|
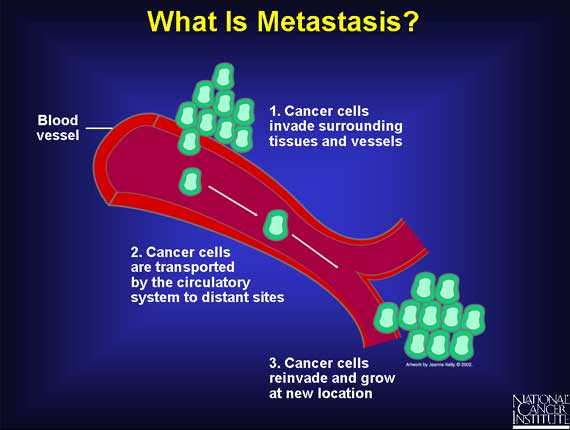
When patients are diagnosed with cancer, they want to know whether their
disease is local or has spread to other locations. Cancer spreads by
metastasis, the ability of cancer cells to penetrate into lymphatic and blood
vessels, circulate through the bloodstream, and then invade and grow in normal
tissues elsewhere.
In large measure, it is this ability to spread to other tissues and organs that
makes cancer a potentially life-threatening disease, so there is great interest
in understanding what makes metastasis possible for a cancerous tumor.
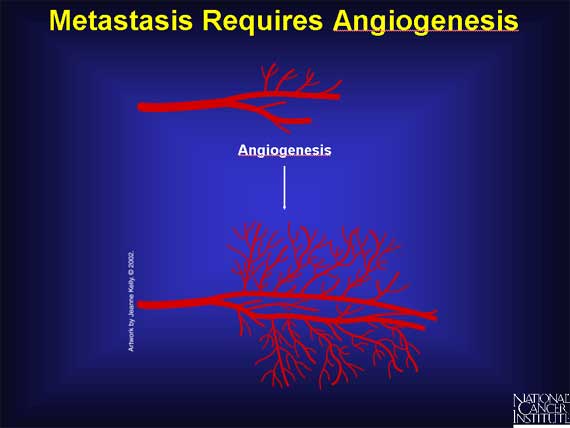
Cancer researchers studying the conditions necessary for cancer metastasis have discovered that one of the critical events required is the growth of a new network of blood vessels. This process of forming new blood vessels is called angiogenesis.
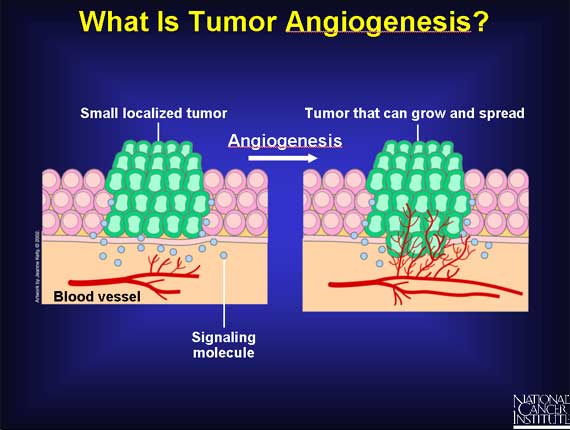
Tumor angiogenesis is the proliferation of a network of blood vessels that penetrates into cancerous growths, supplying nutrients and oxygen and removing waste products. Tumor angiogenesis actually starts with cancerous tumor cells releasing molecules that send signals to surrounding normal host tissue. This signaling activates certain genes in the host tissue that, in turn, make proteins to encourage growth of new blood vessels.
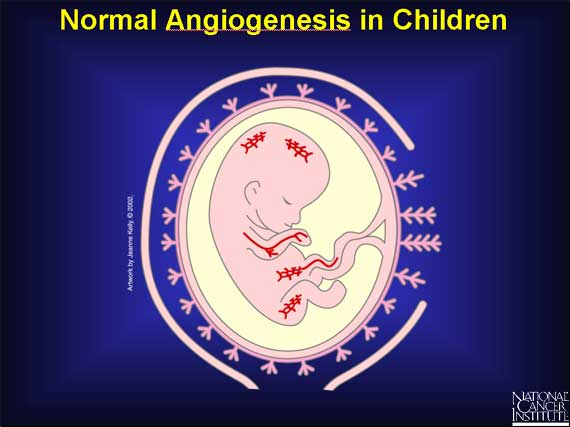
In addition to its role in tumors, angiogenesis occurs normally in the human
body at specific times in development and growth. For example, a developing
child in a mother's womb must create the vast network of arteries, veins, and
capillaries that are found in the human body. A process called vasculogenesis
creates the primary network of vascular endothelial cells that will become
major blood vessels. Later on, angiogenesis remodels this network into the
small new blood vessels or capillaries that complete the child's circulatory
system.
