 |
 |
FDA Home Page | Search
FDA Site | FDA A-Z Index | Contact
FDA
![]()
| Email
this Page To a Friend |
|
By Linda Bren
When he entered his lab on Dec. 23, 2003, Allen Jenny, D.V.M., knew right away that something was wrong. He recalls the solemn expression on the face of a fellow scientist, who said, "I've got a slide to show you." Jenny, a U.S. Department of Agriculture (USDA) pathologist, peered through the microscope. What he saw was a bright red stain seeping into the gray matter of a slice of brain tissue--a telltale sign of bovine spongiform encephalopathy (BSE), otherwise known as mad cow disease.
"Are you sure it's a cow?" Jenny asked, a logical question in light of the fact that the National Veterinary Services Laboratory in Ames, Iowa, also tests sheep, deer, and other animals for mad cow-like diseases.
Yes, it was a cow, and this first diagnosis of BSE in the United States launched an emergency investigation that involved two countries--the United States and Canada--and regulatory changes by two U.S. government agencies to further bolster their effective safeguards to protect public health and livestock.
After U.S. authorities announced on the same day that a single dairy cow in Washington state was infected with the fatal brain-wasting disease, BSE, the Food and Drug Administration and the USDA took immediate action. While the USDA went to work to trace the origin of the cow and to initiate a recall of its meat, the FDA made sure that other portions of the cow, including the infectious brain and spinal cord, didn't get into animal feed or other FDA-regulated products. It is believed that BSE spreads when cows eat feed containing remnants of infected cattle. The FDA, which regulates animal feed, has banned the use of these remnants in feed for cattle and other ruminants, such as sheep and goats, since 1997. Canada implemented a similar ban at the same time.
USDA investigators and Canadian health officials found the herd the infected cow originally came from, identified her former herd mates, and then traced many of them to the herds they were later sent to.
"BSE does not spread from cow-to-cow contact," says Stephen F. Sundlof, D.V.M., Ph.D., director of the FDA's Center for Veterinary Medicine. "But we wanted to find these cows because they may have shared a common feed source when they were young," he says, and might also be infected.
An exhaustive search uncovered no other infected cows.
By the time the BSE investigation was completed in February 2004, the USDA had examined the identification tags and other devices on 75,000 cattle in three states--Washington, Oregon, and Idaho--and had humanely slaughtered 255 adult cattle and tested them for BSE.
Previously, in May 2003, Canadian authorities had reported finding the first native BSE cow in North America. Records indicated that this cow and the one found in Washington were more than six years old. "We now have very good evidence that both of these animals were born prior to the feed ban" in the United States and Canada, says Ron DeHaven, chief veterinarian at the USDA.
Beginning as far back as 1989, the FDA and the USDA had set up a series of safeguards to protect against the spread of BSE. The two agencies have continually evaluated these safeguards and other possible measures to protect public health. After finding the BSE-infected cow in Washington, the agencies introduced some additional measures to further safeguard human and animal health.
The FDA and the USDA work in complementary roles to protect the food supply, and both have regulatory responsibilities. The FDA's responsibility in the human food area generally covers all domestic and imported food except meat, poultry, and frozen, dried, and liquid eggs, which are under the authority of the USDA. But the FDA does regulate certain foods that contain a small amount of meat, such as soups, gravies, and pizza with meat topping. In addition, the FDA regulates animal feed. The USDA also protects and improves the health of the nation's animals by preventing, controlling, and eliminating animal diseases.
Agriculture Secretary Ann M. Veneman announced the USDA's additional measures to protect against BSE on Dec. 30, 2003. These measures include prohibiting all material from non-ambulatory cattle in human food, removing certain cattle tissues from the human food chain, and banning certain nervous system tissues in meat products obtained by an industrial technology to cut meat from bones called advanced meat recovery.
On Jan. 26, 2004, Health and Human Services Secretary Tommy G. Thompson announced the FDA's new measures, including banning certain additional materials from animal feed. "These actions will make strong public health protections against BSE even stronger," said Thompson.
