| |
| Publications: |
A Guide to the Clinical Care of Women with HIV/AIDS, 2005 edition |
<
Previous
| Home
| Next
>
Chapter
IV
Primary Medical Care
Judith Feinberg, MD, and
Janine Maenza, MDr
I. Introduction
TOP
No field in medicine today is moving as swiftly as that of HIV/AIDS. The speed at which new developments occur and the rapidity with which they are superseded by newer data are nothing short of breathtaking. As a consequence, most studies are typically out of date at the time of publication. Because of the rapid turnover of key information, this chapter focuses on the essential principles of care for the HIV-infected woman. “Cutting-edge” treatment strategies currently being studied will be mentioned but not described in detail. To be truly useful, we indicate the general directions in which this field is moving and how to access updated information.
Several studies have demonstrated that positive clinical outcomes are a function of the clinician’s experience in caring for HIV-infected individuals (Kitahata, 1996). Nonspecialists are urged to seek expert advice and consultation whenever there is any question about the best way to manage a specific patient. This is especially important in the setting of antiretroviral treatment failure and in advanced HIV disease when patients are vulnerable to multiple simultaneous opportunistic processes.
There is as yet no compelling evidence that the clinical course of HIV infection in women differs significantly from that in men, with the obvious exception of the associated gynecologic conditions and obstetric issues (described elsewhere, see Chapters VI and VII). Although recent data have indicated that women may have lower HIV viral loads than men with an equivalent degree of immunosuppression, this does not appear to confer benefit in terms of overall survival or complication-free survival (see Chapter I: Epidemiology and Natural History of HIV Infection in Women). At present, the approach to management of HIV-infected women and men is the same. With prolonged survival now possible, general preventive strategies and health maintenance, such as smoking cessation, control of hypertension, minimizing cardiovascular risk factors, and routine screening for malignancy (cervical, breast, colon), are all part of routine care for HIV-seropositive adults.
II. Initial Evaluation TOP
A. HISTORY
A comprehensive database is valuable to the primary caregiver in assessing the patient’s current status and in formulating a management plan. It is critical to remember that most patients are anxious and frightened at their initial encounter for HIV care; the ability to empathize, to share knowledge without being patronizing, to provide reassurance, and to remain nonjudgmental are essential to gaining the patient’s trust and to obtaining accurate information (see Chapter II: Approach to the Patient). In addition to all the usual aspects of history-taking, the following areas are of particular importance in HIV disease and deserve special attention.
- HIV diagnosis: When did you first test positive for HIV? Why were you tested? This neutral, open-ended start permits the patient to ask questions about HIV risk behaviors and possible route(s) of transmission, including sexual partners and practices and alcohol/drug use behaviors. If the patient can identify the source of new infection, it is valuable to know if the source patient has been treated for HIV, as the acquisition of drug-resistant infection has increasingly been reported. Was the patient ever tested for HIV before? If prior test(s) were negative, it is valuable to assess whether HIV has been relatively recently acquired by looking for evidence of the acute seroconversion syndrome within the past 6–9 mo. These symptoms are classically those of seronegative mononucleosis — fever, aches, pharyngitis, lymphadenopathy, and frequently rash, although the range of possible clinical manifestations of acute HIV infection is very broad.
- HIV treatment history: If the patient has already been treated for HIV disease, then it is extremely valuable to know the patient’s pretherapy CD4 cell count, HIV viral load, and specific treatment history. What was her prior antiretroviral therapy, including duration? Were there any difficulties with adherence, response to therapy, adverse effects, or history of treatment-limiting intolerance to any agent? Was resistance testing done and, if so, are results available? It is important to determine what, if any, obstacles she has experienced in taking antiretroviral therapy as prescribed (see Chapter V on Adherence). Has she had any HIV-associated diagnoses and was she treated for these conditions? Has she taken any opportunistic infection prophylaxis? Has she ever been hospitalized? If so, was it for an HIV-related problem?
- History of sexually transmitted infections and other infectious diseases: Including syphilis, gonorrhea, herpes simplex, pelvic inflammatory disease, anogenital warts; tuberculosis (PPD status, exposure to active case, prior prophylaxis or treatment for active disease); hepatitis A, B, or C; prior vaccinations, including those for childhood illnesses, hepatitis A and/or B, pneumococcal infection and influenza; history of chicken pox or shingles; complete gynecologic history (see Chapter VI on Gynecologic Problems), including most recent evaluation, Pap smear, and results.
- History of other medical diagnoses: With particular attention to hypertension, type 2 diabetes, cardiovascular disease, premalignant or malignant conditions.
- Sexual practices: Including use of condoms (male and/or female versions) and/or other forms of birth control, consistency of use; number of current partners and their HIV status (if known); sexual activity with men, women, or both; history of trading sex (oral or intercourse) for drugs or money; history of anal sex.
- Presence of HIV-associated signs and symptoms: Fatigue, lymphadenopathy, weight loss, skin problems, bacterial pneumonia, thrush (oral, vaginal), as well as signs/symptoms more typical of advanced HIV disease, including fevers, night sweats, persistent diarrhea, severe headache, respiratory symptoms (especially progressive dyspnea on exertion and cough, whether productive or nonproductive), mental status changes, difficulty swallowing, midline substernal discomfort with swallowing, and visual changes, particularly the presence of floaters or visual field deficits.
- Mental health history: Past and current problems, evidence of depression (trouble sleeping, early awakening, change in appetite, loss of interest in usual activities, anhedonia).
- Family history: Age and health of children, including HIV test results if performed; HIV in other family members; other medical diagnoses, especially hypertension, type 2 diabetes, cardiovascular disease, malignancy in family members.
- Medications taken regularly: Including prescription and over-the-counter remedies; history of and attitude toward regular medication use; use of alternative (nontraditional) medications for HIV or other conditions; drug allergies.
- Social history: Place of birth, where patient was raised, where and with whom patient lives and relationship to others in the household; childcare responsibilities; history of domestic violence; pets, especially reptiles (risk of salmonellosis) and kittens (risk of toxoplasmosis); extent of formal education; occupational history and potential toxic exposures; travel history; cigarette, alcohol, and illicit drug use in the past or continuing; misuse of prescription medications.
- Sources of support: To whom has the patient disclosed her diagnosis and what were their reactions? Are there friends or family to whom disclosure seems possible either now or perhaps in the future? Are other family members HIV-positive? Are family or friends able to care for the patient’s children in the event of illness? Does she have a job and, if so, does it provide health insurance?
Just as important as the information that the clinician obtains in the history-taking process is the information about HIV disease that is shared with the patient. Counseling and education are important elements of the therapeutic bond with the caregiver, but because this entails an enormous amount of information, it is best broached initially and then reintroduced and reinforced at appropriate intervals.
Many patients are in a state of shock following diagnosis, or may be suffering from situational depression or fear of their partner’s response. Be kind. Be patient. Schedule enough time (1 hr) for the initial visit. Make sure the patient knows your purpose is to support her and care for her. Another key bond is the one between the patient and the office/clinic nurse, which should be encouraged. Ensure that she has a path to reach you or the nurse for any questions, complaints, or symptomatic therapy, especially when starting antiretroviral therapy.
It is important to convey information in lay language at a level of complexity appropriate to the patient’s level of comprehension (remembering that formal educational levels may not necessarily correlate with the patient’s ability to understand complicated medical concepts). These include the following areas.
- HIV pathogenesis: What are CD4 lymphocytes and why are they important? How does HIV infection affect CD4 cells?
- Natural history of HIV disease: How is “AIDS” different from “HIV infection” (or “HIV disease”)? What is the typical time course between acquisition of HIV and the development of HIV-associated problems? AIDS?
- Monitoring the activity of HIV disease: What do CD4 cell counts and HIV viral load tests measure? How are they used, and how often will they be repeated?
- Goals of HIV disease management: To maintain or improve the patient’s immune system, control HIV replication; avoiding or minimizing side effects of medications; preventive care (vaccinations, opportunistic infection prophylaxis, periodic Pap smears, other appropriate screening tests).
- Principles of HIV treatment: Describe the available viral targets and classes of drugs used, and the value of combination therapy in preserving health and prolonging life. Underscore the importance of adherence.
- Preventing spread of HIV infection: Notifying sexual partners and drug use contacts, safer sexual practices, safer needle use including needle exchange programs, ready availability of bleach in the household for cleaning up blood, appropriate wound care for accidental injuries, reassurance about the difficulty of transmitting HIV to casual contacts and to family members even in the close context of everyday family life.
Last, because a diagnosis of HIV infection means a chronic, life-threatening disease and still carries a social stigma, the clinician plays a key role in exploring mental health and psychosocial needs, helping the patient identify potential sources of support, and referring the patient for additional medical, psychiatric, and/or social services.
B. PHYSICAL EXAMINATION
The examination may yield clues to specific HIV-associated conditions. Vital signs should be tracked carefully, particularly temperature and weight. The discovery of hypertension, largely ignored in the past, should trigger appropriate attempts at control, including weight loss, reduction of salt intake, and medication if necessary. Special attention should be paid to the following areas.
- General: Evidence of wasting, often prominent at the temples; fat redistribution syndromes including the development of a buffalo hump, enlarged breasts, and truncal obesity, which may coexist with or be separate from marked subcutaneous fat loss in the extremities, face, and buttocks.
- Eyes: The conjunctival surfaces should be examined for the purplish spots of Kaposi’s sarcoma (KS) and for petechiae. Fundoscopy may reveal “cotton wool” spots (microinfarcts of the retinal nerve fiber layer due to occlusion of retinal capillaries). These must be differentiated from the typical ‘eggs and ketchup’ appearance of the infiltrates and hemorrhages caused by cytomegalovirus (CMV) retinitis in patients with very advanced HIV disease; visual field deficits are common in CMV retinitis and may be uncovered with simple field testing by confrontation.
- Oropharynx: Oral examination often yields the earliest physical evidence of HIV infection with thrush (white plaques on buccal mucosa or posterior pharynx that are readily scraped with a tongue blade) and oral hairy leukoplakia (furry white plaques most often found on the lateral margins of the tongue that cannot be scraped off); purplish spots or plaques on mucosal surfaces, including the area under the tongue, typically indicate Kaposi’s sarcoma but may also be consistent with bacillary angiomatosis. No examination of an HIV-infected person, regardless of disease stage, should be considered complete without a careful assessment of the oropharynx.
- Lymph nodes: Nontender or minimally tender generalized adenopathy may wax and wane and most often is related to HIV infection itself, but may also indicate lymphoma. Regional adenopathy is more frequently associated with local pathology, such as intrathoracic adenopathy in tuberculosis or abdominal adenopathy in disseminated Mycobacterium avium complex (MAC) infection. Extremely tender lymph nodes should trigger an evaluation for the etiology.
- Lungs: Fine, dry “cellophane” rales are classic for Pneumocystis carinii pneumonia (PCP), but are a late finding and may be absent.
- Hepatosplenomegaly: Organomegaly typically reflects disseminated infection with MAC, tuberculosis, or histoplasmosis, or may be a sign of lymphoma.
- Pelvic examination:
- External genitalia/perineum: Sores or ulcers are usually indicative of sexually transmitted infections, especially herpes simplex virus (HSV) or syphilis. In very immunosuppressed patients, ulcers may be caused by other opportunistic pathogens, such as CMV, or may represent aphthous ulcers. Condyloma acuminata may appear as small, fleshy papules or may be exuberant, florid growths reaching several centimeters in diameter; other human papillomavirus-associated lesions may be recognized only with magnification and/or application of acetic acid. Raised and pigmented lesions may represent premalignant changes (vulvar intraepithelial neoplasia).
- Speculum and bimanual pelvic examination: Abnormal vaginal discharge can be caused by various forms of vaginitis (yeast, bacterial vaginosis, or trichomoniasis) or cervicitis. Pap smears should be obtained to rule out cervical dysplasia. Cervical motion, and uterine and adnexal tenderness suggest possible pelvic inflammatory disease. (Gynecologic exam is discussed in detail in Chapter VI.)
- Neurologic: Motor deficits may reflect space-occupying lesions of the central nervous system (CNS) such as toxoplasmosis, CNS lymphoma, and progressive multifocal leukoencephalopathy, or may be due to neurosyphilis. Symmetrical, distal sensory deficits (especially decrease or loss of vibratory or proprioceptive sensation), typically affecting the feet more than the hands, indicate peripheral neuropathy, which may be due to HIV itself or to drug toxicity from the dideoxy nucleoside analogues. Poor short-term memory, diminished concentration, and sensorimotor retardation are the hallmarks of AIDS dementia complex (HIV encephalopathy). Dysphoric mood or flat affect may reveal depression.
- Skin: Like the oropharynx, careful examination of the skin often yields early clues about HIV infection, and should be performed regularly. Early manifestations include pruritic papular eruptions that may be bacterial folliculitis, eosinophilic folliculitis, or scabies. Pearly papules, often with central umbilication, are typical of molluscum contagiosum. A painful vesicular rash may be HSV but in a dermatomal distribution is usually shingles (varicella-zoster virus). Seborrheic dermatitis may be severe and appears as scaly, erythematous areas on the face, especially the nasolabial fold and eyebrows, or may be confined to the scalp and hairline. Psoriasis is another common scaling lesion. Purplish macules or plaques may be either KS or bacillary angiomatosis, similar to their appearance on mucosal surfaces; however, in dark-skinned individuals, KS may appear more brown than purple.
