|
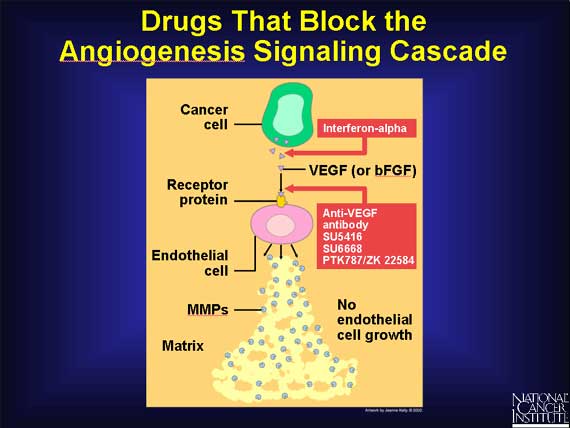
A second group of angiogenesis inhibitors being tested in human clinical trials
are molecules that interfere with steps in the angiogenesis signaling cascade.
Included in this category are anti-VEGF antibodies that block the VEGF receptor
from binding growth factor. Bevacizumab (Avastin), a monoclonal antibody, is the first of these anti-VEGF antibodies to be FDA-approved. This new drug has been proven to delay tumor growth and more importantly, to extend the lives of patients.
Another agent, interferon-alpha, is a naturally
occurring protein that inhibits the production of bFGF and VEGF, preventing
these growth factors from starting the signaling cascade.
Also, several synthetic drugs capable of interfering with endothelial cell
receptors are being tested in cancer patients.
< Previous | Index | Next Slide > |