|
Laboratory Information Bulletin No. 4334
November 2004
This document is also available in PDF (191 Kb).
U.S. Food and Drug Administration, Animal Drugs Research Center, Denver, CO
The Laboratory Information Bulletin is a communication from the Division of Field Science, Office of Regulatory Affairs, U.S. Food and Drug Administration for the rapid dissemination of laboratory methods (or scientific regulatory information) which appear to solve a problem or improve an existing problem. In many cases, however, the report may not represent completed analytical work. The reader must assure, by appropriate validation procedures, that the reported methods or techniques are reliable and accurate for use as a regulatory method. Reference to any commercial materials, equipment, or process does not, in any way, constitute approval, endorsement, or recommendation by the U.S. Food and Drug Administration.
A liquid chromatographic method is presented for the quantitative determination of malachite green in salmon. Malachite green (MG) and leucomalachite green (LMG) residues were extracted from salmon tissue with ammonium acetate buffer and acetonitrile, and then isolated by partitioning into methylene chloride. Leucomalachite green was quantitatively oxidized to the chromic malachite green by reaction with 2,3-dichloro-5,6-dicyano-1,4-benzoquinone. Samples were then cleaned-up by solid phase extraction with alumina and propylsulfonic acid phases. Extracts were analyzed for MG by LC with visible detection at 618 nm, using isocratic elution and a C18 column. The method was validated with fortified farm-raised salmon tissues at 1, 2, 4, and 10 ng/g (ppb), and canned salmon (wild-caught) at 10 ppb. The average recovery of 40 fortified samples was 94.6 % with an RSD of ± 10.7 %. Malachite green was quantitatively determined at the method detection limit of 1 ppb.
Malachite green (MG) is a cationic triphenylmethane dye commercially available as the oxalate and hydrochloride salts. MG is well known as an effective topical fungicide used by the aquaculture industry. Fish treated with malachite green will excrete MG rapidly, but will store the metabolized leuco base, leucomalachite green (LMG), in muscle tissue for months. In trout and catfish, 80 % or more of the MG absorbed is converted to LMG.1, 2 The half-life of LMG residence in trout muscle may be as long as 40 days.1 It is, therefore, likely that the majority of violative residues present in fish will be in the form of LMG.
Malachite green is a suspected mutagen and teratogen.3 For this reason, MG is not approved as an aquaculture veterinary drug in many countries including the United States, Canada and the European Union. The European Commission requires that methods be able to determine the sum of MG and LMG residues at the minimum performance limit of 2 ng/g.4 Between 2001 and 2003, the United Kingdom Veterinary Residues Committee reported that of 343 domestic and imported salmon samples tested, 33 were found to have violative residues of LMG green at levels typically ranging from 2 to 18 ng/g.5
In past years, the U.S. Food and Drug Administration has monitored catfish for the presence of MG and LMG using the method of Roybal et al.6 According to that method, MG and LMG residues are extracted from catfish and analyzed by chromatographically separating the residues, then converting the colorless LMG to MG in a post-column oxidation column, and separately measuring the visible absorbance of MG and MG (converted from LMG) at 618 nm.7
This Laboratory Information Bulletin presents a simplified method for the determination of LMG and MG in salmon that reduces sample size, solvent volume, minimizes sample preparation, and introduces an in-situ oxidation of LMG to MG with 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) to eliminate the need for post-column lead oxide oxidation.8 Visible absorbance at 618 nm is used to determine a single MG chromatographic peak that results from either the presence of LMG or MG in the salmon. This method results in the quantitative determination of LMG and MG in salmon with a residue detection limit of 1.0 ng/g.
Equipment and reagent sources have been provided for information and guidance. Equivalent products may be substituted as appropriate.
MG Standard Solutions for Calibration Curve Only:
(MG1, 1.0 μg/mL): Pipette 1.0 mL of the MG stock solution into a 100 mL volumetric flask, dilute to volume with methanol and mix to prepare MG1 working standard. (MG1-ICV, 1.0 μg/mL): Prepare a second 1.0 μg/mL MG working standard from the MG-ICV stock solution.
(MG2, 0.1 μg/mL): Pipette 1.0 mL of (MG1) into a 15 mL graduated glass centrifuge tube and dilute to 10 mL with methanol to prepare MG2 working standard.
(MG2-ICV, 0.1 μg/mL): Prepare a second 0.1 μg/mL MG working standard from the MG1-ICV working standard solution.