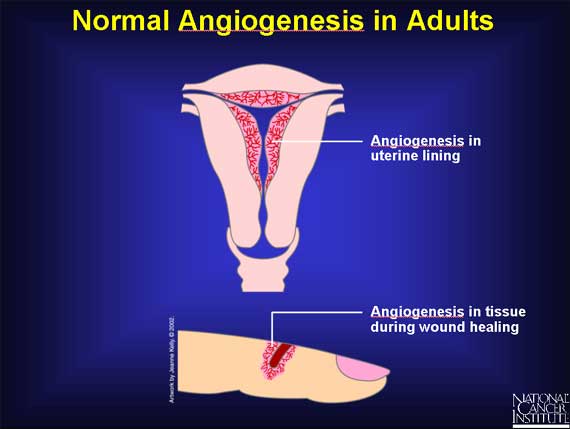
Proliferation of new blood vessels also takes place in adults, although it is a relatively infrequent event. In women, angiogenesis is active a few days each month as new blood vessels form in the lining of the uterus during the menstrual cycle. Also, angiogenesis is necessary for the repair or regeneration of tissue during wound healing.
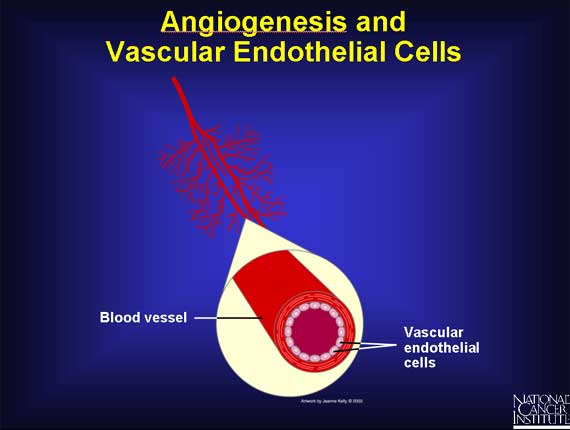
The walls of blood vessels are formed by vascular endothelial cells.
These cells rarely divide, doing so only about once every 3 years on average.
However, when the situation requires it, angiogenesis can stimulate them to
divide.
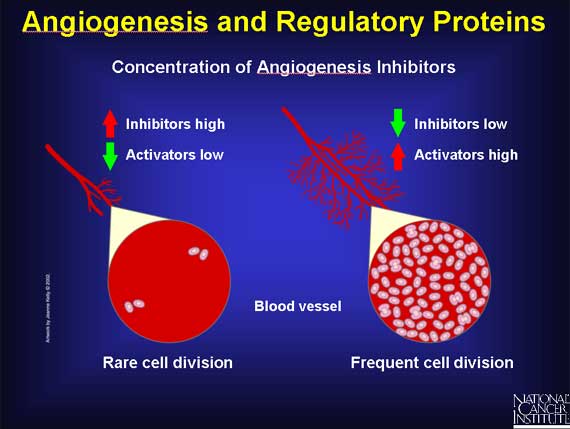
Angiogenesis is regulated by both activator and inhibitor molecules. Normally, the inhibitors predominate, blocking growth. Should a need for new blood vessels arise, angiogenesis activators increase in number and inhibitors decrease. This prompts the growth and division of vascular endothelial cells and, ultimately, the formation of new blood vessels.