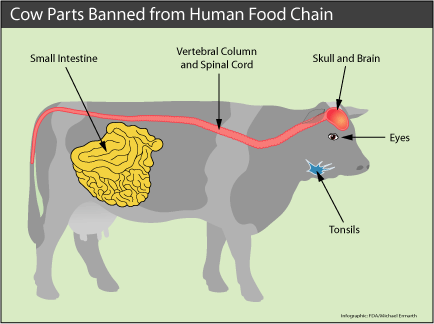 Parts
of cattle at high risk for harboring the infectious agent for bovine spongiform
encephalopathy (BSE), or mad cow disease, include the skull, brain, eyes, vertebral
column, and spinal cord of cows at least 30 months of age. The tonsils and a
portion of the small intestine of all cattle also may contain the agent. Federal
agencies protect public health by prohibiting these cow parts in the human food
supply.
Parts
of cattle at high risk for harboring the infectious agent for bovine spongiform
encephalopathy (BSE), or mad cow disease, include the skull, brain, eyes, vertebral
column, and spinal cord of cows at least 30 months of age. The tonsils and a
portion of the small intestine of all cattle also may contain the agent. Federal
agencies protect public health by prohibiting these cow parts in the human food
supply.
Infographic: FDA/Michael Ermarth
The recent measures to protect against BSE add strength to the federal government's already effective series of safeguards, or "firewalls." If one firewall fails, multiple backup safeguards are in place to continue protecting the public.
The first firewall is the USDA's 1989 ban on importing live ruminants and subsequent USDA and FDA import controls over most ruminant products from countries with BSE or considered to be at risk for BSE.
The second firewall, the USDA's surveillance program to look for BSE in cows, began in 1990. It was through this program that the BSE-infected cow in Washington was found. The USDA has tested more than 20,000 cows for BSE in each of the last two years, and plans on significantly increasing its testing to test every animal in the high-risk cattle population in 2004 and 2005.
Despite this rigorous surveillance program, some people have called for testing all slaughtered cows. But many of these cows are young and the BSE agent doesn't usually show up in animals until after 30 months of age, says DeHaven. "This is a disease with a very long incubation period--typically three to eight years," he says. "To suggest that we would test all animals regardless of age at slaughter is not consistent with the science and what we know about the disease."
"Animals may get exposed at a very young age," says Linda Detwiler, D.V.M., adjunct professor at the University of Maryland. "During the incubation period, the BSE agent replicates in certain tissues of the body and usually after three years makes its way to the brain." The tests used today can detect the infection only in the brain--not in other tissues or blood, so BSE cannot be diagnosed in very young animals, says Detwiler. "The animal may be infected, but it just can't be detected."
Detwiler uses the analogy of looking for a human disease, such as Alzheimer's, to explain the rationale for the current BSE surveillance system. "If you didn't know that Alzheimer's was in the United States, what population would you concentrate on to be able to find it?" asks Detwiler. "Would you start taking brain biopsies from teen-agers, middle-aged, or older people? Testing older folks with dementias increases your chance of finding the disease."
The FDA's 1997 feed rule, which bans most protein from mammals in ruminant animal feed, is the third BSE firewall. In 2001, the Harvard Center for Risk Analysis concluded that the feed rule provided the nation's major protection against BSE becoming established in the United States. In August 2003, Harvard reaffirmed the findings of its initial study and concluded that if infected animals or feed material entered the United States from Canada, the risk of spreading extensively within U.S. herds was extremely low.
The fourth firewall is the additional protective measures recently taken by the FDA and the USDA, and the fifth firewall is the emergency response plan that the agencies initiated immediately after finding the BSE-positive cow in Washington state.