III. Laboratory Testing TOP
A. INITIAL DIAGNOSIS
Because of the advances made in HIV treatment, with associated decreases in HIV-related morbidity and mortality; concerns about possible increases in HIV incidence; and the new availability of a simple rapid HIV test (Oraquick), the CDC recommends that voluntary HIV testing be made a routine part of medical care for all patients in high HIV-prevalence clinical settings and for those with risks for HIV in low HIV-prevalence clinical settings. (CDC, 2003).
HIV infection is usually diagnosed by serologic tests that detect antibody to the virus. Infection may also be detected by nucleic acid-based assays that either measure the number of copies of the virus in plasma (RNA polymerase chain reaction [PCR]) or detect the virus in cells (DNA). Informed consent, with pre- and posttest counseling, is legally mandatory for performing HIV serologic tests in most locations, and should be procured at all times when the test is offered.
- Serology: The most common method of HIV detection is with an enzyme-linked immunosorbent assay (ELISA) test for screening, followed by confirmation with a Western blot. For a positive Western blot, the Centers for Disease Control and Prevention (CDC) and Association of State and Territorial Public Health Laboratory Directors require a band pattern indicating antibodies to two of the following proteins: p24, gp41, and gp120/160. A serologic test may be reported as positive if the ELISA is positive and Western blot criteria are met. The test may also be reported as indeterminate if the ELISA is positive, but only a single band is detected by Western blot. Serologic tests generally become positive 3–12 wk after infection occurs. The interpretation of an indeterminate test during this window period may be clarified by a quantitative virology assay with a PCR-based technique (see below). An indeterminate test may reflect the process of seroconversion, but may also be a constant finding in an uninfected individual. Causes of indeterminate results include:
- seroconversion;
- advanced HIV infection with decreased titers of p24 antibodies (rare)(seroreversion);
- autoantibodies due to autoimmune or collagen vascular diseases or malignancy;
- cross-reactive alloantibodies from pregnancy, blood transfusions, or organ transplantation; and
- previous receipt of an experimental HIV vaccine.
When indeterminate results are obtained, risk assessment is important, since women in low-risk categories with indeterminate tests are unlikely to be infected and can be reassured. Nevertheless, after indeterminate test results, repeat testing should be performed at 1, 2, and 6 months, and precautions should be taken to prevent HIV transmission to others until seroconversion is ruled out. In general, patients with indeterminate tests who are in the process of seroconversion usually have positive Western blots within 1 month. In high-risk patients or in other situations where seroconversion is suspected HIV nucleic acid amplification (HIV-DNA PCR, HIV-RNA PCR) may be considered and has high sensitivity during acute infection, often before antibodies to the virus have developed.
The window period before seroconversion and agammaglobulinemia are possible causes of false-negative results. Although infection with HIV-2 (more common in West Africa) and HIV-1 subtype O have been associated with indeterminate and false negative results on earlier generation serologic tests, most currently used assays will detect these infections.
Accuracy of HIV serologic testing is quite high (>99% sensitivity and specificity), but the predictive value of a positive or negative test depends on the seroprevalence of HIV in the patient population. In a low prevalence population, the rate of false-positive results of combined ELISA and Western blot testing is <.001%. The frequency of indeterminate results in a low prevalence population is .02%.
- Viral detection:
- Nucleic acid amplification. May be used to clarify the diagnosis of HIV infection in acute infection, during the window period (after exposure, before seroconversion), when serologic tests are indeterminant, or with neonatal infection.
- Plasma HIV RNA. Routinely used to monitor the course and treatment of HIV infection (see below). The three most common techniques are reverse transcriptase polymerase chain reaction (RT-PCR), a branched DNA (bDNA) technique, and nucleic acid sequence-based amplification (NASBA). These tests report the number of copies of virus per milliliter of plasma. The assays are considered equally reliable, but vary somewhat in lower levels of detection and dynamic range. Lower limits of detection for standard tests are 100–400 copies/mL, but ultrasensitive assays are available that can detect as few as 20–50 copies/mL. Sensitivity is 90–95% overall, but is increased to 98–100% with CD4 counts <200/mm3. False-positive rates are 2–3%, usually with low HIV RNA titers (Rich, 1999).
- DNA PCR. A qualitative test used to detect intracellular virus, and primarily used for viral detection with neonatal infection and with indeterminant serology. Sensitivity is >99% at all stages of infection and specificity is approximately 98%.
- Viral isolation. Qualitative or quantitative cultures are used primarily for diagnosis in neonatal HIV infection, and for more in-depth viral analysis. The procedure is expensive and labor intensive. Sensitivity is 95–100%.
- Alternative tests
- Home testing: Home Access Express Test is the only available home test for HIV as of May 2003. Filter paper with a blood sample obtained with a lancet is mailed in to a laboratory in a coded, anonymous process. Dried blood samples are tested by the same ELISA and Western blot tests used on venous blood. Sensitivity and specificity approach 100%. Results are provided by phone (a recorded message for those with negative results, counseling for those with positive results).
- Rapid tests: The OraQuick Rapid HIV Test provides results in 10–20 minutes. Sensitivity approaches 100%; specificity is also >99%, but positive results should be confirmed with standard serology. Rapid tests may prove useful in STI clinics or emergency rooms (where patients often do not return for tests results) or on labor and delivery wards for high-risk pregnant women who have not previously been tested.
- Saliva test: The OraSure test uses ELISA and Western blot testing to detect antibodies to HIV in saliva. Sensitivity and specificity are similar to that with standard serology. This test is useful for people with poor venous access or those who want HIV testing, but refuse blood tests.
- Urine test: The only currently available urine test (Calypte HIV-1 Urine EIA) is licensed for screening only and must be administered by a physician; a positive result requires confirmation by another method.
B. BASELINE LABORATORY EVALUATION
After the diagnosis of HIV has been confirmed, a baseline laboratory evaluation is needed to establish the stage of disease, and exposure to other infectious diseases (Table 4-1). In addition, routine tests of hematology, chemistry, and lipid profiles are needed at baseline, because HIV and other concomitant illness may affect these values, and detected abnormalities may also have an impact on the choice of therapy for the individual patient.
- CD4 lymphocyte count: The hallmark of HIV infection is the progressive decline in CD4+ (helper) T lymphocytes. Normal laboratory ranges for CD4 lymphocyte counts are usually 500–1400/mm3. CD4 counts may drop precipitously at the time of primary HIV infection, and then usually rebound to near-baseline levels. The natural history of HIV then involves a progressive loss of CD4 cells, averaging 30–60 cells/yr (Figure 4-1). The risk of opportunistic infections increases with declining counts. (See Chapter I on Epidemiology and Natural History.)
Knowledge of the baseline CD4 count is of vital importance in assessing the patient: staging of HIV infection (Table 1-3 in Chapter I), recommendations for antiretroviral treatment (see Section IV. C), and prophylaxis against specific opportunistic infections (see Section V. A) are based on the degree of immunosuppression as quantified by the CD4 count.
Many factors may cause variability in the CD4 count. These include:
- interlaboratory variations;
- seasonal and diurnal variation (lowest levels at noon, highest in the evening)
- the use of corticosteroids (decreases values)
- intercurrent illness (decreases values)
- HTLV-1 coinfection (increases values).
In addition, because the CD4 count is a value derived by determining the percentage of white blood cells that are lymphocytes, and then the percentage of lymphocytes that are CD4 receptor-positive, there may be variation in other white blood cell compartments (as may occur in pregnancy) that leads to variations in the CD4 count. Because the CD4 percentage is the directly measured value and the absolute CD4 count is the calculated one, it is more useful and accurate to focus on the CD4 percentage to assess trends in this important parameter.
Table 4-1: Baseline Laboratory Evaluation
 Confirm HIV diagnosis (usually with ELISA and Western blot) Confirm HIV diagnosis (usually with ELISA and Western blot)
 CD4 count CD4 count
 Viral load Viral load
 Chemistry panel: including liver and renal function Chemistry panel: including liver and renal function
 Hematology panel: including white blood cell count differential Hematology panel: including white blood cell count differential
 Lipid profile: total cholesterol, HDL, LDL, triglycerides Lipid profile: total cholesterol, HDL, LDL, triglycerides
 Serologies: syphilis, toxoplasmosis, CMV, varicella-zoster virus (if no history of chickenpox or shingles), hepatitis A, hepatitis B, hepatitis C Serologies: syphilis, toxoplasmosis, CMV, varicella-zoster virus (if no history of chickenpox or shingles), hepatitis A, hepatitis B, hepatitis C
 PPD PPD
 G6PD (in selected patients) G6PD (in selected patients)
 Pap smear/STI screening Pap smear/STI screening
|
Figure 4-1: Natural History of HIV Infection without
the Use of Antiretroviral Therapy
- Quantitative virology/viral load assays: The HIV RNA level or “viral load” is also of pivotal importance in assessing the HIV-infected patient. Whereas the CD4 count indicates the current degree of immunosuppression, the viral load indicates the rapidity with which the disease is likely to progress: higher viral loads have repeatedly been shown to be associated with a more rapid rate of disease progression (Figure 4-2). Recent studies have shown that women have lower viral loads than men at comparable CD4 cell counts, although these viral load differences tend to disappear several years after seroconversion and they have not been associated with slower disease progression or longer survival (see Chapter I: Epidemiology and Natural History of HIV Infection in Women).
The most commonly used methods to quantify HIV RNA are RT-PCR, bDNA, and NASBA techniques (see Viral Detection, above). Standard tests have lower limits of detection of 100–400 copies/mL, but current ultrasensitive assays can detect as few as 20 copies/mL. Although results of different viral load assays correlate, absolute values differ and there is no standard multiplication factor to translate between results in the different assays. Therefore, the same assay should be used to follow an individual patient longitudinally. Intraperson variability on viral load assays is <.5 log, but this degree of variability is important to consider when determining clinical significance of a reported change in viral load values for an individual patient.
Indications for plasma HIV RNA testing are shown in Table 4-2. It is also critical to repeat any HIV RNA result that is being used as the basis for a change in patient management.
Figure 4-2: Likelihood of Developing AIDS within 3 Years
Table 4-2: Indications for Plasma HIV RNA Testing*
| Clinical Indication |
Information |
Use |
| Syndrome consistent with acute HIV infection |
Establishes diagnosis when HIV antibody test is negative or indeterminate |
Diagnosis† |
| Initial evaluation of newly diagnosed HIV infection |
Baseline viral load “set point” |
Decision to start or defer therapy |
| Every 3–6 mo in patients not on therapy |
Changes in viral load |
Decision to start therapy |
| 2–8 wk after initiation of antiretroviral therapy |
Initial assessment of drug efficacy |
Decision to continue or change therapy |
| 3–6 mo after start of therapy |
Maximal effect of therapy |
Decision to continue or change therapy |
| Every 3–6 mo in patients on therapy |
Durability of antiretroviral effect |
Decision to continue or change therapy |
| Clinical event or significant decline in CD4+ T cells |
Association with changing or stable viral load |
Decision to continue, initiate, or change therapy |
* Acute illness (e.g., bacterial pneumonia, tuberculosis, HSV, PCP, etc.) and immunizations can cause increase in plasma HIV RNA for 2–4 wk; viral load testing should not be performed during this time. Plasma HIV RNA results should usually be verified with a repeat determination before starting or making changes in therapy.
† Diagnosis of HIV infection made by HIV RNA testing should be confirmed by standard methods such as Western blot serology performed 2–4 mo after the initial indeterminate or negative test.
Source: DHHS, 2005.
- Hematology and chemistry panels: The effects of HIV and related infections may involve hematologic, renal, or hepatic abnormalities. A complete blood count is necessary at baseline to evaluate for leukopenia, anemia, and thrombocytopenia. In addition, the total white blood cell count and lymphocyte count are needed to calculate an absolute CD4 count. A chemistry panel that includes an evaluation of renal and hepatic function is also necessary: HIV-associated nephropathy may be indicated by elevations in blood urea nitrogen/creatinine, and the effects of viral hepatitis, alcohol, or medications may cause abnormalities of liver function tests. Any of these findings provide important information in their own right, but will also have an impact on the patient’s options for antiretroviral therapy.
- Other serologies:
- Syphilis: High rates of coinfection with other STIs necessitate routine syphilis serology in all HIV-infected patients. A reactive nontreponemal assay (RPR or VDRL) must be confirmed with the treponemal-specific FTA or MTPA. Cerebrospinal fluid evaluation is indicated in HIV-infected persons with latent syphilis, treatment failure (when a nontreponemal test does not decline 4-fold within 6–12 mo after treatment), and those patients with neurologic signs or symptoms.
- Toxoplasmosis: Serologic evidence of latent toxoplasmosis infection, as detected by Toxoplasma gondii IgG, may be relevant for decisions on prophylaxis, evaluation of neurologic symptoms in patients with advanced immunosuppression, and avoidance of exposure in those who have not been previously infected. There is great worldwide variation in the prevalence of latent toxoplasma infection: in the United States the rate is approximately 30%.
- CMV: Latent CMV infection is present in most HIV-infected adults. Knowledge of CMV antibody status can guide the medical provider to the use of CMV-negative blood products if transfusions are required.