Prepare a series of MG LC calibration standards by aliquoting into individual 15 mL graduated glass centrifuge tube, 50 μL, 100 μL, and 200 μL of the 0.1 μg/mL MG2 solution and 50 μL and 100 μL of the 1.0 μg/mL MG1 solution, as summarized in Table 1. Dilute each to 5.0 mL with (1:1 vol) acetonitrile/ammonium acetate buffer (mobile phase A), vortex mix or stopper and invert to mix thoroughly. Based on a 5 g sample weight, these solutions will generate a standard curve that will have a range of 0, 1, 2, 4, 10 and 20 ppb of MG. Prepare a 4 ppb ICV standard by diluting 200 μL of the 0.1 μg/mL MG2-ICV solution to 5 mL with mobile phase A.
| Calibration Standard (ppb) |
Use MG Working Standard (MG1 or MG2) |
Volume of Working Standard Used (μL) |
Final Volume (mL) Dilute with Mobile Phase A |
|---|---|---|---|
| 0 | - | - | 5 |
| 1 | MG2 | 50 | 5 |
| 2 | MG2 | 100 | 5 |
| 4 | MG2 | 200 | 5 |
| 4 - ICV | MG2-ICV | 200 | 5 |
| 10 | MG1 | 50 | 5 |
| 20 | MG1 | 100 | 5 |
(LMG1, 1.0 μg/mL): Pipette 1.0 mL of the LMG stock solution into 100 mL volumetric flask, dilute to volume with methanol and mix to prepare LMG1 fortification solution.
(LMG2, 0.1 μg/mL): Pipette 1.0 mL of (LMG1) into a 15 mL graduated glass centrifuge tube and dilute to 10 mL with methanol to prepare LMG2 fortification solution.
Fresh or frozen salmon samples were composed of muscle tissue and skin (scales were removed from the skin). Thawed fish was cut into 1 to 2 inch cubes and placed in a freezer (-20 to -30 °C) for at least an hour until slightly firm or frozen. Canned salmon samples were composed of the entire contents of the can including muscle tissue, skin, bone, and juice. Can contents were emptied into a bowl, coarsely chopped with a spoon, and frozen for at least an hour until slightly firm. Fish samples were blended with dry ice in blender/homogenizer with pulsed action until contents were uniform and had the consistency of a fine powder. Homogenate was placed in Whirl-Pak bags, loosely sealed and stored in the freezer (-20 to -30 °C) overnight to allow the carbon dioxide to dissipate. Whirl-Pak bags were then tightly sealed until analysis.
Salmon samples consisting of 5.0 g of completely thawed tissue composite were fortified by spiking with the necessary volume of LMG1 or LMG2 fortification solutions to produce samples containing 1, 2, 4 and 10 ppb LMG. Fortified samples at 1, 2, and 4 ppb were obtained by spiking 5 g of salmon with 50, 100, and 200 μL aliquots, respectively, of the 0.1 μg/mL LMG2 fortification solution. The fortified samples at 10 ppb were obtained by spiking 5 g of salmon with 50 μL aliquots of the 1.0μg/mL LMG1 fortification solution. Samples were allow to sit at room temperature for at least 15 minutes before proceeding.
Accurately weigh 5.0 g prepared salmon into a 50 mL centrifuge tube. Add 5 mL of ammonium acetate buffer, 1 mL of HAH solution (0.25g/mL) and 100 μL of p-TSA solution (1 M). Cap the centrifuge tube and mix by vortexing vigorously for 30 seconds. Add 25 mL of acetonitrile, cap and shake vigorously for 30 seconds. Add 5 to 6 g (but not less than 5 g) of alumina, cap and shake vigorously for 15 seconds. Centrifuge at 0 °C for 5 minutes at 4000 rpm.
Add 50 mL water and 2 mL DEG to a 250 mL separatory funnel. Decant the sample supernatant into the separatory funnel. Add another 25 mL of acetonitrile to the solids remaining in the 50 mL centrifuge tube, cap, mix by vortexing for 30 seconds, and then shake vigorously for 30 seconds. Centrifuge at 0 °C for 5 minutes at 4000 rpm. Decant supernatant into original 250 mL separatory funnel containing first extract. Add 25 mL methylene chloride to separatory funnel, stopper, invert and open stopcock to release pressure. Close stopcock and extract by mixing funnel in bicycle motion for 30 seconds. Allow phases to separate (for at least 15 minutes). Note, if a stubborn emulsion persists, or if the organic phase has inverted and remains on top of the aqueous phase, one may add an additional 20 mL of water or 10-15 mL of methylene chloride, and re-agitate the phases to obtain the necessary separation; the sample should not be allowed excessive time to separate. Drain the lower pale-orange methylene chloride layer into a 150 mL pear-shaped boiling flask. The last few drops of the organic phase should be collected either by touching the tip of the separatory funnel to the boiling flask, or by rinsing the tip of the separatory funnel with 3 mL methanol into the boiling flask. Add an additional 25 mL of methylene chloride to the 250 mL separatory funnel, and repeat the liquid extraction as before. After letting the phases separate for 15 minutes, collect the organic phase in the original 150 mL boiling flask, combining the first and second extracts. At this point, sample may be stoppered and stored overnight at room temperature and protected from light.