Some critics have suggested that the federal government take further actions, such as tightening slaughter controls and feed regulations, similar to practices used by European governments in their efforts to combat BSE. "You shouldn't equate the actions that the Europeans had to take with the actions that we need to take because the situation in the two continents was very different when BSE was discovered in each continent," says Murray Lumpkin, M.D., the FDA's deputy commissioner for special programs. "In Europe, BSE was at an epidemic stage in their cattle population with ultimately thousands of cases identified. So they had to put in place measures not only to keep BSE from spreading but to contain an epidemic. Based on their experience, we put many BSE firewalls in place before we had any evidence of disease in this country."
Since the feed rule went into effect in 1997, FDA and state inspectors have conducted more than 26,000 inspections involving more than 13,000 firms that handle animal feed.
"Regulations are only as good as your enforcement activities," says Sundlof. When violations are found, the firm must quickly address them and undergo a prompt follow-up inspection. Depending on the nature of the violation, a firm's products could be recalled, it could receive an FDA warning letter that demands a response from the firm about how it will correct the violations, or it could find itself in court. Renderers, feed mills, and protein blenders that process materials prohibited for ruminants are inspected at least annually, and more frequently if they are not in compliance with the rule, says Sundlof.
From 1997 to the end of 2003, 47 feed firms had recalled a total of 280 feed products, the FDA had issued 63 warning letters, and the court had ordered one permanent injunction against a feed company. The recall track record is improving: Only 12 of the 280 recalls occurred during 2003.
Sundlof says a strong education program on the feed rule for industry and inspectors has contributed to the high rate of compliance, which has climbed from 75 percent when inspections first began in 1997 to more than 99 percent today.
"The compliance rate is the highest of any FDA compliance program in all of the categories of products that we regulate," says Acting Commissioner of Food and Drugs Dr. Lester M. Crawford. "It is probably the most effective regulatory program that FDA has had in its 100-year history."
All of the firms involved in the investigation of the BSE-infected cow in Washington were in compliance with the FDA's feed rule.
The agency is stepping up its inspections of feed facilities to further ensure compliance. The FDA, along with state agencies, plans to conduct 6,600 inspections in 2004, several hundred more than the previous year.
Protecting the food supply from BSE includes ensuring not only the safety of meat, but also of milk and other foods, including dietary supplements, which may contain ingredients from cows.
There is no scientific evidence that milk and dairy products pose any risk for transmitting BSE to humans, and people should not be concerned about consuming milk and milk products, says the FDA's Center for Food Safety and Applied Nutrition.
Other cow-derived foods, such as gelatin, are also protected from BSE through FDA regulations and guidance to manufacturers. And since 1992, the FDA has advised dietary supplement manufacturers and distributors to take steps to ensure that supplement ingredients come from BSE-free herds. The agency also has directed manufacturers of soaps, lipsticks, and other cosmetics that may contain fat or other ingredients from cows to use only ingredients from BSE-free cattle.
Until recently, the possibility of getting the human form of mad cow disease, called variant Creutzfeldt-Jakob disease (vCJD), through tainted blood was only a theory. But in December 2003, authorities in the United Kingdom announced the death of an adult from vCJD whom they believe was infected by a blood transfusion during surgery seven and a half years earlier. The blood had been donated by a young, apparently healthy person in 1996, who died three years later from vCJD.
"We've taken the theoretical risk of blood-borne infection seriously for years, even before the U.K. transfusion case appeared," says David Asher, M.D., head of an FDA laboratory that studies contaminants in blood and tissues. Although there are no rapid, reliable tests to screen the blood supply for vCJD, the FDA has worked with blood centers since 1999 to exclude blood donations by people who lived in the U.K. during a high-risk period for BSE. In 2002, the agency added exclusions of people who might have been exposed to BSE while living elsewhere in Europe.
Certain cattle parts are used to produce a variety of medical products regulated by the FDA, including some blood products, drugs, devices, vaccines, and human cell- and tissue-based products. For example, some oral medications contain amino acids from cow tissues. Vaccines may be made by growing viruses in cell cultures using a highly diluted cow blood product called fetal bovine serum. And diseased heart valves in people may be replaced with medical devices made from tissues surrounding the heart of a cow.