- Varicella: In patients who do not have a known history of chickenpox or shingles, varicella serology should be obtained. The knowledge that a patient is varicella IgG-negative is important in the event of a subsequent exposure: postexposure prophylaxis with varicella immune globulin could then be given.
- Hepatitis: Hepatitis A serology will identify those who are not immune, and are therefore vaccine candidates. Hepatitis A vaccine should be given to patients with hepatitis C co-infection, other chronic liver disease, and perhaps to all HIV-infected patients. Hepatitis B serologies should be performed routinely: hepatitis B surface antigen (HBsAg) and hepatitis B core or surface antibodies (anti-HBc and anti-HBs) allow determination of active hepatitis (HBsAg-positive) and of those who are not immune to hepatitis B (anti-HBc-negative, anti-HBs-negative). Hepatitis B vaccination is then recommended in those who are not immune; and antiviral therapy, such as lamivudine (which has anti-hepatitis B activity), can be considered in those who are HBsAg-positive. Hepatitis C virus (HCV) serology (anti-HCV IgG) is also routinely recommended. Recombinant immunoblot assays (RIBA) for HCV are used to confirm the diagnosis if a screening ELISA is positive. HCV RNA, as detected by RT-PCR or bDNA assay, can also be used to confirm the diagnosis and allows determination of active HCV infection. Knowledge of hepatitis C antibody status is needed to guide therapeutic decision for possible HCV treatment and may also be relevant for decisions regarding antiretroviral therapy and other potentially hepatotoxic agents, and frequency of assessment of liver function tests during such therapy (see Table 4-3)
Table 4-3: Laboratory Tests for Hepatitis Viruses
| Hepatitis Virus |
Laboratory Test |
Interpretation |
A |
Anti-HAV IgM |
Recent HAV infection |
| |
Anti-HAV IgG |
Immunity to HAV |
B |
HBsAg |
Current (acute or chronic) HBV infection |
| |
HBeAg |
Current HBV infection with high risk of infectivity |
| |
Anti-HBc |
Past or present HBV infection |
| |
Anti-HBs |
Immunity to HBV (past infection or after vaccination) |
C |
Anti-HCV IgG (ELISA) |
Past or present HCV infection |
| |
Anti-HCV IgG (RIBA) |
Confirms HCV ELISA |
| |
HCV RNA |
Current HCV infection |
- Tuberculosis: A baseline PPD should be obtained in all patients who do not have a history of a positive PPD in the past. A positive PPD is considered >5mm induration in the setting of HIV infection.
- Glucose-6-phosphate dehydrogenase: A relative deficiency of glucose-6-phosphate dehydrogenase (G6PD) may be found in up to 2% of African American women and an absolute deficiency is occasionally found in women of Mediterranean descent. Absolute G6PD deficiency predisposes to hemolytic anemia upon exposure to certain medications, including several that are commonly used in HIV treatment: dapsone, sulfonamides, primaquine. A relative deficiency is not usually clinically significant. Baseline testing in selected patients is helpful so that these agents may be safely administered at a later date without needing to determine G6PD levels at that point.
- Lipid profile: Many antiretroviral agents have been associated with the development of hypertriglyceridemia and hypercholesterolemia. A baseline fasting lipid profile should be performed to determine total cholesterol, triglycerides, high-density lipoprotein (HDL), and low-density lipoprotein (LDL) levels before beginning any antiretroviral therapy.
- Pap smear/STI screening: A Pap smear should be obtained, and testing done for gonorrhea and Chlamydia (see Chapter VI Gynecologic Problems).
C. INTERVAL MONITORING
In an asymptomatic patient not taking antiretroviral therapy with a high (>500/mm3) CD4 count, follow-up every 6 mo may be appropriate. For those patients who are symptomatic and/or receiving antiretroviral therapy, visits should occur at least every 3 mo. For those who have just initiated or changed antiretroviral therapy, follow-up in 4–6 wk may be appropriate. Laboratory evaluation at each of these visits should routinely include the following: complete blood count with differential, CD4 lymphocyte count, and viral load. Chemistry panels may be done less frequently (every 6 mo) in a patient with prior normal values who remains clinically stable.
Hematology and chemistry values are needed to monitor possible medication toxicities, complications of HIV, and other possible illnesses. The CD4 count and viral load allow assessment of disease progression and effects of antiretroviral therapy. In following the CD4 count over time, it is important to recognize the causes of variability discussed above. Use of the CD4 percentage, rather than absolute CD4 count, may help eliminate some of this variability to clarify CD4 response to medications. In interpreting viral load changes over time, the variability of tests results must be noted: .3–.5 log. In a patient who previously had a viral load below the limit of detection of the assay being used (“undetectable”), who now has quantifiable virus, a repeat test should be performed as soon as possible, rather than waiting until routinely scheduled follow-up.
The frequency with which lipid profiles are checked will vary by individual patient characteristics. In patients not taking antiretroviral therapy, a baseline lipid profile should be done with the initial evaluation or before beginning antiretroviral therapy. The profile should include total cholesterol, HDL, LDL, and triglycerides. If the baseline is normal and the patient is not on antiretroviral therapy, there is no need for interval monitoring beyond that which would be done in an HIV-uninfected adult. In patients taking combination antiretroviral therapy, general guidelines are:
- Get a baseline lipid profile (fasting) before starting therapy.
- Follow total cholesterol with routine chemistry panels.
- Obtain a complete fasting lipid profile annually or if the total cholesterol begins to increase on routine testing.
- Follow complete lipid profiles every 3–6 mo for patients in whom a lipid abnormality has been detected, both before starting any antihyperlipidemic therapy and once such therapy has been started.
Recommendations for management of hyperlipidemia may be found at http://www.americanheart.org.
Annual monitoring of syphilis serology for reactivation or new infection is generally recommended. PPDs should also be checked annually if the patient belongs to a population with high epidemiologic risk of tuberculosis.
Baseline data and interval monitoring may be followed by the use of a flow sheet such as the one developed at the Johns Hopkins Outpatient HIV Clinic (Figure 4-3).
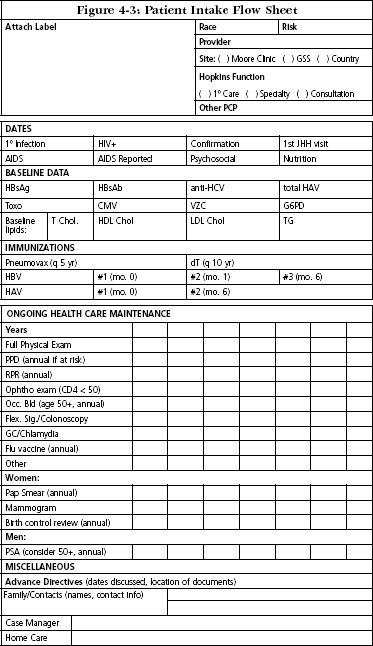
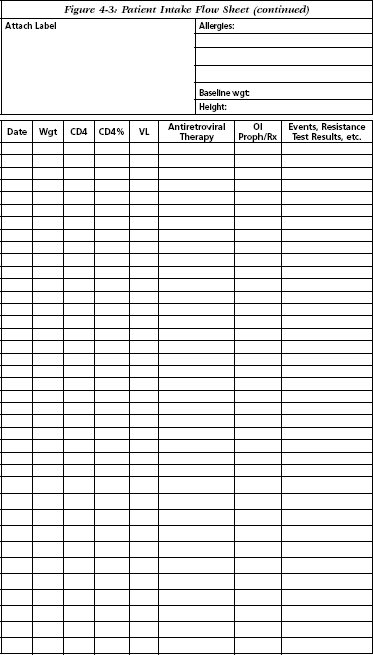
Johns Hopkins Outpatient HIV Clinic. Baltimore, MD. Reprinted with permission.
IV. Antiretroviral Therapy TOP
A. GENERAL PRINCIPLES
Three characteristics of HIV infection have significant implications for antiretroviral therapy (see Chapter VII HIV and Reproduction for discussion of antiretroviral therapy in pregnancy).
- Between the time of initial infection and the development of clinical disease there is progressive immunosuppression as evidenced by a decline in CD4 lymphocyte counts.
- Viral replication is extremely rapid: the half-life of HIV in plasma is less than 48 hr and there is turnover of up to 1 billion virions per day (Ho, 1995).
- HIV has a high degree of inherent genetic mutability: mutations that may confer resistance to antiretroviral therapy arise rapidly.
Thus, there is a rationale for initiating antiretroviral therapy before the onset of symptoms (i.e., to prevent immunosuppression), and therapy must be maintained to prevent viral replication. Strategies of antiretroviral therapy have therefore evolved to prevent the development of viral resistance. Although monotherapy with any of the antiretroviral agents will increase CD4 count, the clinical benefit of such therapy is very limited, largely because of the development of viral resistance. Combination antiretroviral therapy has been shown to have superior effectiveness in controlling viral replication and in limiting the emergence of resistant virus. These effects translate into greater clinical benefit: combination therapy reduces the risk of HIV progression and death. In addition, patients with levels of circulating virus that are below 400–500 copies/mL (the limit of detection in the past few years), but greater than 20–50 copies/mL (the limit of detection in the newest generation of tests) will experience virologic failure sooner that those with viral loads below 20–50 copies/mL (Raboud, 1998). Therefore, achievement of the lowest possible viral load should be a guiding principle in the selection of a treatment regimen.
The specific combination of antiretroviral therapy selected for a patient must take into account many factors. These include the specific side effects, dosing schedules, drug-drug interactions of different medications, and history of antiretroviral therapy. See Chapter XIV on Pharmacologic Considerations in HIV-infected Pregnant Patients for information on highly active antiretroviral therapy in pregnancy and Chapter XV on Resources for sources of complete updated information on antiretroviral therapy.
B. ANTIRETROVIRAL AGENTS
Nucleoside Analogues
Nucleoside analogue reverse transcriptase inhibitors (NRTIs) were the first class of agents shown to be effective in the treatment of HIV infection. The target enzyme for this group of drugs is HIV reverse transcriptase, an RNA-dependent DNA polymerase (see Figure 4-4).
Figure 4-4: Sites of Action of Antiretroviral Agents
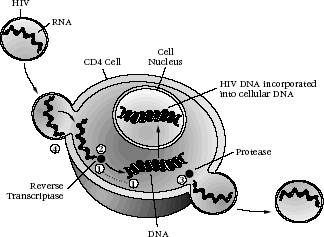
- Site of Action of NRTIs: Incorporate into DNA and block reverse transcriptase
- Site of Action of NNRTIs: Bind to reverse transcriptase
- Site of Action of PIs: Bind to protease to inhibit viral protein cleavage and therefore release of virus from cell
- Site of Action of Fusion Inhibitors: Interact with virus to inhibit virus-cell fusion
Seven NRTIs are currently licensed in the United States: zidovudine (AZT), didanosine (ddI), zalcitibine (ddC), stavudine (d4T), lamivudine (3TC), abacavir, (ABC) and emtricitabine (FTC). There are also 3 combination NRTIs (Combivir, Trizivir, Epzicom), a nucleotide reverse transcriptase inhibitor (tenofovir) and a nucleoside-nucleotide reverse transcriptase inhibitor combination (Truvada) (Table 4-4).
Table 4-4: Nucleoside/Nucleotide Analogue Reverse Transcriptase Inhibitors
| Generic Name |
Trade
Name |
Standard Dosing |
Common Side Effects |
| Zidovudine (AZT) |
Retrovir |
200 mg tid or 300 mg bid (2–6 pills/day) |
Anemia, nausea, headache |
| Didanosine (ddI) |
Videx/Videx - EC |
200 mg bid or 400 mg qd (125 mg bid if <60 kg) (1–4 pills/day) |
GI symptoms (diarrhea), peripheral neuropathy, pancreatitis |
| Zalcitibine (ddC) |
Hivid |
0.75 mg tid (3 pills/day) |
Peripheral neuropathy, pancreatitis |
| Stavudine (d4T) |
Zerit |
40 mg bid (30 mg bid if <60 kg) (2 pills/day) |
Peripheral neuropathy, pancreatitis |
| Lamivudine (3TC) |
Epivir |
150 mg bid or 300 mg qd (1–2 pills/day) |
Headache |
| Lamivudine/zidovudine |
Combivir |
1 pill bid |
As for 3TC and AZT |
| Abacavir |
Ziagen |
300 mg bid
(2 pills/day) |
Hypersensitivity,* rash, GI symptoms |
Abacavir/lamivudine/ zidovudine |
Trizivir |
1 pill bid |
As per abacavir (including hypersensitivity), 3TC, and AZT |
| Tenofovir disoproxil fumarate |
Viread |
300 mg qd (1 pill/day) |
GI symptoms |
| Emtricitabine |
Emtriva |
200 mg qd (1 pill/day) |
Headache, nausea, diarrhea, rash |
| Abacavir/lamivudine |
Epzicom |
1 tablet qd |
As for 3TC and abacavir |
Emtricitabine/ tenofovir DF
|
Truvada |
1 tablet qd |
As for FTC and TDF |
* 3-4% of patients will develop a hypersensitivity reaction to abacavir with symptoms that include fever, rash, myalgias. Rechallenge with abacavir after hypersensitivity reaction may be life-threatening and should never be done. In addition to the side effects listed for each medication, lactic acidosis with hepatic steatosis is a rare but potentially life-threatening toxicity with the use of NRTIs.