Rotoevaporate the contents of the boiling flask to dryness under reduced pressure while heating flask in a water bath set at 50 °C. Add 3 mL of acetonitrile to the dried oily residue and swirl to dissolve the residue. At this point, sample may be stoppered and stored overnight at room temperature and protected from light. Add 3 mL of 0.001 M DDQ solution and swirl to mix. Sample will immediately change from orange to a dark red-purple color. Allow oxidation reaction to proceed for 30 minutes, with periodic sample agitation. The color of the sample may lighten over time; however, if the sample turns yellow, one should expect to obtain a lower than average recovery, as discussed in greater detail below.
While the oxidation reaction is occurring, prepare the SPE columns. Condition a disposable ALN-SPE alumina column and a PRS-SPE column with 5 mL of methanol followed by 5 mL of acetonitrile. Add an additional 5 mL of acetonitrile to the PRS-SPE to serve as a solvent reservoir. Connect the ALN-SPE column on top of the PRS-SPE column using a column adapter and insert the SPEs in a vacuum elution system. Transfer the oxidized sample solution to the upper ALN-SPE column, and adjust vacuum to allow sample to elute onto the PRS-SPE column at a drop rate of approximately 4 mL/min. Wash the boiling flask with two sequential portions of 5 mL acetonitrile and add each wash to the upper ALN-SPE column just as the previous liquid portion clears the upper column. Remove the ALN-SPE column from PRS-SPE column and discard. Wash the PRS-SPE with 5 mL acetonitrile. Pull vacuum on the PRS-SPE column for 2-3 seconds to remove most of the residual acetonitrile (column should not be fully dried). Remove PRS-SPE from the vacuum elution system and elute PRS-SPE column, by gravity, into 15 mL graduated centrifuge tube with 4 mL of mobile phase A (1:1 ammonium acetate buffer:acetonitrile). With hand bulb or syringe, blow out remaining solvent from column into centrifuge tube. Dilute sample to 5.0 mL with mobile phase A, and transfer a portion of the sample to a chromatographic vial for LC-visible analysis. If desired, the remaining portion of this sample may be transferred to a second vial for later residue confirmation by LC-MS.
Inject 100 μL volumes of standards and samples into the LC, using a needle wash if available. A six-point calibration curve was prepared from the peak area response of the MG LC standards shown in Table 1 (calibration curve does not include ICV). A 100 μL injection of the 4 ppb calibration standard should give an approximate peak area response for MG of 5.3 mAU·s (0.26 mAU peak height) with a retention time of approximately 8.7 minutes. Correlation coefficients for acceptable standard curves should be ≥0.995. Typical calibration data for the six standards are shown in Table 2.
| 0 ppb | 1 ppb | 2 ppb | 4 ppb | 10 ppb | 20 ppb | |
|---|---|---|---|---|---|---|
|
Actual MG Concentration (ng/mL) |
0 | 1.1259 | 2.2518 | 4.5036 | 11.259 | 22.518 |
|
MG Peak Area (mAU·s) |
ND* | 1.09773 | 2.47438 | 5.92023 | 13.90750 | 28.03680 |
slope = 1.2507; y-intercept = -0.1107; r 2 = 0.9995
* ND = None detected
As indicated, although the fortified samples are spiked with LMG, the DDQ oxidation quantitatively converts all of the LMG in the sample to MG. Recovery is therefore calculated based on the total residue as MG, and is determined from the MG calibration curve. After injecting all of the calibration standards and samples, the 2 or 4 ppb standard should be reinjected as the Continuous Calibration Verification (CCV). The 4 ppb ICV standard should be within ± 10 % of the peak area counts of the 4 ppb standard and CCV should be within ± 10 % of the peak area counts of the initial injection of that standard. After the injection of each set of standards and samples, or at the end of each day, the chromatography column should be flushed with 100 % methanol for 30 minutes.