In regulating these and other medical products, the FDA has advised manufacturers to use cow materials only from BSE-free herds and has recommended that non-animal materials be used in their products whenever possible. Human medical products--whether they're devices or biologics or drugs--have pre-approval requirements, says Lumpkin. When companies come to the FDA for approval, "we work with them on their manufacturing processes, what materials go into the products, and where those materials come from," he says.
The FDA continues to evaluate its regulations and guidances and seeks advice from experts outside the agency to help keep pace with the evolving science relating to transmissible spongiform encephalopathies (TSEs), the family of degenerative diseases of the nervous system that includes BSE and vCJD.
One forum for soliciting independent opinions is the TSE Advisory Committee, a group of scientists and medical experts who met most recently in Silver Spring, Md., in February 2004. In this two-day public meeting, the committee discussed additional measures that the agency might take to further minimize the risk of getting a TSE disease from FDA-regulated medical products. The agency will consider the recommendations of this committee in its regulatory decision-making to keep medical products safe.
No one knows for certain what causes BSE, but the leading scientific theory is that an abnormal form of a protein called a prion is responsible. All humans and other mammals have prion proteins in their cells that are harmless in their normal form, but become potentially damaging when they fold into a different shape, clump together, and accumulate in brain tissue. In BSE, abnormal prions are believed to enter the bodies of mammals when they eat tissues contaminated with these prions.
Researchers worldwide are studying how prions cause BSE and other TSEs and how to block the conversion of normal prion protein to the abnormal form, which may lead to methods to prevent or treat these deadly diseases.
Other areas of research focus on developing tests to diagnose TSEs in humans and animals and developing methods to detect the abnormal prions in animal feed and human food. Scientists are trying to find ways to detect BSE in live cattle, since current tests can detect it only by examining brain tissue after death.
Diagnosis of the human form of BSE, vCJD, is also confirmed by looking at brain tissue. In humans, this tissue may be collected through a biopsy while the person is ill or collected after the person has died.
Probable cases can be diagnosed in living people based on their symptoms and the results of either a tonsil biopsy or two non-invasive tests of the brain, electroencephalogram and magnetic resonance imaging. The tonsil biopsy is invasive and requires anesthetizing the patient, says Lawrence Schonberger, M.D., M.P.H., epidemiologist and assistant director of the Centers for Disease Control and Prevention's National Center for Infectious Diseases. "What we're really looking for is a urine or blood test," he says, and research continues to identify less invasive tests.
FDA researchers are developing tests to detect the prohibited proteins in animal feed for cows. These tests will help the agency enforce the feed ban, allowing samples of feed from processing facilities to be checked to ensure that they do not contain the protein that may carry the BSE agent.
FDA scientists are also evaluating decontamination techniques to try to rid TSE agents from surgical instruments, hospital rooms, and other areas where patients and health care workers may be accidentally exposed. Using an evaluation method they developed, FDA scientists have found that conventional decontamination procedures, such as heating with steam augmented by soaking in solutions of lye or using chlorine bleach, "are extremely effective in removing most of the infectivity," says Asher. "This is contrary to the mythology that boiling, heating, or other sterilization doesn't kill the agent," he says. "They don't remove 100 percent of infectivity under worst-case circumstances, but they are successful in removing huge amounts of infective material."
U.S. Department of Agriculture
USDA Meat and Poultry Hotline (English and Spanish)
(888) MP-HOTLINE (674-6854)
On Dec. 30, 2003, the USDA announced additional measures to bolster safeguards against mad cow disease:
As of February 2004, 156 cases of the human form of mad cow disease, known as variant Creutzfeldt-Jakob disease (vCJD), have been reported worldwide, according to the Centers for Disease Control and Prevention (CDC). But there has never been a case of vCJD contracted in the United States. One resident of Florida was diagnosed in 2002 with a probable case of vCJD, but it is believed she acquired it in the United Kingdom, where she lived for more than 12 years during an epidemic of bovine spongiform encephalopathy (BSE), or mad cow disease. It is believed that the vCJD victims got the human variant by eating beef products that came from BSE-infected cattle.