Nonnucleoside Reverse Transcriptase Inhibitors
Nonnucleoside reverse transcriptase inhibitors (NNRTIs) noncompetitively inhibit HIV reverse transcriptase by binding to a site distant from the enzyme’s active site. Three NNRTIS are currently available in the United States: nevirapine, delavirdine, and efavirenz (Table 4-5).
Table 4-5: Nonnucleoside Reverse Transcriptase Inhibitors
| Generic Name |
Trade
Name |
Standard Dosing |
Common Side Effects |
| Nevirapine |
Viramune |
200 mg qd x 14 days, then 200 mg bid
(2 pills/day) |
Rash, hepatitis |
| Delavirdine |
Rescriptor |
400 mg tid
(12 pills/day) |
Rash |
| Efavirenz |
Sustiva |
600 mg qd (qhs administration may limit CNS side effects) (1 or 3 pills/day) |
Headache, dizziness, cognitive effects, rash |
Protease Inhibitors
Protease inhibitors (PIs) prevent maturation of virus protein by competitively inhibiting HIV protease, an enzyme essential for viral protein cleavage. When this enzyme is blocked, immature, noninfectious virus particles are produced. The other important properties that protease inhibitors share include their limited central nervous system penetration and their metabolism by the cytochrome P450 enzyme system and resultant multiple drug-drug interactions (Table 4-6).
Table 4-6: Protease Inhibitors
| Generic Name |
Trade
Name |
Standard Dosing |
Common Side Effects |
| Saquinavir (hard gel capsules) |
Invirase |
600 mg tid (9 pills/day) |
Diarrhea, nausea, abdominal discomfort |
| Saquinavir (soft gel capsules) |
Fortovase |
1200 mg tid (18 pills/day) |
Diarrhea, nausea, abdominal discomfort |
| Ritonavir |
Norvir |
600 mg bid (12 pills/day) |
Nausea, abdominal discomfort, circumoral paresthesias |
| Indinavir |
Crixivan |
800 mg q8h (6 pills/day) |
Nephrolithiasis, GI symptoms |
| Nelfinavir |
Viracept |
750 mg tid (or 1250 mg bid) (4–10 pills/day) |
Diarrhea |
| Amprenavir |
Agenerase |
1200 mg bid (16 pills/day) |
GI symptoms, rash |
| Lopinavir/ritonavir |
Kaletra |
Lopinavir 400 mg/ ritonavir 100mg (3 capsules or 5mL) bid (6 capsules/day) |
Diarrhea, nausea, abdominal discomfort |
| Atazanavir |
Reyataz |
400 mg qd (2, 200 mg capsules/day) 300 mg qd (2, 150 mg capsules/day) if combined with ritonavir 100 mg qd (1 capsule/day) |
Nausea, diarrhea, unconjugated hyperbilirubinemia |
| Fosamprenavir |
Lexiva |
1400 mg bid (2, 700 mg tablets twice daily) 1400 mg qd (2, 700 mg tablets/day) if combined with ritonavir 200 mg qd (2 capsules/day) 700 mg bid (1, 700 mg tablet twice daily) if combined with ritonavir 100 mg bid (1 capsule twice daily) Adjustment of ritonavir dose needed when fosamprenavir plus ritonavir are administered with efavirenz |
GI symptoms, headache, rash |
In addition to the medication-specific side effects listed here, a number of abnormalities are associated with protease inhibitors as a class. Patients taking protease inhibitors may develop serum lipid abnormalities (hyperlipidemia, hypertriglyceridemia), redistribution of body fat (lipodystrophy), and/or glucose intolerance.
Fusion Inhibitors
Fusion inhibitors interact with HIV directly, rather than with the host cell. This interaction prevents fusion of HIV to the cell. The first fusion inhibitor, enfuvirtide (T-20, Fuzeon) was licensed in the U.S. in March 2003. Enfuvirtide must be given by subcutaneous injection (twice daily). The most common side effect is local injection site reactions.
C. ADVERSE CLINICAL EVENTS ASSOCIATED WITH ANTIRETROVIRAL THERAPY
There are several significant adverse clinical events that have been associated with use of antiretroviral therapy. In some cases these are drug specific, while in others an increased risk of a specific adverse event appears to involve an entire class of antiretroviral drugs. The complications discussed below are of particular clinical significance or concern. In general, decisions about management, including future antiretroviral management, should be made in consultation with an HIV expert.
- Lactic Acidosis/Hepatic Steatosis: This is a rare but life-threatening complication associated with use, often prolonged, of NRTIs and appears to more commonly seen in women. The initial clinical signs and symptoms are nonspecific and may include nausea and vomiting, diarrhea, anorexia, abdominal pain, generalized weakness, myalgias, ascending neuromuscular weakness, and hepatomegaly. In addition to elevated lactate levels, laboratory evaluation may reveal elevated liver function tests, creatine phosphokinase, lipase, and amylase or increased anion gap. There are technical difficulties associated with serum lactate testing and routine testing is not recommended. However, providers should have a low threshold for measuring serum lactate in the presence of suggestive signs or symptoms or other associated laboratory abnormalities. When interpreting serum lactate levels, levels of 2-5 mmol/dL are considered elevated and need to be correlated with symptoms; levels >5mmol/dL are abnormal; and levels > 10 mmol/dL indicated serious and possibly life-threatening situations. Antiretroviral treatment should be stopped if clinical and laboratory manifestations of lactic acidosis occur.
- Hepatotoxicity: Hepatotoxicity, defined as a 3–5 fold increase in serum transaminases, may occur with or without clinical hepatitis. It has been reported with all NNRTIs and PIs and may be present with lactic acidosis associated with NRTI use. Most patients with hepatotoxicity are asymptomatic. Nevirapine has the greatest potential for causing hepatotoxicity (up to 12%) and this complication appears to be more common in women (Martinez, 2001; Bartlett, 2003). Nevirapine-associated hepatitis might also be part of a hypersensitivity syndrome, associated with other symptoms such as skin rash, fever, and eosinophilia. In a recent retrospective analysis of controlled and uncontrolled clinical trials, women with CD4 cell counts > 250 cells/mm3, including pregnant women, receiving chronic treatment for HIV infection, were at significantly higher risk (12-fold) of hepatotoxicity. In some cases hepatic injury progressed despite discontinuation of treatment and fatalities have occurred. (Boehringer-Ingelheim, 2004). Approximately two-thirds of nevirapine-associated clinical hepatitis occurs within the first 12 weeks of treatment and the greatest risk for severe hepatotoxicity in the recent analysis occurred in the first six weeks of treatment and was often associated with rash. However, risk continues after this time and patients should be closely monitored for the first 18 weeks of treatment. Initial presentation may include nonspecific gastrointestinal and flu-like smptoms, and liver enzyme abnormalities may or may not be present. However, this syndrome can progress rapidly to fulminant hepatic failure. A two-week lead-in dosing with 200 mg once daily before dose escalation to 200 mg twice daily is recommended to reduce the incidence of hepatotoxicity. Many clinicians advise close monitoring of clinical symptoms and liver enzymes after starting nevirapine (e.g., every 2 weeks for the first month, then monthly for the first 12 weeks, and every 1–3 months thereafter). Nevirapine should not be used in future regimens in women who experience severe liver toxicity while taking nevirapine. PI-associated hepatotoxicity can occur any time during the treatment course. Risk factors for liver toxicity include hepatitis B or C infection, alcohol abuse, baseline elevated liver enzymes, stavudine (d4T) use, and concomitant use of other hepatotoxic agents (DHHS, 2003; http://AIDSinfo.nih.gov) .
- Hyperglycemia: Hyperglycemia has been reported in 3–17% of patients on PI-containing antiretroviral regimens (DHHS, 2003. http://AIDSinfo.nih.gov). Preexisting diabetes may be exacerbated. Although routine use of glucose tolerance testing is not recommended, patients should be advised about symptoms of hyperglycemia (i.e., polydipsia, polyphagia, polyuria) and fasting blood glucose measurements should be considered at 3–4 month intervals during the first year of PI treatment for patients with no prior history of diabetes. Patients with preexisting diabetes should be monitored closely.
- Lipodystrophy (Fat Maldistribution Syndromes): Recognition of fat maldistribution syndromes has increased in the era of combination antiretroviral therapy; they are characterized by fat wasting (lipoatrophy) or fat accumulation. The absence of standard case definitions makes it difficult to estimate prevalence. Lipodystrophy might be associated with serum dyslipidemias, glucose intolerance, or lactic acidosis (Joffe, 2001; Carr, 1998). Fat accumulation is most commonly seen in the abdomen, the dorsocervical fat pad, and the breasts. This complication has been most associated with use of PI-containing regimens and prevalence increases with duration of therapy (Miller, 1998). Lipoatrophy most commonly affects the face and extremities and risk has been reported to increase with long-term NRTI exposure (Mallal, 1999). Women seem particularly prone to developing truncal obesity (increased abdominal girth, increased breast size). The etiology of these syndromes is unknown and at the present time, there is no clearly effective therapy. Women who perceive significant changes in body habitus related to their antiretroviral regimen may be at increased risk for nonadherence. It may be useful to obtain some standard measurements, such as minimum waist, maximum hip, and neck circumference at an early visit, before antiretroviral therapy is started. It is important to question the patient at regular intervals about any perceived changes in body shape or changes in clothing and brassiere size, and anthropomorphic measurements may be repeated to document any changes.
Detailed descriptions of medications, drug-drug interactions, and medication use in pregnancy may be found in Chapter XIV on Pharmacologic Considerations in HIV-infected Pregnant Patients.
- Hyperlipidemia: Combination antiretroviral therapy, primarily regimens containing protease inhibitors, has been associated with elevation in total serum cholesterol and low-density lipoprotein (LDL) and in increases in fasting triglycerides (Thiebaut 2000; Romeu 1999). Therapeutic intervention may be needed and, although data remain inconclusive, lipid elevations seen with antiretroviral therapy may be associated with increased risk of cardiovascular complications. Indications for monitoring and treatment of antiretroviral-associated dyslipidemias are the same as among uninfected persons (Adult Treatment Panel III, 2001), although patients with additional risks for atherosclerotic disease should be especially closely monitored (Dube 2000). Low-fat diet, regular exercise, control of hypertension, and smoking cessation should be routinely recommended for all patients, including those treated with antiretroviral agents. When treatment is indicated, statins are generally considered first-line therapy, although potential drug-drug interactions between statins and PIs must be kept in mind and agents that are less affected by the inhibitory effect of PIs via the cytochrome P450 system are preferred (e.g., pravastatin). If lipid elevations are severe or do not respond to other therapy, a change in antiretroviral regimen may be indicated, such as replacement of the PI component with an NNRTI.
- Bone Disorders: There is evidence that avascular necrosis involving the hips and decreased bone density (osteopenia, osteoporosis) may be linked to combination antiretroviral therapy regimens in adults and children (Tebas 2000; Scribner 2000). Diagnosis of osteonecrosis is generally made with CT or MRI ordered in response to complaints of pain. Diagnosis of osteopenia or osteoporosis is made with bone densitometry (dual energy X-ray absorptiometry or DEXA; quantitative ultrasound). Losses in bone mineralization appear to be more common in PI-containing regimens (Tebas 2000). Women are at increased risk for decreased bone density and adequate intake of calcium and vitamin D and appropriate weight-bearing exercise should be recommended. There are no recommendations for routine monitoring of bone density among asymptomatic HIV-infected persons, but additional risk factors, including estrogen deficiency (e.g., menopausal women), alcohol or tobacco abuse, sedentary lifestyle or immobilization, Caucasian or Asian race, wasting, and thin body habitus, should prompt consideration of screening. When significant decreases in bone density are recognized, treatment with bisphosphonates, raloxifene, or calcitonin may be indicated.
- Rash: Skin rash most commonly occurs with NNRTI-containing regimens, and is most frequent and most severe with nevirapine. Most cases are mild to moderate and occur within the first weeks of therapy. Women appear to be at increased risk for more serious skin rashes (Bersoff-Matcha, 2001). More serious cutaneous manifestations (e.g., Stevens-Johnson Syndrome and toxic epidermal necrosis) should result in prompt and permanent discontinuation of NNRTI or other offending agents. A severe and potentially life-threatening syndrome consisting of drug rash, eosinophilia, and systemic symptoms (DRESS) has been described (Bourezane, 1998). Outside of the NNRTI drug class, skin rash occurs most frequently with abacavir and amprenavir. If rash is determined to be from an abacavir-associated systemic hypersensitivity reaction, then abacavir should be discontinued and not restarted. Amprenavir is a sulfonamide and should be used with caution in patients with history of sulfa allergy.
D. TREATMENT GUIDELINES
The Department of Health and Human Services (DHHS) Panel on Clinical Practices for Treatment of HIV Infection continuously updates treatment guidelines. Updated recommendations are available at http://www.aidsinfo.nih.gov. The guidelines detail indications for therapy in chronically infected patients, recommendations for initial therapy, considerations for changes in therapy, and possible regimens for such changes (Table 4-7).