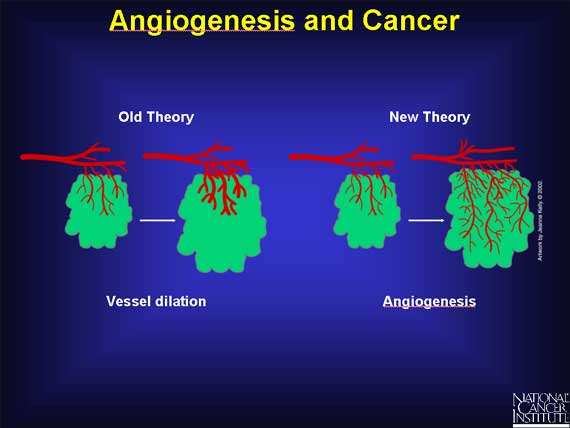
Before the 1960s, cancer researchers believed that the blood supply reached tumors simply because pre-existing blood vessels dilated. But later experiments showed that angiogenesis--the growth of the new blood vessels--is necessary for cancerous tumors to keep growing and spreading.
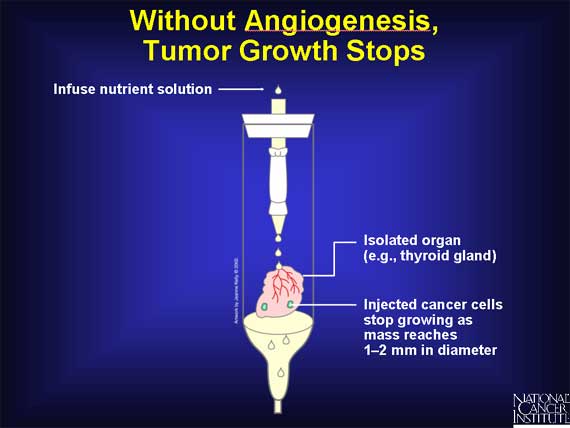
In early experiments, researchers asked whether cancer growth requires angiogenesis. Scientists removed a cancerous tumor from a laboratory animal and injected some of the cancer cells into a normal organ removed from the same strain of animal. The organ was then placed in a glass chamber and a nutrient solution was pumped into the organ to keep it alive for a week or two. Scientists found that the cancer cells grew into tiny tumors but failed to link up to the organ's blood vessels. As a result, tumor growth stopped at a diameter of about 1-2mm. Without angiogenesis, tumor growth stopped.
 |
In another experiment designed to find out whether cancer growth can continue when angiogenesis occurs, researchers compared the behavior of cancer cells in two regions of the same organ. Both locations in the eye had nutrients available, but only one could support angiogenesis. Scientists found that the same starting injection of cancer cells grew to 1-2mm in diameter and then stopped in the region without nearby blood vessels, but grew well beyond 2 mm when placed in the area where angiogenesis was possible. With angiogenesis, tumor growth continued.

 |
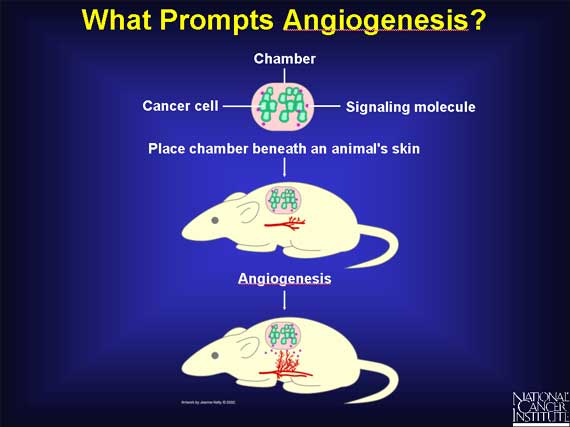
In an experiment designed to find out whether molecules from the cancer cells or from the surrounding host tissues are responsible for starting angiogenesis, scientists implanted cancer cells in a chamber bounded by a membrane with pores too small for the cells to exit. Under these conditions, angiogenesis still began in the region surrounding the implant. Small activator molecules produced by the cancer cells must have passed out of the chamber and signaled angiogenesis in the surrounding tissue.
Once researchers knew that cancer cells could release molecules to activate the
process of angiogenesis, the challenge became to find and study these
angiogenesis-stimulating molecules in animal and human tumors.
From such studies more than a dozen different proteins, as well as several
smaller molecules, have been identified as "angiogenic," meaning that they are
released by tumors as signals for angiogenesis. Among these molecules, two
proteins appear to be the most important for sustaining tumor growth: vascular
endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF).
VEGF and bFGF are produced by many kinds of cancer cells and by certain types
of normal cells, too.

