions of the American Fisheries Society 116:641-645.
Brandt, S.B., D.M. Mason, E.V. Patrick, R.L. Argyle, L. Wells, P.A. Unger, D.J. Stewart. 1991. Acoustic measures of the abundance and size of pelagic planktivores in Lake Michigan. Canadian Journal of Fisheries and Aquatic Sciences 48:894-908.
Brooks, J.L., and S.I. Dodson. 1965. Predation, body size, and composition of plankton. Science. 150: 28-35.
Brown, E.H., Jr. 1972. Population biology of alewives, Alosa pseudoharengus, in Lake Michigan, 1949-70. Journal of the Fisheries Research Board of Canada 29:477-500.
Charlebois, P.M., L.D. Corkum, D.J. Jude, and C. Knight. 2001. The round goby invasion: current research and future needs. Journal of Great Lakes Research 27: 263-266.
Clapp, D.F., P.J. Schneeberger, D.J. Jude, G. Madison, and C. Pistis. 2001. Monitoring round goby population expansion in eastern and northern Lake Michigan. Journal of Great Lakes Research 27: 335-341.
Corkum, L.D., M. R. Sapota, and K.E. Skora. 2004. The round goby, a fish invader on both sides of the Atlantic Ocean. Biological Invasions 6: 173-181.
Djuricich, P. and J. Janssen. 2001. Impact of round goby predation on zebra mussel size distribution at Calumet Harbor, Lake Michigan. Journal of Great Lakes Research 27: 312-318.
Eshenroder, R.L., M. E. Holey, T.K. Gorenflo, and R.D. Clark Jr. 1995. Fish-community objectives for Lake Michigan. Great Lakes Fishery Commission Special Publication. 95-3. 56 p.
Evans, M.S. 1992. Historic changes in Lake Michigan zooplankton community structure- the 1960's revisited with implications for top-down control. Canadian Journal of Fisheries and Aquatic Sciences 49: 1734-1749.
Flath, L.E. and J.S. Diana. 1985. Seasonal energy dynamics of the alewife in southeastern Lake Michigan. Transactions of the American Fisheries Society 114:328-337.
Garvey, J.E. and E.A. Marschall. 2003. Understanding latitudinal trends in fish body size through models of optimal seasonal energy allocation. Canadian Journal of Fisheries and Aquatic Sciences 60: 938-948.
Hanson, P.C., T.B. Johnson, D.E. Schindler, and J.F. Kitchell. 1997. Fish Bioenergetics 3.0. Center for Limnology, University of Wisconsin-Madison. University of Wisconsin Sea Grant Institute.
Hewett, S.W. and D.J. Stewart. 1989. Zooplanktivory by alewives in Lake Michigan: ontogenetic, seasonal, and historical patterns. Transactions of the American Fisheries Society 118: 581-596.
Höök, T.O., E.S. Rutherford, S.J. Brines, D.M. Mason, D.J. Schwab, M.J. McCormick, G.W. Fleischer, and T.J. DeSorcie. 2003.Spatially explicit measures of production of young alewives in Lake Michigan: Linkage between essential fish habitat and recruitment. Estuaries. 26: 21-29
Höök, T.O., E.S. Rutherford, S.J. Brines, D.J. Schwab, and M.J. McCormick. 2004. Relationship between surface water temperature and steelhead distributions in Lake Michigan. North American Journal of Fisheries Management. 24: 211-221.
Höök, T.O. 2005. Habitat-mediated production and recruitment of young alewives in Lake Michigan. PhD Dissertation. University of Michigan, Ann Arbor, MI.
Houde, E.D. 1997. Patterns and consequences of selective processes in teleost early life histories. In R.C. Chambers and E.A. Trippel (eds.), Early Life History and Recruitment in Fish Populations. Chapman and Hall, New York. 173-196.
Houde, E.D. 2002. Mortality. in, Fishery Science: The unique contributions of early life stages. L. A. Fuiman and R.G. Werner eds. Blackwell Sciences Ltd.
Hurst, T.P., and D.O. Conover. 2003. Seasonal and interannual variation in the allometry of energy allocation in juvenile striped bass. Ecology. 84: 3360-3369.
Ihssen, P.E., G.W. Martin, and D.W. Rodgers. 1992. Allozyme variation of Great Lakes alewife, Alosa pseudoharengus: Genetic differentiation and affinities of a recent invader. Canadian Journal of Fisheries and Aquatic Science. 49: 1770-1777.
Janssen, J., and D.J. Jude. 2001. Recruitment failure of mottled sculpin in Calumet Harbor, southern Lake Michigan, induced by the newly introduced round goby. Journal of Great Lakes Research 27: 319-328.
Jude, D.J., F.J. Tesar, S.F. Deboe, and T.J. Miller. 1987. Diet and selection of major prey species by Lake Michigan salmonines, 1973-1982. Transactions of the American Fisheries Society 116: 677-691.
Jude, D.J., R.H. Reider, and G.R. Smith. 1992. Establishment of Gobiidae in the Great Lakes Basin. Canadian Journal of Fisheries and Aquatic Science. 49: 416-421.
Lee, V.A. and T.B. Johnson. 2005. Development of a bioenergetics model for the round goby. Journal of Great Lakes Research 31: 125-134.