Table 4-7: Indications for Initiating Antiretroviral Therapy
for the Chronically HIV-1 Infected Patient
| Clinical Category |
CD4+ Cell |
Plasma HIV RNA |
Recommendation |
| Symptomatic (AIDS or severe symptoms) |
Any value |
Any value |
Treat |
| Asymptomatic, AIDS |
CD4+ cells <200/mm3 |
Any value |
Treat |
| Asymptomatic |
CD4+ cells >200/mm3 but  350/mm3 350/mm3 |
Any value |
Treatment should be offered, although controversial.* |
| Asymptomatic |
CD4+ T cells >350/mm3 |
>100,000 (by RT-PCR or bDNA) |
Some experienced clinicians recommend initiating therapy, recognizing that the 3-year risk for untreated patients to develop AIDS is >30%; in the absence of increased levels of plasma HIV RNA, other clinicians recommend deferring therapy and monitoring the CD4+ T cell count and level of plasma HIV RNA more frequently; clinical outcome data after initiating therapy are lacking. |
| Asymptomatic |
CD4+ T cells >350/mm3 |
<100,000 (by RT-PCR or bDNA) |
Most experienced clinicians recommend deferring therapy and monitoring the CD4+ T cell count, recognizing that the 3-year risk for untreated patients to experience AIDS is <15%. |
Source: Adapted from DHHS, 2004.
* Clinical benefit has been demonstrated in controlled trials only for patients with CD4+ T cells <200/mm3, however, the majority of clinicians would offer therapy at a CD4+ T cell threshold <350/mm3.
The strength of the recommendation for starting therapy in an asymptomatic patient must take into account prognosis for disease-free survival, potential benefits and risks of therapy, and the willingness of the patient to take, and adhere to, therapy (see Chapter V on Adherence to HIV Therapies). Prognosis for disease-free survival may be assessed by utilizing the data in Table 4-8. However, given the sex-based differences in viral load and CD4 count (See Chapter 1 Epidemiology and Natural History of HIV Infection in Women), these data, generated from a prospective cohort of men who have sex with men (MSM), should be extrapolated with caution to women.
Among the benefits of therapy are:
- prevention of progressive immunosuppression by control of viral load,
- delayed progression of clinical disease/progression to AIDS,
- prolongation of life, and
- possible decreased risk of transmission (Quinn, 2000).
The risks of starting therapy include:
- a decrease in quality of life associated with adverse drug effects and inconvenience of dosing,
- limitations of future options for therapy if resistance develops to current agents,
- potential long-term toxicity of therapy,
- unknown duration of effectiveness of therapy, and
- possible transmission of drug-resistant virus.
Table 4-8: Risk for Progression to AIDS-Defining Illness
Among a Cohort of Men Who Have Sex with Men,
Predicted by Baseline CD4+ T Cell Count and Viral Load*
CD4  200 cells/mm3 200 cells/mm3
Plasma Viral Load (copies/mL)† |
Percentage of AIDS-defining illness |
| bDNA |
RT-PCR |
n |
3 years |
6 years |
9 years |
 500 500 |
 1,500 1,500 |
0§ |
|
|
|
501 - 3,000 |
1,501 - 7,000 |
3 |
|
|
|
3,001 - 10,000 |
7,001 - 20,000 |
7 |
14.3 |
28.6 |
64.3 |
10,001 - 30,000 |
20,001 - 55,000 |
20 |
50.0 |
75.0 |
90.0 |
> 30,000 |
> 55,000 |
70 |
85.5 |
97.9 |
100.0 |
CD4 201 – 350∞ cells/mm3
Plasma Viral Load (copies/mL)† |
Percentage of AIDS-defining illness |
| bDNA |
RT-PCR |
n |
3 years |
6 years |
9 years |
 500 500 |
 1,500 1,500 |
3 |
|
|
|
501 - 3,000 |
1,501 - 7,000 |
27 |
0 |
20.0 |
32.2 |
3,001 - 10,000 |
7,001 - 20,000 |
44 |
6.9 |
44.4 |
66.2 |
10,001 - 30,000 |
20,001 - 55,000 |
53 |
36.4 |
72.2 |
84.5 |
> 30,000 |
> 55,000 |
104 |
64.4 |
89.3 |
92.9 |
CD4 >350 cells/mm3
PlasmaViral Load (copies/mL)† |
Percentage of AIDS-defining illness |
| bDNA |
RT-PCR |
n |
3 years |
6 years |
9 years |
 500 500 |
 1,500 1,500 |
119 |
1.7 |
5.5 |
12.7 |
501 - 3,000 |
1,501 - 7,000 |
227 |
2.2 |
16.4 |
30.0 |
3,001 - 10,000 |
7,001 - 20,000 |
342 |
6.8 |
30.1 |
53.5 |
10,001 - 30,000 |
20,001 - 55,000 |
323 |
14.8 |
51.2 |
73.5 |
> 30,000 |
> 55,000 |
262 |
39.6 |
71.8 |
85.0 |
* Adapted from data from the Multicenter AIDS Cohort Study (MACS). (Source: Mellors, JW, Rinaldo CR Jr, Gupta P, et al. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science 1996; 272:1167-70. Erratum: Science 1997; 275:14; adapted by Alvaro Muñoz, PhD, Johns Hopkins University, Baltimore, MD, 2001.)
† MACS numbers reflect plasma HIV RNA values obtained by version 2.0 bDNA testing. RT-PCR values are consistently 2–2.5-fold higher than first-generation bDNA values, as indicated. The version 3.0 bDNA assay provides similar HIV-1 RNA values as RT-PCR, except at the lower end of the linear range (<1,500 copies/mL).
§ Too few subjects were in the category to provide a reliable estimate of AIDS risk.
∞ A recent evaluation of data from the MACS cohort of 231 persons with CD4+ T cell counts >200 and <350 cells/mm3 demonstrated that of 40 (17%) persons with plasma HIV RNA <10,000 copies/mL, none progressed to AIDS by 3 years (Phair, 2002). Of 28 individuals (12%) with plasma viremia of 10,000 – 20,000 copies/mL, 4% and 11% progressed to AIDS at 2 and 3 years, respectively. Plasma HIV RNA was calculated as RT-PCR values from measured bDNA values.
Source: DHHS, 2004. Recommendations For Initial Treatment Regimens
Recommendations for antiretroviral treatment continue to evolve with the development of new medications and additional data from clinical trials. The most recent guidelines from the DHHS are shown in Table 4-9.
Although these guidelines illustrate generally recommended regimens, nonspecialists should consider expert consultation regarding initiation of a specific regimen whenever there is any question about patient management.
Although there are multiple possible effective regimens, individualized decisions about therapy should take into account considerations such as pill burden, dosing frequency, toxicities and side effects, drug-drug interactions, and specific patient variables (e.g., pregnancy, co-morbid conditions, plasma HIV-RNA level, lifestyle, etc).
Table 4-9a: Antiretroviral Regimens Recommended for
Treatment of HIV-1 Infection in Antiretroviral-Naive Patients
| This table is a guide to treatment regimens for patients who have no previous experience with HIV therapy. Preferred regimens are in bold type; these regimens have been selected by experts based on the totality of virologic, immunologic, and toxicity data. Clinicians initiating antiretroviral regimens in the HIV-1 infected pregnant patient should refer to Chapter VII HIV and Reproduction and to “Recommendations for Use of Antiretroviral Drugs in Pregnant HIV-1 Infected Women for Maternal Health and Interventions to Reduce Perinatal HIV-1 Transmission in the United States” (http://www.aidsinfo.nih.gov/guidelines/). |
NNRTI-Based Regimens |
# of pills per day |
| Prefered Regimenns |
efavirenz + (lamivudine or emtricitabine) + (zidovudine or tenofovir DF) – except for pregnant women or women with pregnancy potential |
2–3 pills/day |
| Alternative Regimens |
efavirenz + (lamivudine or emtricitabine) + abacavir or (didanosine or stavudine*) – except for pregnant women or women with pregnancy potential** |
2–4 pills/day |
| nevirapine + (lamivudine or emtricitabine) + (zidovudine or stavudine or didanosine or abacavir or tenofovir) |
3–6 pills/day |
PI-Based Regimens |
# of pills per day |
| |
lopinavir/ritonavir (co-formulated as Kaletra®) + (lamivudine or emtricitabine) + (zidovudine) |
8–9 pills/day |
| atazanavir + (lamivudine or emtricitabine) + (zidovudine or stavudine* or abacavir or didanosine) or (tenofovir + ritonavir 100mg/d) |
3–6 pills/day |
| indinavir/ritonavir† + (lamivudine or emtricitabine) + (zidovudine or stavudine* or abacavir or tenofovir or didanosine) |
7–12 pills/day |
| lopinavir/ritonavir (co-formulated as Kaletra® + (lamivudine or emtricitabine) + (stavudine* or abacavir or tenofovir or didanosine) |
7–10 pills/day |
| nelfinavir§ + (lamivudine or emtricitabine) + (zidovudine or stavudine* or abacavir or tenofovir or didanosine) |
5–8 pills/day |
| saquinavir (sgc or hcg)ø/ritonavir† + (lamivudine or emtricitabine) + (zidovudine or stavudine* or abacavir or tenofovir or didanosine) |
13–16 pills/day |
| fosamprenavir + (lamivudine or emtricitabine) + (zidovudine or stavudine* or abacavir or tenofovir or didanosine) |
5–8 pills/day |
| fosamprenavir/ritonavir + (lamivudine or emtricitabine) + (zidovudine or stavudine* or abacavir or tenofovir or didanosine) |
5–8 pills/day |
Triple NRTI Regimen – Only when an NNRTI- or a PI-based regimen cannot or should not be used as first line therapy |
# of pills per day |
| |
abacavir + lamivudine + zidovudine |
2 pills /day |
* Higher incidence of lipoatrophy, hyperlipidemia, and mitochondrial toxicities reported with stavudine than with other NRTIs
** Women with child bearing potential implies women who want to conceive or those who are not using effective contraception
† Low-dose (100–400 mg) ritonavir
§ Nelfinavir available in 250 mg or 625 mg tablet
ø sgc = soft gel capsule; hgc = hard gel capsule
Source: Adapted from DHHS, 2004.
Table 4-9b: Advantages and Disadvantages of Antiretroviral Components
Recommended as Initial Antiretroviral Therapy
| ARV Class |
Antiretroviral Agent(s) |
Advantages |
Disadvantages |
| NNRTIs |
|
NNRTI Class Advantages:
- Less fat maldistribution and dyslipidemia than PI-based regimens
- Save PI options for future use
|
NNRTI Class Disadvantages
- Low genetic barrier to resistance
- Cross-resistance among NNRTIs
- Skin rash
- Potential for CYP450 drug interaction
|
| Efavirenz |
- Potent antiretroviral activity
- Low pill burden and frequency ( 1-tablet per day)
|
- Neuropsychiatric side effects
- Teratogenic in nonhuman primates, contraindicated in pregnancy and avoid use in women with pregnant potential
|
| Nevirapine |
- No food effect
- No evidence of increased adverse hepatic events with single-dose NVP for prevention of mother-to-child transmission
|
- Higher incidence of rash than with other NNRTIs, including rare serious hypersensitivity reaction
- Higher incidence of hepatotoxicity than with other NNRTIs; including serious cases of hepatic necrosis
- Women with CD4 > 250 cells/mm3 are at higher risk of symptomatic hepatic events
|
| PIs |
|
PI Class Advantages:
- NNRTI options saved for future use
- Longest prospective study data including data on survival benefit
|
PI Class Disadvantages:
- Metabolic complications – fat maldistribution, dyslipidemia, insulin resistance
- CYP3A4 inhibitors & substrates – potential for drug interactions (esp. with ritonavir-based regimens)
|
| Lopinavir/ ritonavir |
- Potent antiretroviral activity
- Co-formulated as Kaletra®
|
- Gastrointestinal intolerance
- Hyperlipidemia
- Little experience in pregnant women
- Food requirement
|
| Atazanavir |
- Less adverse effect on lipids than other PIs
- Once-daily dosing
- Low pill burden
|
- Hyperbilirubinemia (indirect)
- PR interval prolongation – generally inconsequential unless combined with another drug with similar side effect
- Interaction with tenofovir and efavirenz – avoid concomitant use unless combined with RTV (ATV 300 mg qd + RTV 100 mg qd)
- Food requirement
|
| Fosamprenavir |
- Lower pill burden than amprenavir (4 vs 16 capsules/day)
- No food effect
|
|
| |
- Lower pill burden than amprenavir/ ritonavir
- Once daily regimen in patients with no history of PI failure
- No food effect
|
|
| |
- Low-dose ritonavir/indinavir T1⁄2 & Cmin allows for twice-daily instead of 3-times-daily dosing
- Eliminates food restriction of indinavir
|
- Possibly higher incidence of nephrolithiasis than with IDV alone
- High fluid intake required (1.5–2 liters of fluid per day)
|
| Nelfinavir |
- More favorable safety and pharmacokinetic profile in pregnant women than with other PIs
|
- Diarrhea
- Higher rate of virologic failure than with other PIs in comparative trials
- Food requirement
|
| Saquinavir (hgc or sgc) + ritonavir |
- Low-dose ritonavir reduces saquinavir daily dose and frequency -
 Cmax, Cmin, Cmax, Cmin,
& T1⁄2
|
- Gastrointestinal intolerance (sgc worse than hgc)
|
| NRTIs |
|
- Established backbone of combination antiretroviral therapy
|
- Rare but serious cases of lactic acidosis with hepatic steatosis reported with most NRTIs
|
| Triple NRTI regimen |
Abacavir + zidovudine + lamivudine only |
- Abacavir + zidovudine + lamivudine - Co-formulated as Trizivir®
- Minimal drug-drug interactions
- Low pill burden
- Saves PI & NNRTI for future option
|
- Inferior virologic response when compared to efavirenz-based and indinavir-based regimens
- Potential for abacavir hypersensitivity reaction
|
| Dual NRTIs backbone of three or more drug combination therapy |
Zidovudine + lamivudine |
- Most extensive and favorable virological experience
- Co-formulated as Combivir® – ease of dosing
- No food effect
- Lamivudine – minimal side effects
|
- Bone marrow suppression with zidovudine
- Gastrointestinal intolerance
|
| Stavudine + lamivudine |
- No food effect
- Once-daily dosing (when extended release stavudine formulation becomes available)
|
Adverse effects associated with stavudine:
- Peripheral neuropathy, lipoatrophy, hyperlactatemia and lactic acidosis, reports of progressive ascending motor weakness, potential for hyperlipidemia
- Higher incidence of mitochondrial toxicity with stavudine than with other NRTIs
|
| Abacavir + lamivudine |
- No food effect
- Once-daily dosing
- Co-formulation (Epzicom)
|
- Potential for abacavir hypersensitivity reaction
- Higher incidence of severe hypersensitivity reactions with once daily dosing reported in 1 study
|
| Tenofovir + lamivudine |
- Good virologic response when used with efavirenz
- Well tolerated
- Once-daily dosing
|
- Data lacking for tenofovir use in patients with renal insufficiency
- Tenofovir – some reports of renal impairment
- Drug interactions with atazanavir and didanosine requiring dose adjustment
|
| Didanosine + lamivudine |
|
- Peripheral neuropathy, pancreatitis – associated with didanosine
- Food effect – needs to be taken on an empty stomach
- Requires dosing separation from most PIs
- Potential increase in toxicities when used with ribavirin, tenofovir, hydroxyurea
|
| NRTI + emtricitabine |
- Long half-life of emtricitabine allows for once daily dosing (of emtricitabine)
- Co-formulation with tenofovir (Truvada)
|
|
Source: Adapted from DHHS, 2004. Recommendations For Antiretroviral Therapy In The Treatment-Experienced Patient
The need for a change in antiretroviral therapy most commonly arises in two situations: medication toxicity and treatment failure.