The classic form of CJD has been found in the United States. Unlike the variant, the classic form is not known to be food-related. "CJD and vCJD are best thought of as two different diseases," says Lawrence Schonberger, M.D., M.P.H., epidemiologist and assistant director of the CDC's National Center for Infectious Diseases. "CJD was around long before the emergence of BSE in cattle." Both diseases are brain disorders, but the patterns of the brain lesions they leave are distinct.
Variant CJD is found in younger patients and the length of illness is longer. There is no treatment for either disease, and they always result in death. The average age for death of vCJD is under 30 years versus the mid- to late 60s for classic CJD.
Neither vCJD nor CJD is spread through direct contact with others with the infection. The classic form of CJD may be inherited (familial), transmitted by infectious surgical instruments or tissues (iatrogenic), or occur among people with no known environmental risk factors (sporadic). The sporadic form generally occurs at a rate of about 1 case per million people per year, and familial and iatrogenic cases are even rarer, according to the CDC.
Centers for Disease Control and Prevention
Creutzfeldt-Jakob Disease Foundation
Inc.
(800) 659-1991
Cats are the only pets known to be affected by the infectious agent that causes bovine spongiform encephalopathy (BSE), known as mad cow disease, in cattle. No evidence of BSE has been found in dogs, horses, birds, or reptiles.
About 90 cats in the United Kingdom and several cats in other European countries have been diagnosed with feline spongiform encephalopathy (FSE). Before it was recognized that they could be affected by the BSE agent, cats were exposed to it through commercial cat food or meat scraps provided by butchers. The number of reported cases of FSE in Europe has been declining annually since 1994 after European countries implemented feed bans that lowered the risk of BSE transmission.
To date, no case of FSE has been found in the United States. The FDA believes that the safeguards it has put in place to prevent BSE in this country have also protected cats.
Infections are more likely to be transmitted within a species than from one species to another, a concept known as the species barrier. Bovine spongiform encephalopathy (BSE), or mad cow disease, was once thought to infect only cows. But now scientists know it jumps the species barrier to infect humans in the form of a brain-wasting disease called variant Creutzfeldt-Jakob disease (vCJD). BSE and vCJD belong to a family of degenerative diseases of the nervous system called transmissible spongiform encephalopathies (TSEs).
European authorities suggest that the cattle disease may be 10 to 100,000 times less infectious in humans. As of March 3, 2004, the United Kingdom reported 146 cases of vCJD, yet more than 180,000 cattle in Great Britain were diagnosed with BSE. But some scientists fear that many more people are incubating vCJD and signs of the disease just haven't shown up yet.
"It's not appropriate at this stage to totally rely on the species barrier to protect the population against BSE," says Lawrence Schonberger, M.D., M.P.H., epidemiologist and assistant director of the Centers for Disease Control and Prevention's National Center for Infectious Diseases. "We don't know for sure how well the population is protected. We must try to reduce exposures to the maximum extent possible."
Understanding which animals can get and transmit BSE and how likely they are to get the disease helps government agencies set policies to protect human and animal health. BSE has never been found to occur naturally in pigs or to be transmissible to pigs by feeding them the BSE agent. However, researchers have been able to infect pigs by injecting them with the BSE agent. Researchers have also infected sheep and goats by feeding them BSE-contaminated food. Chickens and turkeys have not been shown to develop the disease naturally or through injection.
But why are scientists trying to infect animals with BSE through non-natural routes, such as injecting it into the brain? "If a TSE is not transmitted by the most direct route--injection into the brain--one may have more assurance that the species is resistant through other routes, such as orally," explains Linda Detwiler, D.V.M., adjunct professor at the University of Maryland. "But I think we have to be so careful that by constant exposure we do not force infectivity into another species just because we think they have a resistance," she says. "The resistance may, in some cases, be able to be overpowered or the TSE agent might adapt."
![]()