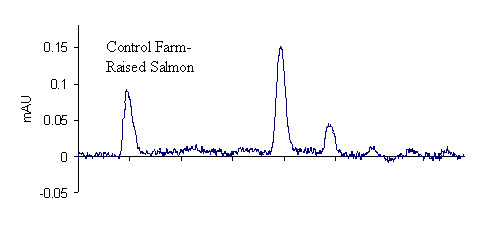
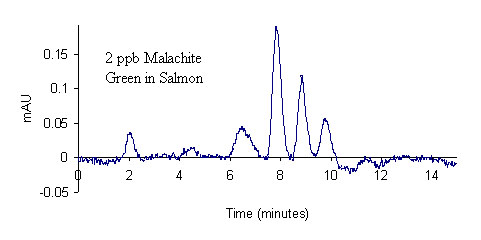
Results for the extraction of LMG from fortified salmon are shown in Table 3. A total of 40 salmon samples were spiked with varying levels of LMG to produce an overall residue recovery (as MG) of 94.6 % with an RSD of 10.7 %. Typical chromatograms are shown in Figure 1 for the salmon control, 2 ppb standard, and 2 ppb spike. No MG residue was found in any of the eleven control salmon samples or the two reagent blanks. For the 1 ppb spikes, MG was recovered for all samples in excess of 100 %. This outcome is likely a result of greater uncertainty in the chromatographic baseline noise for these low concentration samples. The signal to noise ratio obtained for the 1 ppb standards and spikes was approximately 3.9, suggesting that it may be possible to obtain quantitative results for samples with slightly lower MG concentrations; however, we did not include lower concentration spikes in this study. The method detection limit was therefore designated as 1 ppb in accordance with the lowest calibration and spike levels. The average recovery of fifteen 2 ppb and 10 ppb spikes (five of each extracted and analyzed on different days) was 97.2 ± 8.1 % and 86.2 ± 4.4 %, respectively, showing excellent inter-day reproducibility of the method. To verify method linearity, two 5.0 g salmon samples were each spiked with 250 μL of the 1.0μg/mL LMG1 fortification solution, and then extracted, to produce samples with MG concentrations of 50 ppb. These samples had an average recovery of 82.8 %, showing good method linearity.
This method requires the oxidation of the colorless LMG to MG, which can be detected at the method absorbance wavelength of 618 nm. By using a post-column lead-oxide oxidation column6 to monitor the DDQ conversion of LMG to MG, we found that the addition of 3 mL of 0.001 M DDQ to the salmon extracts provides 100 % conversion of LMG to MG. The in-situ DDQ oxidation, therefore, provides efficient LMG conversion, eliminating the need for post-column lead oxide oxidation. This method cannot distinguish between LMG and MG residues that may be present, but rather allows quantification of the sum of the residues, both of which are prohibited. In addition to the elimination of the lead oxide column, this method also has reduced or eliminated the sample size, solvent volume, and some of the sample preparation steps described in the original method.6 The current method requires approximately 5 to 6 hours of sample preparation for a set of six salmon samples. The single solvent evaporation step is the most time consuming, requiring approximately 15 to 20 minutes to evaporate each sample. Greater sample throughput would be realized if the analyst had more than one rotoevaporation unit available to use parallel evaporation.
| Spikes | Control | Recovery of spikes (%) | Average (%) | RSD (%) | ||||
|---|---|---|---|---|---|---|---|---|
| SP1 | SP2 | SP3 | SP4 | SP5 | ||||
| 1 ppb LMG | ND* | 111.0 | 109.8 | 105.3 | 117.3 | 115.3 | 111.7 | 4.2 |
| 2 ppb LMGa | ND | 99.8 | 94.8 | 98.1 | 115.3 | 100.6 | 101.7 | 7.8 |
| 2 ppb LMGa | ND | 95.5 | 86.7 | 87.0 | 97.3 | 101.7 | 93.6 | 7.0 |
| 2 ppb LMGa | ND | 98.7 | 101.1 | 81.5 | 97.5 | 102.1 | 96.2 | 8.8 |
| 4 ppb LMG | ND | 90.9 | 89.0 | 88.3 | 100.8d | 104.1d | 94.6 | 7.7 |
| 10 ppb LMGb | ND | 90.6 | 84.7 | 85.5 | 85.2 | 90.5 | 87.3 | 3.4 |
| 10 ppb LMGb | ND | 80.7 | 86.9 | 80.6 | 82.1 | 81.2 | 82.3 | 3.2 |
| 10 ppb LMGb,c | ND | 92.5 | 86.1 | 89.0 | 88.4 | 88.5 | 88.9 | 2.6 |
| Other Experiments | ||||||||
| 1, 2, 2, 4, and 10 ppb MG | ND | 73.4 | 59.1 | 63.4 | 78.1 | 81.4 | 71.1 | 13.4 |
| 50 ppb LMG | ND | 76.1 (fresh) | 89.4 (can) | |||||
| Reagent blanks | ND | ND | ND | |||||
aEach set of five 2 ppb spikes and 1 control was extracted and analyzed on 3 different days.