- Medication toxicity: When the need to change therapy arises because of medication toxicity, it may be possible to simply change one component of a regimen. If the toxicity occurs in a regimen that has provided effective virologic control, the goal is to continue effective therapy by changing the component that causes toxicity. For example, in a patient taking an effective regimen of AZT/3TC/PI, the development of anemia could be attributed to AZT. A different NRTI that does not commonly cause bone marrow suppression (e.g., d4T) could be substituted. The similar toxicities of certain agents must be remembered when making such changes: for example, in a patient taking ddI, the development of peripheral neuropathy would not be expected to be alleviated by substituting d4T.
There are other situations in which the toxicity is not as easily attributed to a single component of a regimen (e.g., rash, GI symptoms). In these instances, a “drug holiday” (temporary discontinuation) of the entire regimen may be necessary to allow symptoms to resolve, and a new regimen initiated with some change in components. In most cases reinitiation of antiretroviral therapy would not be expected to be associated with any increase in side effects. However, in the case of certain serious or life-threatening toxicities (see C. on page 115), such as abacavir–associated hypersensitivity or severe liver toxicity with nevirapine, the offending agent should never be resumed.
- Treatment failure: Changes in regimen for lack of efficacy may be triggered by evidence of clinical progression, progressive decline in CD4 count, and, most commonly, for incomplete virologic suppression or virologic rebound. Clinical failure is defined by the occurrence of HIV-related events after at least 3 months on an antiretroviral regimen, excluding immune reconstitution syndromes. Immunologic failure is the failure to increase 25–50 cells/mm3 above the baseline CD4 count in the first year of therapy or a decrease in the CD4 count below baseline while on therapy. Virologic failure is the failure to achieve HIV RNA <400 copies/mL by 24 weeks or <50 copies/mL by 48 weeks on therapy or repeated detection of viremia after complete virologic suppression. When changes in therapy are contemplated, an increase in viral load or a downward trend in CD4 count should be confirmed with repeat determinations before changes are made. In general virologic failure occurs first, followed by immunologic failure, and then by clinical failure, although these events may be separated by months to years.
There are a number of possible reasons for treatment failure: patient factors (e.g., age (in some cohorts), pretreatment HIV-RNA level and CD4 count, active substance use, depression, baseline drug resistance or resistance related to prior treatment regimens); suboptimal adherence (including running out of medications); medication side effects or toxicity; pharmacokinetics (e.g., absorption, metabolism, penetration into body reservoirs, food or fasting requirements, drug-drug interactions); potency of the current regimen; and other unknown reasons. Sex, race, pregnancy and history of substance abuse have not been associated with treatment failure. It is important to try to distinguish among these different causes of treatment failure, since approaches to management will differ.
Failure increases the risk of disease progression and should be addressed aggressively. Assessing and managing a patient with extensive prior antiretroviral experience and treatment regimen failure is complex and expert advice is critical. Table 4-10 summarizes guidelines for patient assessment and management with suspected treatment failure. In general a distinction is made between patients with limited prior treatment and those with extensive prior treatment, since those with more limited antiretroviral experience have a greater likelihood of achieving maximal viral suppression with an appropriate change in regimen. In these patients changing therapy sooner rather than later is recommended to minimize continued selection of resistance mutations.
Table 4-10: Guidelines for Changing an Antiretroviral Regimen
for Suspected Treatment Regimen Failure
Patient Assessment
- Review antiretroviral treatment history (drugs, doses, duration, adherence, tolerability, prior resistance testing).
- Perform physical exam to assess for signs of clinical progression.
- Assess adherence, tolerability, and pharmacokinetic issues.
- Distinguish between first or second, and multiple treatment regimen failures.
- Perform resistance testing while patient is taking therapy.
- Identify susceptible drugs and drug classes.
Patient Management: Specific Clinical Scenarios
- Limited prior treatment with low (but not suppressed) HIV RNA level (e.g., up to 5000 copies/mL: The goal of treatment is to re-suppress viral replication. Consider intensifying with one drug (e.g., tenofovir) or pharmacokinetic enhancement (use of ritonavir boosting of a protease inhibitor) or most aggressively, change to a completely new regimen. If continuing the same treatment regimen, need to follow HIV RNA levels more closely, because ongoing viremia will lead to the accumulation of resistance mutations.
- Limited or intermediate prior treatment with single drug resistance: Consider changing one drug pharmacokinetic enhancement (few data available) or, most aggressively, change to a completely new regimen.
- Limited or intermediate prior treatment with more than 1 drug resistance: The goal of treatment is to suppress viremia to prevent further selection of resistance mutations. Consider optimizing regimen by changing classes (e.g., PI-based to NNRTI-based and vice versa) and/or adding new active drugs.
- Prior treatment with no resistance identified: Consider the timing of obtaining the drug resistance test (e.g., was the patient off antiretroviral medications?) and/or nonadherence. Consider resuming the same regimen or starting a new regimen and then repeating genotypic testing early (e.g., 2–4 weeks) to see if a resistant strain has been selected.
- Extensive prior treatment and drug resistance: It is reasonable to continue the same antiretroviral regimen if there are few or no treatment options. In general, avoid adding a single active drug because of the risk of the development of resistance to that drug. In advanced disease with a high likelihood of clinical progression, adding a single drug may reduce the risk of immediate clinical progression. In this complicated scenario, expert advice should be sought.
|
Source: Adapted from DHHS, 2004
Viral suppression may be difficult to achieve in patients with extensive prior drug exposure and the primary goals are to preserve immune function and prevent clinical progression. In a patient with extensive prior treatment and lower CD4 count (e.g., <200/mm3) a change in therapy is indicated to prevent clinical progression; with higher CD4 counts, risk of progression is less and a well-tolerated regimen may be continued, if it is impossible to construct a new regimen that is effective. Discontinuing therapy, even in the presence of ongoing viremia, leads to a rapid increase in viral load, a decrease in CD4 count, and increases the risk of clinical progression (Deeks, 2001; Lawrence, 2003) and is therefore not recommended (DHHS, 2004).
When a regimen is changed for lack of efficacy, the goal is to use medications that will minimize the likelihood of further viral resistance. Prior to the general availability of resistance testing, regimen changes to improve efficacy were made empirically. Some of the general principles from that empiric practice are still worth emphasizing, e.g. often an entire regimen (not just one medication) must be changed, and there is often significant cross-resistance among agents within a class. Now, however, when antiretroviral therapy is changed for lack of efficacy, information from resistance testing can be of pivotal importance in choosing a new regimen (see “Resistance Testing” below). Recommendations for regimen changes may also be found at http://www.aidsinfo.nih.gov.
“Intensification” refers to the theory that some patients with a suboptimal decrease in viral load from an early regimen, may benefit from the early addition of a single new agent. This may also refer to the situation in which rebound occurs after complete viral suppression with limited prior treatment. In these patients, low levels of detectable virus (usually <1000 c/mL) are present, generally too low to obtain accurate resistance testing. This may be a unique situation in which it may be reasonable to add only a single medication, or to change only a portion of the combination, adding an agent to which the patient will likely be sensitive.
Resistance Testing
There are two main techniques to assess the development of viral resistance to antiretroviral therapy. Phenotypic assays directly determine the amount of a medication required to inhibit HIV. Genotypic assays determine changes in the nucleotide sequences of the genes that code for the protease and reverse transcriptase enzymes. Interpretations of genotypic results require knowledge of which specific changes are associated with resistance. Results are reported as a string of three pieces of information for each mutation detected:
- wild-type amino acid,
- codon involved, and
- amino acid coded for by mutated codon.
Table 4-11 shows the mutations known to be associated with resistance to specific agents (Bartlett, 2003). Updated listings of mutations and associated resistance can be found at: http://hiv-web.lanl.gov or http://hivdb.stanford.edu.
Table 4-11 Resistance Mutations Adapted from IAS-USA
| Drug |
Codon Mutations* |
| Nucleosides and Nucleotides |
| AZT |
41, 67, 70, 210, 215, 219 |
Mutations are “TAMs”** reduces susceptibility to AZT, d4T, ABC, ddl, ddC, TDF, 3TC. |
| 3T |
184 (44, 118) |
184 – high level 3TC resistance, increases activity of d4T, AZT, and TDF, reduces susceptibility to ddl, ddC, ABC. |
| ddC |
65, 69, 74, 184 |
|
| ddI |
65, 74 |
Presence of 74 or 65 alone or combined with TAMs is associated with resistance to ddl. Multiple TAMs also decrease ddl susceptibility |
| dd4T |
41, 67, 70, 75, 210, 215, 219 |
The d4T-specific mutation at 75 is seen mostly in vitro. In vitro resistance depends on number of TAMs, which reduce susceptibility to all NRTIs. |
| ABC |
41, 65, 67, 70, 74, 115, 184, 210, 215, 219 |
Resistance depends on number of TAMs ± M184V; 184 alone does not confer resistance. Presence of M184V plus  3-4 TAMs associated with ABC resistance. ABC selects for mutations that may confer cross-resistance to 3TC, ddl, and TDF. 3-4 TAMs associated with ABC resistance. ABC selects for mutations that may confer cross-resistance to 3TC, ddl, and TDF. |
| TDF |
65, 69 insertion,  3 TAMs including 41L or 210W 3 TAMs including 41L or 210W |
Reduced activity with K65R and resistance with 69 insertion. |
| Multinucleoside resistance – A Q151M complex |
151, 62, 75, 77, 116 |
Occurs with or without TAMs. Confers resistance to all NRTIs except tenofovir. |
| Multinucleoside resistance – B T69 insertion |
69 (insertion), 41, 62, 67, 70, 210, 215, 219 |
Requires TAMs. Confers resistance to all NRTIs and TDF but not to DAPD. |
| Multinucleoside resistance – Multiple TAMs |
41, 67, 70, 210, 215, 219 |
Confers resistance to all NRTI including TDF. |
| NNRTIs |
| NVP |
100, 103, 106, 108, 181, 188C/L/H, 190 |
Y181C is favored mutation with NVP, unless combined with AZT, in which case K103N is favored. |
| DLV |
103, 181, 236, 318 |
|
| EFV |
100, 103, 108, 181, 188L, 190, 225 |
181C is not selected, but its presence contributes low-level cross resistance. Resistance with 188L but not 188C or 188H. |
| Multi-NNRTI resistance |
103, 188L |
Either mutation substantially reduces activity of all NNRTIs |
| Multi-NNRTI resistance accumulation |
100, 106, 181, 190, 230 |
 2 of the mutations substantially reduces activity of all NNRTIs 2 of the mutations substantially reduces activity of all NNRTIs |
Drug |
Major Mutation† |
Minor Mutation‡ |
Comment |
| PIs |
| IDV |
46, 82, 84 |
10, 20, 24, 32, 36, 54, 71, 73, 77, 90 |
At least 3 mutations required for resistance (4x decreases in susceptibility). |
| NFV |
30, 90 |
10, 36, 46, 71, 77, 82, 84, 88 |
D30N most common mutation: No PI cross-resistance. L90M occurs in some, leading to greater PI cross-resistance. |
| RTV |
82, 84 |
10, 20, 32, 33, 36, 46, 54, 71, 77, 90 |
Cross resistance with IDV common. |
| SQV |
48, 90 |
10, 54, 71, 73, 77, 82, 84 |
90 develops 1st, then 48; Codon 48 mutation unique, but L90M contributes to PI cross-resistance. |
| APV |
50V, 84 |
10, 32, 46, 47, 54, 73, 90 |
I50V is associated with cross-resistance to LPV. |
| LPV/r |
73 |
10, 20, 24, 32, 33, 46, 47, 50V, 53, 54, 63, 71, 73, 82, 84, 90 |
 6 mutations cause reduced response; the number may be as low as 4. I50V (selected by APV) decreases LPV susceptibility. 6 mutations cause reduced response; the number may be as low as 4. I50V (selected by APV) decreases LPV susceptibility. |
| Atazanvir |
50L |
32, 46, 54, 71, 82, 84, 88, 90 |
Selects for 50L and 71 when initial PO; in PI experienced patients selects for 54 and 84. |
| Multi-PI resistance |
46, 82, 84, 90 |
10, 54 |
 4 or 5 usually cause multiple PI resistance. 4 or 5 usually cause multiple PI resistance. |
| Fusion Inhibitors |
| Enfuvirtide (T20) |
|
|
Resistance in the gp41 envelope gene at positions 36-45. |
Source: Adapted from D’Acquilla, 2002. Updated October, 2003 by the Drug Resistance Mutations Group, International AIDS Society–USA. Available at http://www.iasusa.org. (Accessed 3/04.)