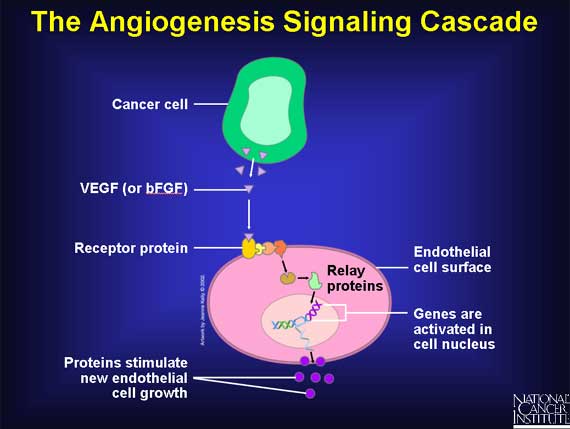
VEGF and bFGF are first synthesized inside tumor cells and then secreted into the surrounding tissue. When they encounter endothelial cells, they bind to specific proteins, called receptors, sitting on the outer surface of the cells. The binding of either VEGF or bFGF to its appropriate receptor activates a series of relay proteins that transmits a signal into the nucleus of the endothelial cells. The nuclear signal ultimately prompts a group of genes to make products needed for new endothelial cell growth.
 |
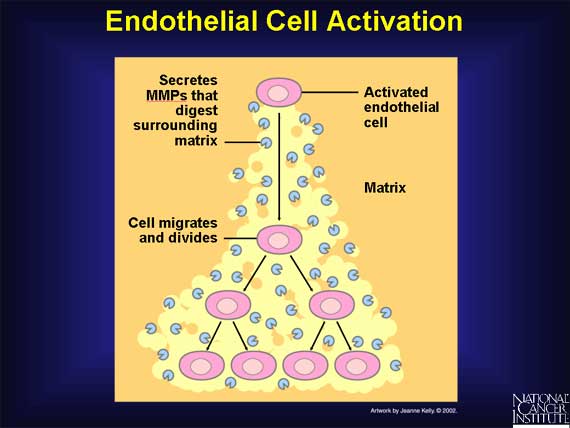
The activation of endothelial cells by VEGF or bFGF sets in motion a series of
steps toward the creation of new blood vessels. First, the activated
endothelial cells produce matrix metalloproteinases (MMPs), a special class of
degradative enzymes. These enzymes are then released from the endothelial cells
into the surrounding tissue. The MMPs break down the extracellular
matrix--support material that fills the spaces between cells and is made of
proteins and polysaccharides. Breakdown of this matrix permits the migration of
endothelial cells. As they migrate into the surrounding tissues, activated
endothelial cells begin to divide. Soon they organize into hollow tubes that
evolve gradually into a mature network of blood vessels.
 |
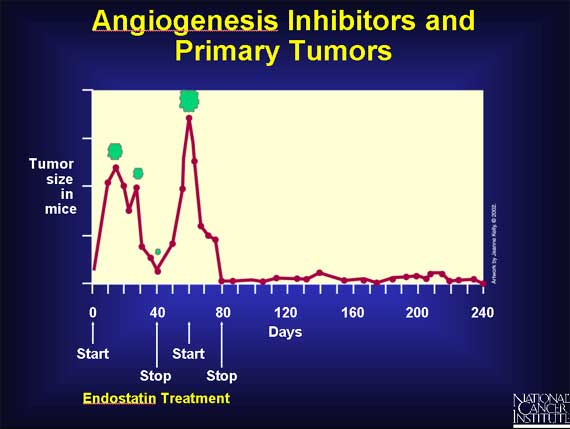
The discovery of angiogenesis inhibitors raises the question of whether such molecules might therapeutically halt or restrain cancer's growth. Researchers have addressed this question in numerous experiments involving animals. In one striking study, mice with several different kinds of cancer were treated with injections of endostatin. After a few cycles of treatment, the initial (primary) tumor formed at the site of the injected cancer cells almost disappeared, and the animals did not develop resistance to the effects of endostatin after repeated usage.
 |
The discovery of angiogenesis inhibitors raises the question of whether such molecules might therapeutically halt or restrain cancer's growth. Researchers have addressed this question in numerous experiments involving animals. In one striking study, mice with several different kinds of cancer were treated with injections of endostatin. After a few cycles of treatment, the initial (primary) tumor formed at the site of the injected cancer cells almost disappeared, and the animals did not develop resistance to the effects of endostatin after repeated usage.
