|
Home >
Environment > Emission of Greenhouse Gases > High-GWP Gases
|
Emissions of Greenhouse Gases Report
|
Full Printer-Friendly Version |
High-GWP Gases
Total Emissions | Hydrofluorocarbons | Perfluorocarbons | Sulfur Hexafluoride |
|
|
Total Emissions |
|
|
 |
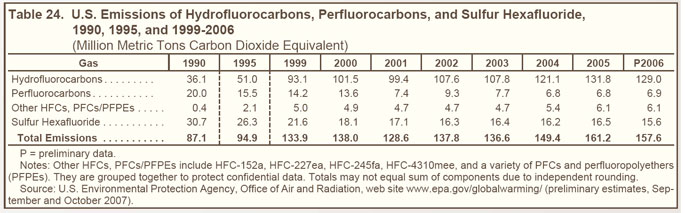
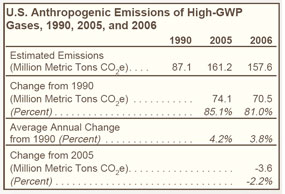
Greenhouse gases with high global warming potential (high-GWP gases) are hydrofluorocarbons (HFCs), perfluorocarbons (PFCs), and sulfur hexafluoride (SF6), which together represented just over 2 percent of U.S. greenhouse gas emissions in 2006. |
 |
Emissions estimates for the high-GWP gases are provided to EIA by the EPA’s Office of Air and Radiation. The estimates are derived from the EPA Vintaging Model. |
 |
Emissions of high-GWP gases have increased steadily since 1990 (see Figure 22 on right and Table 24 below), largely because HFCs are being used to replace chlorofluorocarbons (CFCs), hydrochlorofluorocarbons (HCFCs), and other ozone-depleting substances that are being phased out under the terms of the Montreal Protocol, which entered into force on January 1, 1989. |
 |
PFC emissions have declined since 1990 as a result of production declines in the U.S. aluminum industry as well as industry efforts to lower emissions per unit of output. |
|
|
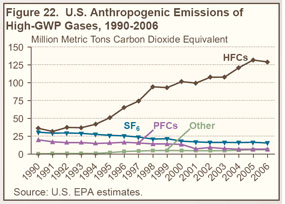
figure data
|
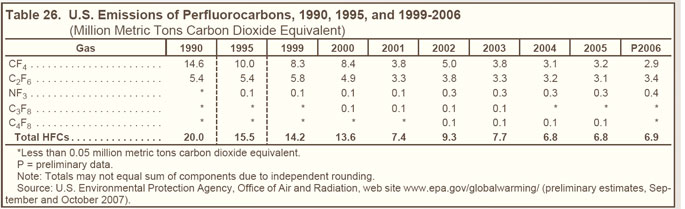
| Hydrofluorocarbons |
|
|
 |
HFCs are compounds that contain carbon, hydrogen, and fluorine. Although they do not destroy stratospheric ozone, they are powerful greenhouse gases. |
 |
HFCs are used in many applications, such as solvents, residential and commercial refrigerants, firefighting agents, and propellants for aerosols. |
 |
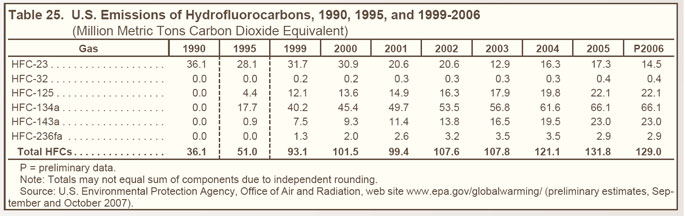
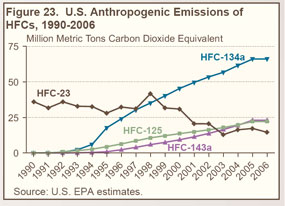
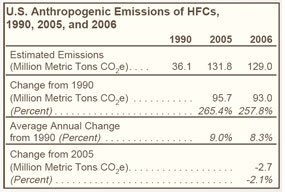
Emissions of substitutes for ozone-depleting substances, including HFC-32, HFC-125, HFC-134a, and HFC-236fa, have grown from trace amounts in 1990 to more than 110 MMTCO2e in 2006 (see Figure 23 on right). |
 |
Nearly 90 percent of the growth in HFC emissions since 1990 can be attributed to the use of HFCs as replacements for ozone-depleting substances. The market is expanding, with HFCs used in fire protection applications to replace Halon 1301 and Halon 1211. |
 |
Since 1999, HFC-134a—used as a replacement for CFCs in air conditioners for passenger cars, trucks, trains, and buses—has accounted for the largest share of HFC emissions (see Table 25 below). |
|
|
 |
| Perfluorocarbons |
|
|
 |
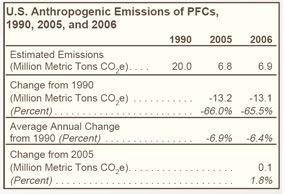
The two principal sources of PFC emissions are domestic aluminum production and semiconductor manufacture, which yield perfluoromethane (CF4) and perfluoroethane (C2F6) (see Figure 24 on right and Table 26 below). |
 |
While PFC emissions from aluminum production have declined markedly since 1990, the decline has been offset in part by increased emissions from semiconductor manufacturing. |
 |
Emissions from process inefficiencies during aluminum production (known as “anode effects”) have been greatly reduced; in addition, high costs for alumina and energy have led to production cutbacks. |
 |
Perfluoroethane is used as an etchant and cleaning agent in semiconductor manufacturing. The portion of the gas that does not react with the materials is emitted to the atmosphere. |
|
|
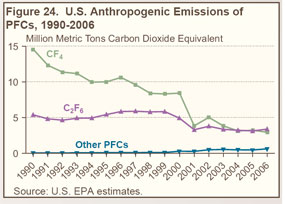
figure data
 |
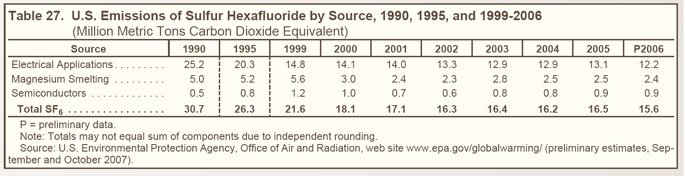
| Sulfur Hexafluoride |
|
|
 |
SF6, an excellent dielectric gas for high-voltage applications, is used primarily in electrical applications— as an insulator and arc interrupter for circuit breakers, switch gear, and other equipment in electricity transmission and distribution systems. |
 |
SF6 is also used in magnesium metal casting, as a cover gas during magnesium production, and as an atmospheric tracer for experimental purposes. |
 |
Other, minor applications of SF6 include leak detection and the manufacture of loudspeakers and lasers. |
 |
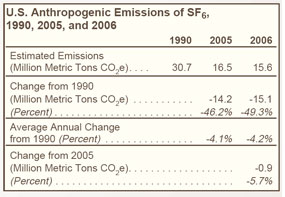
Industry efforts to reduce emissions of SF6 from electrical power systems have led to a decline in emissions since 1990 (see Figure 25 on right and Table 27 below). |
|
|
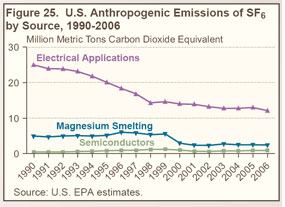
figure data
 |
| |
|
|
|
|