bEach set of five 10 ppb spikes and 1 control was extracted and analyzed on 3 different days.
cCanned salmon, rather than fresh farm-raised, was used for this set of fortified samples.
dDue to a problem of low recovery for two of the 4 ppb spikes, these two additional 4 ppb spikes were analyzed by a different analyst on the following day.
* ND = None detected
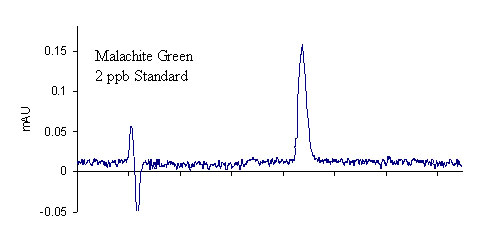
This validation study was based on the analysis of LMG residues in salmon since this metabolite is expected to be the major compound found in tissue.1,2 Several MG spikes were also included in the study to determine how the extraction and DDQ oxidation might affect residual MG that may also be present in the tissue. As shown in Table 3, five 5.0 g salmon samples were spiked with a 50, 100, 100, or 200 μL aliquot of the 0.1 μg/mL MG2 working standard solution, or a 50 μL aliquot of the 1.0 μg/mL MG1 working standard solution, to produce salmon samples fortified with MG in concentrations of 1, 2, 2, 4, and 10 ppb, respectively. These samples were extracted according to the method (including the DDQ oxidation) and the average MG recovery was found to be 71.1 ± 13.4 %. The original Roybal et al. method also found somewhat lower recoveries for MG as compared to LMG.6
The majority of the validation data in this study were generated using fresh farmed Atlantic salmon fillets (color added) that were frozen immediately after purchase from a retail grocery story. Of the three sets of 10 ppb validation data, as indicated in Table 3, one set was generated from canned Alaskan pink salmon, obtained from a retail source and presumably wild-caught. The results for all three sets of 10 ppb data indicate that the method produces equivalent recoveries for either type of salmon. As shown in Figure 1, control farmed salmon has a considerable number of chromatographic background peaks. By contrast, wild caught salmon extracts were free from interference. It should be noted that during method development, we initially used fresh wild-caught salmon as the control matrix. For the wild salmon, good recoveries could be obtained without defatting the sample with alumina. Conversely, for farmed salmon, which has a higher fat content,9 the alumina defatting step is critical to obtain good extraction and high recoveries. Many of the samples included in this study were extracted with little emphasis on the actual amount of alumina used, as long as it was approximately 5 g. In a few cases, however, less than 5 g of alumina was added to the samples. For these samples, when DDQ was added to the salmon extracts (control or spike), the sample initially turned dark red-purple, and then faded to yellow within approximately 5 minutes. This color change always corresponded to a much lower recovery of MG, indicating that a minimum amount of alumina is required to ensure complete conversion of LMG to MG. While it is still not necessary to use an exact quantity of alumina, we recommend that no less than 5 g of alumina be used. For all subsequent validation measurements, 6 g of alumina was added to the samples to ensure high recovery.
An efficient method has been presented to determine the sum of LMG and MG residues in farmed salmon with a method detection limit of 1.0 ng/g (ppb). The method was streamlined for efficiency, reduction in the quantity of reagents, and ease of analysis for use in a regulatory laboratory environment. The method relies on the in-situ conversion of LMG to MG using the oxidizing agent 2,3-dichloro-5,6-dicyano-1,4-benzoquinone. LMG residues in salmon were validated over the concentration range of 1.0 to 10.0 ppb, with overall recoveries (as MG) of 94.6 % with an RSD of 10.7 %. Malachite green residues were also extracted from salmon with an average recovery of 71.1 ± 13.4 % over the concentration range 1.0 to 10.0 ppb.