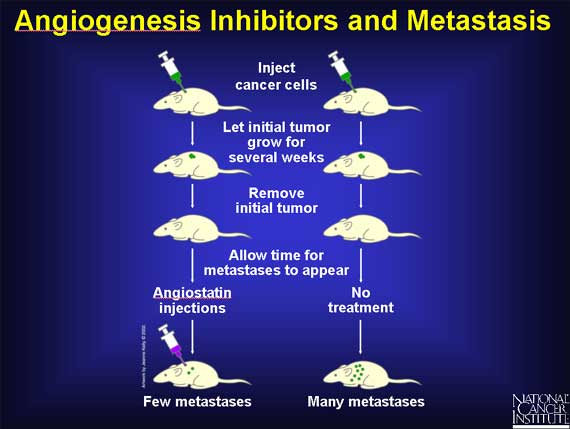
The discovery that angiogenesis inhibitors such as endostatin can restrain the growth of primary tumors raises the possibility that such inhibitors might also be able to slow tumor metastasis.
To test this hypothesis, researchers injected several kinds of mouse cancer cells beneath the animals' skin and allowed the cells to grow for about two weeks. The primary tumors were then removed, and the animals checked for several weeks. Typically, mice developed about 50 visible tumors from individual cancer cells that had spread to the lungs prior to removal of the primary tumor. But mice treated with angiostatin developed an average of only 2-3 tumors in their lungs. Inhibition of angiogenesis by angiostatin had reduced the rate of spread (metastasis) by about 20-fold.
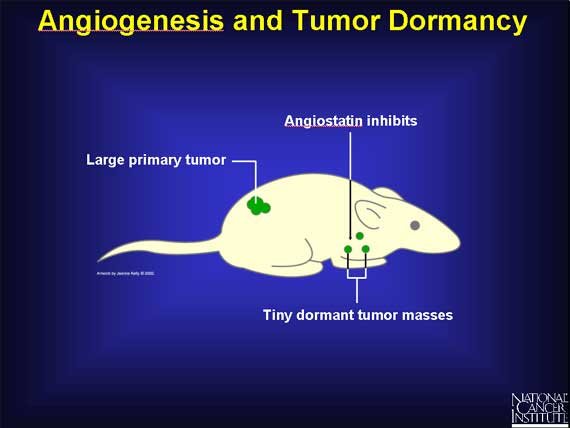
It has been known for many years that cancer cells originating in a primary tumor can spread to another organ and form tiny, microscopic tumor masses (metastases) that can remain dormant for years. A likely explanation for this tumor dormancy is that no angiogenesis occurred, so the small tumor lacked the new blood vessels needed for continued growth.
One possible reason for tumor dormancy may be that some primary tumors secrete the inhibitor angiostatin into the bloodstream, which then circulates throughout the body and inhibits blood vessel growth at other sites. This could prevent microscopic metastases from growing into visible tumors.
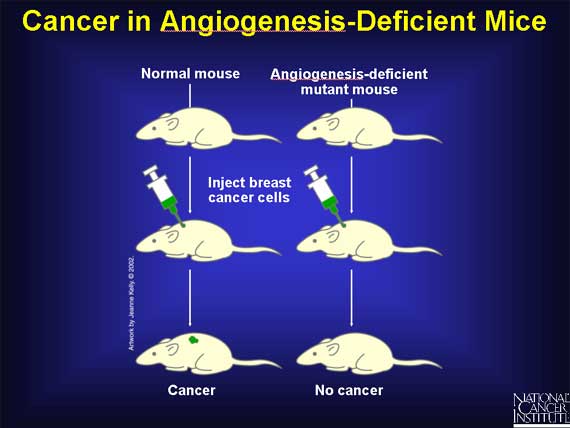
Additional support for the idea that interfering with the process of angiogenesis can restrain tumor growth has come from genetic studies of mice. Scientists have recently created strains of mice that lack two genes, called Id1 and Id3, whose absence hinders angiogenesis. When mouse breast cancer cells are injected into such angiogenesis-deficient mutant mice, there is a small period of tumor growth, but the tumors regress completely after a few weeks, and the mice remain healthy with no signs of cancer. In contrast, normal mice injected with the same breast cancer cells die of cancer within a few weeks.