Klumb, R.A., L.G. Rudstam, and E.L. Mills. 2003. Comparison of alewife young-of-the-year and adult respiration and swimming speed bioenergetics parameters: implications of extrapolation. Transactions of the American Fisheries Society 132: 1089-1103.
Madenjian, C.P. and 14 co-authors. 2002. Dynamics of the Lake Michigan food web, 1970-2002. Canadian Journal of Fisheries and Aquatic Science. 59: 736-753.
Madenjian, C.P., J.D. Holuszko, and T.J. Desorcie. 2003. Growth and condition of alewives in Lake Michigan, 1984-2001.
Madenjian, C.P, T.O. Höök, E.S. Rutherford, E. Szalai, J.R. Bence, D.M. Mason, and T.E. Croley II. 2005. Recruitment variability of alewives in Lake Michigan. Transactions of the American Fisheries Society 134: 218-230.
Mansfield, P.J. and D.J. Jude. 1986. Alewife (Alosa pseudoharengus) survival during the first growth season in southeastern Lake Michigan. Canadian Journal of Fisheries and Aquatic Science. 43: 1318-1326.
Mason, D.M. and S.B. Brandt 1996. Effect of alewife predation on survival of larval yellow perch in an embayment of Lake Ontario. Canadian Journal of Fisheries and Aquatic Science 53: 1609-1617.
Metcalfe, N.B., C.D. Bull, and M. Mangel. 2002. seasonal variation in catch-up growth reveals state-dependent somatic allocations in salmon. Evolutionary Ecology Research. 4: 871-881.
Miller, T.J., L.B. Crowder, J.A. Rice, and E.A. Marschall. 1988. Larval size and recruitment mechanisms in fishes: toward a conceptual framework. Canadian Journal of Fisheries and Aquatic Science. 45: 1657-1670.
O'Gorman, R. and C.P. Schneider. 1986. Dynamics of alewives in Lake Ontario following a mass mortality. Transactions of the American Fisheries Society. 115:1-14.
O'Gorman, R., E.L. Mills, and J. DeGisi. 1991. Use of zooplankton to assess the movement and distribution of alewife (Alosa pseudoharengus) in south-central Lake Ontario in spring. Canadian Journal of Fisheries and Aquatic Science. 48: 2250-2257.
Post, J.R., and E.A. Parkinson. 2001. Energy allocation strategy in young fish: allometry and survival. Ecology. 82: 1040-1051.
Pothoven, S.A., and H.A. Vanderploeg. 2004. Diet and prey selection of alewives in Lake Michigan: seasonal, depth, and interannual patterns. Transactions of the American Fisheries Society. 133:1068-1077.
Rand, P.S., B.F. Lantry, R. O'Gorman, R.W. Owens, and D.J. Stewart. 1994. Energy density and size of pelagic prey fishes in Lake Ontario, 1978-1990: implications for salmonine energetics. Transactions of the American Fisheries Society 123: 519-534.
Rand, P.S., D.J. Stewart, B.F. Lantry, L.G. Rudstam, O.E. Johansson, A.P. Goyke, S.P. Brandt, R. O'Gorman, and G.W. Eck. 1995. Effect of lake-wide planktivory by the pelagic prey fish community in Lakes Michigan and Ontario. Canadian Journal of Fisheries and Aquatic Science 52: 1546-1563.
Scavia, D., G.L. Fahnenstiel, M.S. Evans, D.J. Jude, and J.T. Lehman. 1986. Influence of salmonine predation and weather on long-term water quality trends in Lake Michigan. Canadian Journal of Fisheries and Aquatic Science 43: 435-443.
Schultz, E.T., and D.O. Conover. 1997. Latitudinal differences in somatic energy storage: adaptive responses to seasonality in an estuarine fish (Atherinidae: Menidia menidia). Oecologia. 109: 516-529.
Scott, W.B. and E.S. Crossman. 1973. Freshwater Fishes of Canada. Fisheries Research Board of Canada. Ottawa.
Smith, S.H. 1970. Species interactions of the alewife in the Great Lakes. Transactions of the American Fisheries Society 99: 754-765.
Steinhart, G.B., R.A. Stein, and E.A. Marschall. 2005. High growth rate of young-of-the-year smallmouth bass in Lake Erie: a result of the round goby invasion?. Journal of Great Lakes Research 30: 381-389.
Stewart, D.J., J.F. Kitchell, and L.B. Crowder. 1981. Forage fish and their salmonid predators in Lake Michigan. Transactions of the American Fisheries Society. 110: 751-763.
Stewart, D.J and F.P. Binkowski. 1986. Dynamics of consumption and food conversion by the Lake Michigan alewives: an energetics-modeling synthesis. Transactions of the American Fisheries Society 115:643-661.
Stewart, D.J. and M. Ibarra. 1991. Predation and production by salmonine fishes in Lake Michigan, 1978-88. Canadian Journal of Fisheries and Aquatic Sciences 48:909-922.
Wells, L. 1970. Effects of alewife predation on zooplankton populations in Lake Michigan. Limnology and Oceanography. 15: 556-565.
Last updated: 2006-07-24 mbl