* The distinction between primary and secondary mutations has been eliminated for NRTIs and NNRTIs by the International AIDS Society Expert Committee; this distinction has been retained for PIs, but with the terms “Major” or “Minor”.
** Thymidine-associated mutations
† Major Mutations develop first or are associated with decreased drug binding or reduced viral activity; these effect phenotype resistance.
‡ Minor Mutations appear later and, by themselves, do not significantly change phenotype resistance. Both phenotypic and genotypic assays are difficult to perform if the viral copy number is less than 1000 c/mL. Their utility is also limited by an inability to detect resistant virus that makes up less than 20% of the total viral burden in a sample. It is also critical to recognize that these assays will only reliably detect mutations conferring resistance to medications the patient is taking at the time the assay is performed; samples from patients who are off therapy at the time of resistance testing are likely to show reversion to wild-type (sensitive) virus as the predominant circulating viral strain. Thus, resistance testing is insensitive to mutations secondary to selective pressure that is no longer present after a change in regimen. Virions with these mutations likely still exist as a small percentage of circulating virus and may lead to clinical resistance if inactive drugs that test “sensitive” but are vulnerable to these resistance mutations are used; current assays will not detect their presence. A comparison of genotypic and phenotypic assays is shown in Table 4-12.
Table 4-12: Comparison of Genotypic and Phenotypic Assays
| Advantages |
Disadvantages |
Genotypic Assays |
- Less expensive ($300 to $480/test)
- Short turn-around of 1 to 2 weeks
- May detect presence of resistance mutations before they have resulted in phenotypic resistance
|
- Detect resistance only in dominant species (>20%)
- Interpretation requires knowledge of mutational changes, i.e., expertise
- Technician experience influences results
- May show discrepancy with phenotype
- Require viral load > 1000 c/mL
|
Phenotypic Assays |
- Interpretation more analogous to resistance testing of bacteria
- Assesses total effect, including mutations, mutational interactions
- Reproducibility is good
- Advantage over genotype when there are multiple mutations
|
- More expensive (usually $800 to $1000)
- Report takes 2 to 3 weeks
- Thresholds to define susceptibility are arbitrary and do not account for boosted PI levels
- Detects resistance only to single drug, not combinations
- Detect resistance only in dominant species (>20%)
- Require viral load >500-1000 c/mL
|
Source: Bartlett, 2003. Many studies have shown that patients for whom resistance analysis is done before a change in antiretroviral therapy have a better virologic response to the new regimen than do patients in whom a change in therapy is based solely on antiretroviral history (Baxter, 1999; Durant, 1999, Zolopa 1999). Resistance testing may be useful in the following ways.
- To determine the role of resistance in patients with virologic failure and maximize the number of active medications in a new regimen.
- To determine the role of resistance in patients with suboptimal virologic control on a HAART regimen (and again maximize the number of active medications in a new regimen).
- To determine whether there is drug resistance in a patient with acute HIV who is considering antiretroviral therapy (see “Treatment of Acute HIV Infection,” below).
Patients with pan-sensitive virus in the face of virologic failure should be questioned carefully, but nonjudgmentally, about their medication-taking behaviors. Therapeutic drug monitoring (TDM) can also be considered, although data are not yet available demonstrating that TDM improves clinical outcome. Current USPHS recommendations for the use of drug resistance assays are outline in Table 4-13.
Table 4-13: Recommendations for Using Drug-Resistance Assays
| Clinical Setting/Recommendations |
Rationale |
Drug-resistance assay recommended |
Virologic failure during combination antiretroviral therapy (AI)* |
Determine the role of resistance in drug failure and maximize the number of active drugs in the new regimen |
Suboptimal suppression of viral load after antiretroviral therapy initiation (BIII)
|
Determine the role of resistance and maximize the number of active drugs in the new regimen, if indicated |
Acute human immunodeficiency virus (HIV) infection, if decision is made to initiate therapy (AIII)
|
Determine if drug-resistant virus was transmitted and change regimen accordingly |
Drug-resistance assay should be considered
|
Chronic HIV infection before therapy initiation (CIII) |
Available assays might not detect minor drug-resistant species. However, should consider if significant probability that patient was infected with drug-resistant virus (i.e., if the patient is thought to have been infected by a person receiving antiretroviral drugs). |
Drug-resistance assay usually not recommended
|
After discontinuation of drugs (DIII) |
Drug-resistance mutations might become minor species in the absence of selective drug pressure, and available assays might not detect minor drug-resistant species. If testing is performed in this setting, the detection of drug resistance may be of value, but its absence does not rule out the presence of minor drug-resistant species |
Plasma viral load <1,000 HIV RNA copies/mL (DIII) |
Resistance assays cannot be consistently performed because of low copy number of HIV RNA; patients/providers may incur charges and not receive results |
Source: DHHS, 2004.
* See Notes for Table 4-16 for explanation of evidence grading. Treatment Interruptions (Drug Holiday)
Interruption of antiretroviral therapy has been considered in a variety of different scenarios. Intermittent therapy was previously hypothesized by some to improve HIV-specific immunity. A clinical trial failed to show evidence for this. There do remain two situations where treatment interruptions are being assessed: 1) as a way to reduce total exposure to medication (which could provide a cost or toxicity benefit), and 2) prior to attempts at salvage therapy in patients with virologic failure and multiple resistance mutations. At the current time, however, treatment interruption should be considered experimental in both of these situations.
D. TREATMENT OF ACUTE HIV INFECTION
To consider treatment of acute HIV infection, the clinician must first recognize its presence. In more than half of all patients who acquire HIV infection, there are clinical symptoms 1–6 wk after exposure. The symptoms vary in severity, but commonly include fever, lymphadenopathy, fatigue, rash, myalgias, and pharyngitis — a symptom complex that mimics mononucleosis. HIV antibodies will not yet be present at this point, but techniques that detect viral nucleic acids (see “Initial Diagnosis,” above) will confirm the diagnosis: a negative or indeterminate antibody test in conjunction with a positive HIV RNA or HIV DNA test is diagnostic of acute HIV infection. It is important to note, however, that a low level of HIV RNA (e.g., <5000 c/mL) may be a false-positive result and should be repeated (Rich, 1999). In addition, an HIV DNA assay could be performed to clarify the diagnosis; this should almost always be positive in an infected person, regardless of RNA level. Relatively recent infection may also be diagnosed in a patient with negative HIV serologies in the previous 6–9 mo and a first positive result, even in the absence of a seroconversion syndrome.
The benefits of treating acute HIV infection are not completely defined. The rationale for early treatment is that there will be early suppression of viremia, which may preserve CD4 cell number and function including HIV-specific CD4 cells. There are also risks associated with early treatment that include the toxicities of the medications used and the possibility of early development of resistance. These unanswered questions about risks and benefits of early therapy should be addressed with the patient; enrollment in clinical trials and observational studies of acute HIV should be considered. In treating acute HIV, it is always important to use a three- or four-drug regimen that would be expected to provide complete viral suppression. In addition, after considering the source of exposure and local epidemiologic information, genotypic resistance testing may prove useful in this setting. In acute HIV infection, the patient’s predominant virus will be the strain that was transmitted, without reversion to the wild-type (pan-sensitive) virus seen in chronically infected patients who have stopped treatment. The potential risks and benefits of treating acute HIV are summarized in Table 4-14. Postexposure Prophylaxis
See Chapter XIII on Occupational Exposure.
Treatment In Pregnancy
Antiretroviral treatment is indicated in pregnancy to reduce the risk of perinatal transmission whether or not therapy is yet needed to treat maternal disease. Specific guidelines for optimal therapy are addressed in Chapter VII (HIV and Reproduction). Information is also provided at http://www.aidsinfo.nih.gov.
Table 4-14: Risks and Benefits of Early Initiation of Antiretroviral Therapy
in Acute HIV Infection
Potential Benefits
- Control of viral replication and rate of mutation
- Prevention of progressive immunodeficiency; potential maintenance of a normal immune system
- Delayed progression to AIDS and prolongation of life
- Possible decreased risk of viral transmission
- Decreased severity of acute infection symptoms
- Possibly decreased viral setpoint (which may have a subsequent effect on rate of disease progression)
|
Potential Risks
- Reduction in quality of life from adverse drug effects and inconvenience of current maximally suppressive regimens
- Earlier development of drug resistance, if therapy fails to effectively suppress viral replication
- Transmission of drug resistant virus
- Limitation in future choices of antiretroviral agents due to development of resistance
- Drug-related short- or long-term serious toxicity
- Unknown duration of effectiveness of current antiretroviral therapies
|
Immune-Based Therapy
Therapy to augment the immune response to HIV may be possible through the use of HIV vaccines or cytokines, such as interleukin-2. Such strategies to enhance the control of HIV already provided by antiretroviral medications are being assessed in clinical trials, but are not part of current standard care.
Alternative Or Complementary Therapy
Some patients may present with knowledge or questions about alternative or complementary therapy or may indicate that they are already taking such therapy. All patients should be specifically asked about their use of such therapies, as they may not consider these to be medications and may not volunteer the information to their provider. Specific complementary therapies change rapidly, and their use varies widely with geography and patient demographics. For patients who do choose such therapies it is important to make sure that agents that have overlapping toxicities with a patient’s prescribed therapy are avoided and that discussions of alternative therapy are held in a way that does not alienate the patient from her involvement in medical care.
V. Complications: Opportunistic Diseases TOP
The risk for various opportunistic processes — so called because they take advantage of patients with a weakened immune system — is defined by the total CD4 lymphocyte count. They include opportunistic infections (OIs) and certain malignancies, and are similar to the diseases seen in other immunocompromised hosts such as recipients of solid organ transplants. In fact, AIDS was first recognized as a new entity by the characteristic pattern of opportunistic diseases — especially Pneumocystis pneumonia and Kaposi’s sarcoma — that were being diagnosed in young, previously healthy gay men. The pattern and sequence of OIs that are seen as the total CD4 cell count decreases is so reliable that in most cases the total CD4 cell count limits the differential diagnosis (see Table 4-15).
Table 4-15: Correlation of Complications With CD4 Cell Counts
| CD4 Cell Count* |
Infectious Complications |
Noninfectious† Complications |
| >500/mm3 |
- Acute retroviral syndrome
- Candidal vaginitis
|
- Persistent generalized lymphadenopathy (PGL)
- Guillain-Barré syndrome
- Myopathy
- Aseptic meningitis
|
| 200-500/mm3 |
- Pneumococcal and other bacterial pneumonia
- Pulmonary tuberculosis
- Herpes zoster
- Oropharyngeal candidiasis (thrush)
- Cryptosporidiosis, self-limited
- Kaposi’s sarcoma
- Oral hairy leukoplakia
|
- Cervical intraepithelial neoplasia
- Cervical cancer
- B-cell lymphoma
- Anemia
- Mononeuritis multiplex
- Idiopathic thrombocytopenic purpura
- Hodgkin’s lymphoma
- Lymphocytic interstitial pneumonitis
|
| <200/mm3 |
- Pneumocystis carinii pneumonia
- Disseminated histoplasmosis and coccidioidomycosis
- Miliary/extrapulmonary TB
- Progressive multifocal leukoencephalopathy (PML)
|
- Wasting
- Peripheral neuropathy
- HIV-associated dementia
- Cardiomyopathy
- Vacuolar myelopathy
- Progressive polyradiculopathy
- Non-Hodgkin’s lymphoma
|
| <100/mm3 |
- Disseminated herpes simplex
- Toxoplasmosis
- Cryptococcosis
- Cryptosporidiosis, chronic
- Microsporidiosis
- Candidal esophagitis
|
|
| <50/mm3 |
- Disseminated cytomegalovirus (CMV)
- Disseminated Mycobacterium avium complex
|
- Central nervous system (CNS) lymphoma
|
* Most complications occur with increasing frequency at lower CD4 cell counts.