When lung cancer cells are injected into the same strain of angiogenesis-deficient mutant mice, the results are slightly different. The lung cancer cells do develop into tumors in the mutant, but the tumors grow more slowly than in normal mice and fail to spread (metastasize) to other organs. As a result, the mutant mice live much longer than normal mice injected with the same kinds of lung cancer cells.
Researchers are now asking if inhibiting angiogenesis can slow down or prevent
the growth and spread of cancer cells in humans.
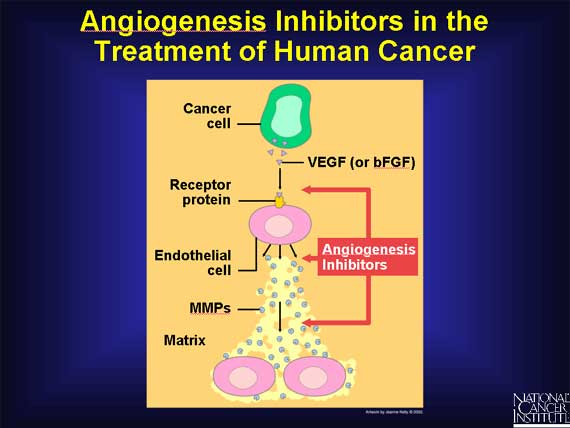
To answer this question, almost two dozen angiogenesis inhibitors are currently
being tested in cancer patients. The inhibitors being tested fall into several
different categories, depending on their mechanism of action. Some inhibit
endothelial cells directly, while others inhibit the angiogenesis signaling
cascade or block the ability of endothelial cells to break down the
extracellular matrix.
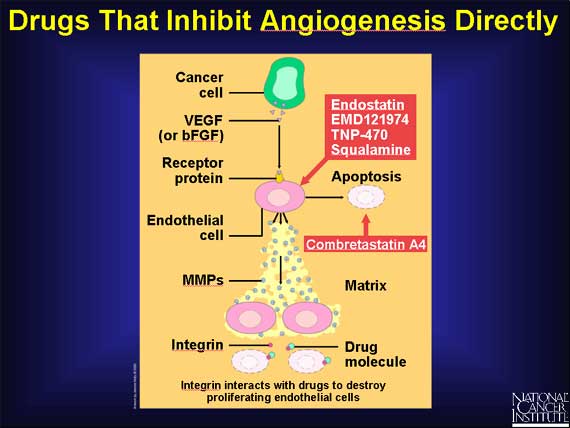
One class of angiogenesis inhibitors being tested in cancer patients are molecules that directly inhibit the growth of endothelial cells. Included in this category is endostatin, the naturally occurring protein known to inhibit tumor growth in animals. Another drug, combretastatin A4, causes growing endothelial cells to commit suicide (apoptosis). Other drugs, which interact with a molecule called integrin, also can promote the destruction of proliferating endothelial cells.
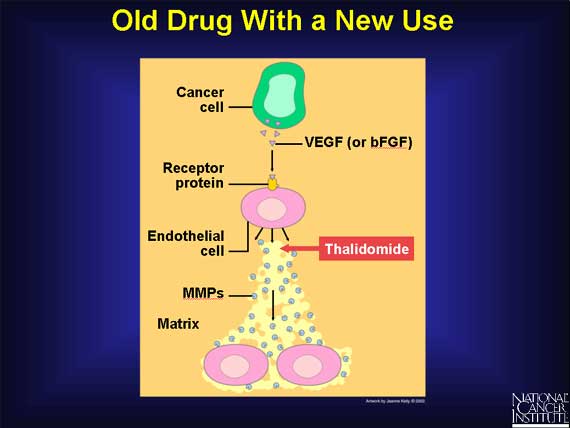
Another interesting drug is thalidomide, a sedative used in the 1950s that was subsequently taken off the market because it caused birth defects when taken by pregnant women. Although this drug clearly would not be suitable for pregnant women, its ability to prevent endothelial cells from forming new blood vessels might make it useful in treating non-pregnant cancer patients.
 |
A second group of angiogenesis inhibitors being tested in human clinical trials
are molecules that interfere with steps in the angiogenesis signaling cascade.
Included in this category are anti-VEGF antibodies that block the VEGF receptor
from binding growth factor. Bevacizumab (Avastin), a monoclonal antibody, is the first of these anti-VEGF antibodies to be FDA-approved. This new drug has been proven to delay tumor growth and more importantly, to extend the lives of patients.
Another agent, interferon-alpha, is a naturally
occurring protein that inhibits the production of bFGF and VEGF, preventing
these growth factors from starting the signaling cascade.
Also, several synthetic drugs capable of interfering with endothelial cell
receptors are being tested in cancer patients.
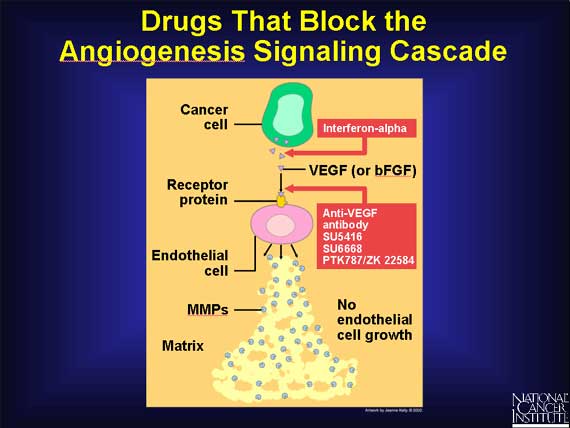
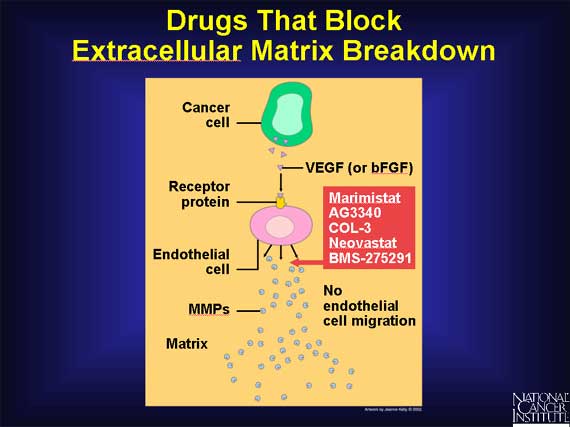
A third group of angiogenesis inhibitors are directed against one of the initial products made by growing endothelial cells, namely, the MMPs, enzymes that catalyze the breakdown of the extracellular matrix. Because breakdown of the matrix is required before endothelial cells can migrate into surrounding tissues and proliferate into new blood vessels, drugs that target MMPs also can inhibit angiogenesis.
Several synthetic and naturally occurring molecules that inhibit the activity of MMPs are currently being tested to see if interfering with this stage in the process of angiogenesis will prolong the survival of cancer patients.
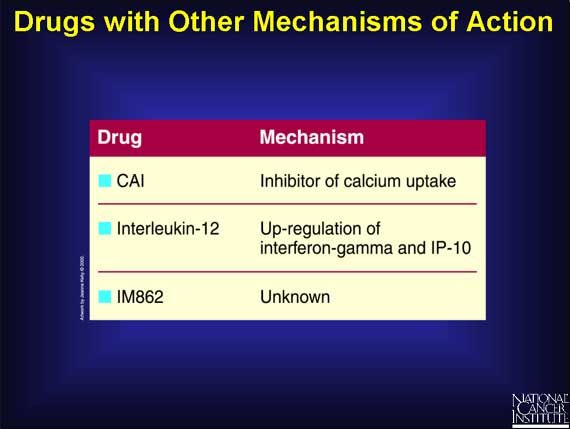
A miscellaneous group of drugs, whose ability to inhibit angiogenesis involves mechanisms that are either nonspecific or are not clearly understood, is also being tested in cancer patients. One example, a drug called CAI, exerts its effects by inhibiting the influx of calcium ions into cells. While this inhibition of calcium uptake suppresses the growth of endothelial cells, such a general mechanism may affect many other cellular processes.
Researchers have answered many questions about angiogenesis, but many questions still remain. Scientists do not know whether using angiogenesis inhibitors to treat cancer will trigger unknown side effects, how long treatment will need to last, or whether tumor cells will find alternative routes to vascularization. To answer such questions, human clinical trials are currently under way.

|