† Some conditions listed as “non-infectious” are probably associated with transmissible microbes. Examples include lymphoma (Epstein-Barr virus [EBV]) and cervical cancer (human papillomavirus [HPV]).
Source: Bartlett, 2003. Reprinted with permission. At total CD4 cell counts above 500, illnesses are rarely specifically associated with the patient’s HIV serostatus. Non-Hodgkin’s lymphoma and mucocutaneous KS are occasional exceptions; they can occur at varying CD4 cell counts, but are more frequently diagnosed at lower values. Infections that are virulent among HIV-seronegative individuals, such as tuberculosis and bacterial pneumonia, may, of course, occur at any CD4 cell count but are increasingly more common and more severe as the CD4 count declines. Between 200 and 500 cells, less serious HIV-associated problems begin to manifest themselves, such as oral hairy leukoplakia, various skin problems, shingles, and oral or vaginal candidiasis (thrush). Candida vaginitis, which is also common among women who do not have HIV, may be the first indication of HIV infection (Imam, 1990).
According to the 1993 version of the CDC case definition, AIDS may be defined by a number of serious opportunistic illnesses or by a decline in the total CD4 cell count below 200 (see Table 1-3 in Chapter I). This CD4 cell count criterion acknowledges an important threshold for OI risk. Pneumocystis carinii pneumonia (PCP), the most common AIDS-defining OI and leading cause of death, is usually diagnosed as patients approach and drift below this critical number of total CD4 cells. Other OIs, such as toxoplasmosis, cryptococcal meningitis, and disseminated histoplasmosis, tend to occur as the CD4 cell count declines from less than 200 to below 100 cells. Typically, end-stage illnesses such as CNS lymphoma, CMV end-organ disease, and disseminated MAC, tend to occur at very low CD4 cell counts, often less than 25 cells.
Antimicrobial therapy works in concert with the individual’s immune system to clear infection. Before the advent of potent combination antiretroviral therapy, HIV-associated opportunistic diseases could not be controlled without ongoing suppressive therapy, because the patients’ immune function was too weak to effect that control. Once an OI was diagnosed and treated acutely (“induction” therapy, borrowing from the language of oncology), treatment would be continued at lower “maintenance” levels or the OI would inevitably recur. “Cure” of OIs was not part of the vocabulary of HIV disease management. With potent combination antiretroviral therapy resulting in dramatic improvement in CD4 cell counts and immune function, both prophylactic and chronic suppressive therapies are being withdrawn successfully in responders. This has opened an entirely new era in the care of people with advanced HIV (see below). A. OPPORTUNISTIC INFECTION PROPHYLAXIS
One of the early significant advances in the management of HIV/AIDS was the demonstration that chemoprophylaxis could prevent PCP and thereby improve survival. Before the development of potent combination antiretroviral therapy an important focus of the clinical research effort was to identify effective prophylactic agents for the other common OIs. The success of this research was in part responsible for the slowing of the death rate from AIDS that was first apparent near the end of 1995, just before the era of potent combination antiretroviral therapy began.
Recommendations for prophylaxis for specific OIs depend on a number of factors: the CD4 threshold that defines the greatest risk, the overall effectiveness of a given approach, the risk of resistance development, the presence of pregnancy, toxicity, and cost. The USPHS/IDSA guidelines for OI prophylaxis are updated periodically to reflect the most current understanding of disease risk and prevention. Current recommendations for initiating primary OI prophylaxis can be found at http://www.aidsinfo.nih.gov, or in 2003 Medical Management of HIV Infection by John Bartlett, listed in the Resources appendix. (USPHS/IDSA, 2002) Table 4-16 summarizes recommendations for primary prophylaxis of the most common OIs.
Table 4-16: Prophylaxis to Prevent First Episode of Opportunistic Disease
in Adults and Adolescents Infected with HIV
| Pathogen |
Preventive Regimens |
| Indication |
First Choice |
Alternatives |
| I. Strongly Recommended as Standard of Care |
| Pneumocystis carinii[1] |
CD4+ count <200/µL or oropharyngeal candidiasis |
Trimethoprim-sulfamethoxazole (TMP-SMX), 1 DS po q.d. (AI) TMP-SMX, 1 SS po q.d. (AI) |
Dapsone, 50 mg po b.i.d. or 100 mg po q.d. (BI); dapsone, 50 mg po q.d. plus pyrimethamine, 50 mg po q.w. plus leucovorin 25 mg po q.w. (BI); dapsone 200 mg po plus pyrimethamine, 75 mg po plus leucovorin, 25 mg po q.w. (BI); aerosolized pentamidine, 300 mg q.month via Respirgard II(TM) nebulizer (BI); atovaquone, 1500 mg po q.d. (BI); TMP-SMX, 1 DS po t.i.w. (BI) |
| Mycobacterium tuberculosis Isoniazid-sensitive[2] |
TST reaction  5 mm 5 mm
or prior positive TST result without treatment
or contact with case of active tuberculosis regardless of TST result (BIII) |
Isoniazid, 300 mg po plus pyridoxine, 50 mg po q.d. x 9 mo (AII) or isoniazid, 900 mg po plus pyridoxine, 100 mg po b.i.w. x 9 mo (BII) |
Rifampin, 600 mgpo q.d. (BIII) x 4 mo or rifabuin 300 mg po q.d. (CIII) x 4 mo Pyrazinamide, 15-20 mg/kg po q.d. x 2 mo plus either rifampin, 600 mg po q.d. (BI) x 2 mo or rifabutin, 300 mg po q.d. (CIII) x 2 mo |
| Isoniazid-resistant |
Same as above; high probability of exposure to isoniazid-resistant tuberculosis |
Rifampin 600 mg po (AIII) or rifabutin, 300 mg po (BIII) q.d. x 4 mo |
Pyrazinamide 15-20 mg/kg po q.d. plus either rifampin, 600 mg po (BI) or rifabutin, 300 mg po (CIII) q.d. x 2 mo |
| Multidrug-(isoniazid and rifampin) resistant |
Same as above; high probability of exposure to multidrug-resistant tuberculosis |
Choice of drugs requires consultation with public health authorities. Depends on susceptibility of isolate from source patient |
---- |
| Toxoplasma gondii[3] |
IgG antibody to Toxoplasma and CD4+ count <100/µL |
TMP-SMX, 1 DS po q.d. (AII) |
TMP-SMX, 1 SS po q.d. (BIII): dapsone, 50 mg po q.d. plus pyrimethamine, 50 mg po q.w. plus leucovorin, 25 mg po q.w. (BI); dapsone, 200 mg po plus pyrimethamineˆ, 75 mg po plus leucovorin, 25 mg po q w (BI); atovaquone, 1500 mg po q.d. with or without pyrimethamine, 25 mg po q.d. plus leucovorin, 10 mg po q.d. (CIII) |
| Mycobacterium avium complex[4] |
CD4+ count <50/µL |
Azithromycin, 1,200 mg po q.w., (AI) q.d. or clarithromycin,4 500 mg po b.i.d. (AI) |
Rifabutin, 300 mg po (BI);azithromycin, 1,200 mg po q.w. plus rifabutin, 300 mg poq.d. (CI) |
| Varicella zoster virus |
Significant exposure to chickenpox or shingles for patients who have no history of either condition or, if available, negative antibody to VZV |
Varicella zoster (VZV) immune globulin (VZIG), 5 vials (1.25 mL each) im, administered  96 h after exposure, ideally within 48 h (AIII) 96 h after exposure, ideally within 48 h (AIII) |
|
| II. Generally Recommended |
| Streptococcus pneumoniae[5] |
CD4+ count > 200/µL |
23 valent poly-saccharide vaccine, 0.5 mL im [BII] |
None |
| Hepatitis B virus[6,7] |
All susceptible (anti-HBc-negative) patients |
Hepatitis B vaccine: 3 doses (BII) |
None |
| Influenza virus[6,8] |
All patients (annually, before influenza season) |
Inactivated trivalent influenza virus vaccine: one annual dose (0.5 mL) im (BIII) |
Oseltamivir, 75 mg po q.d. (influenza A or B) (CIII); rimantadine, 100 mg po b.i.d. (CIII), or amantadine, 100 mg po b.i.d. (CIII) (influenza A only) |
Hepatitis A virus[6,7] |
All susceptible (anti-HAV-negative) patients at increased risk for HAV infection (e.g., illicit drug users, men who have sex with men, hemophiliacs) or with chronic liver disease, including chronic hepatitis B or hepatitis C |
Hepatitis A vaccine: two doses (BIII) |
None |
| III. Evidence for Efficacy but Not Routinely Indicated |
| Bacteria |
Neutropenia |
Granulocyte-colony-stimulating factor (G-CSF), 5-10 µg/kg sc q.d. x 2-4 w or granulocyte-macrophage colony-stimulating factor (GM-CSF), 250 µg/m2 sc iv x 2-4 w (CII) |
None |
| Cryptococcus neoformans |
CD4+ count <50/µL |
Fluconazole, 100-200 mg po q.d (CI) |
Itraconazole capsule, 200 mg po q.d. (CIII) |
| Histoplasma capsulatum[9] |
CD4+ count <100/µL, endemic geographic area |
Itraconazole capsule, 200 mg po q.d.(CI) |
None |
| Cytomegalo-virus(CMV)[10] |
CD4+ count <50/µL (and CMV antibody positivity |
Oral ganciclovir, 1g po t.i.d. (CI) |
None |
NOTES: Information included in these guidelines might not represent Food and Drug Administration (FDA) approval or approved labeling for the particular products or indications in question. Specifically, the terms “safe” and “effective” might not be synonymous with the FDA-defined legal standards for product approval. The Respirgard II nebulizer is manufactured by Marquest, Englewood, Colorado.
Letters and roman numerals in parentheses after regimens indicate the strength of the recommendation and the quality of evidence supporting it. Categories reflecting the quality of evidence forming the basis for recommendations regarding the use of a product or measure for the prevention of opportunistic infection in HIV-infected persons: I — Evidence from at least one properly randomized, controlled trial; II — Evidence from at least one well-designed clinical trial without randomization, from cohort or case-controlled analytic studies (preferably from more than one center), or from multiple time-series studies or dramatic results from uncontrolled experiments; III — Evidence from opinions of respected authorities based on clinical experience, descriptive studies, or reports of expert committees.
ABBREVIATIONS: Anti-HBc = antibody to hepatitis B core antigen; b.i.w.= twice a week; DS = double-strength tablet; HAART = highly active antiretroviral therapy; HAV = hepatitis A virus; HIV = human immunodeficiency virus; im = intramuscular; iv = intravenous; po = by mouth; q.d. = daily; q.m. = monthly; q.w. = weekly; SS= single-strength tablet; t.i.w. = three times a week; TMP-SMX = trimethoprim-sulfamethoxazole; sc = subcutaneous; and TST = tuberculin skin test.
[1] Prophylaxis should also be considered for persons with a CD4+ percentage of <14%, for persons with a history of an AIDS-defining illness, and possibly for those with CD4+ counts >200 but <250 cells/µL. TMP-SMX also reduces the frequency of toxoplasmosis and some bacterial infections. Patients receiving dapsone should be tested for glucose-6 phosphate dehydrogenase deficiency. A dosage of 50 mg q.d. is probably less effective than100 mg q.d. The efficacy of parenteral pentamidine (e.g., 4 mg/kg/month) is uncertain. Fansidar (sulfadoxine-pyrimethamine) is rarely used because of severe hypersensitivity reactions. Patients who are being administered therapy for toxoplasmosis with sulfadiazine-pyrimethamine are protected against Pneumocystis carinii pneumonia and do not need additional prophylaxis against PCP.
[2] Directly observed therapy is recommended for isoniazid, e.g., 900 mg b.i.w.; isoniazid regimens should include pyridoxine to prevent peripheral neuropathy. If rifampin or rifabutin are administered concurrently with protease inhibitors or non-nucleoside reverse transcriptase inhibitors, careful consideration should be given to potential pharmacokinetic interactions (54). See discussion of rifamycin interactions in paragraph 11 in section on Tuberculosis. There have been reports of fatal and severe liver injury associated with the treatment of latent TB infection in HIV-uninfected persons treated with the 2 month regimen of daily rifampin and pyrazinamide; therefore it may be prudent to use regimens that do not contain pyrazinamide in HIV-infected persons whose completion of treatment can be assured (CDC. Update: Fatal and Severe Liver Injuries Associated with Rifampin and Pyrazinamide for Latent Tuberculosis Infection and Revisions in American Thoracic Society/CDC Recommendations, United States 2001 MMWR 50 (No. 34), Aug 31, 2001). Exposure to multidrug-resistant tuberculosis might require prophylaxis with two drugs; consult public health authorities. Possible regimens include pyrazinamide plus either ethambutol or a fluoroquinolone.
[3] Protection against toxoplasmosis is provided by TMP-SMX, dapsone plus pyrimethamine, and possibly by atovaquone. Atovaquone may be used with or without pyrimethamine. Pyrimethamine alone probably provides little, if any, protection.
[4] See paragraph 9, in section on “Disseminated Infection with Mycobacterium avium complex” and references 53-54 for discussion of drug interactions. *During pregnancy, azithromycin is preferred over clarithromycin because of the teratogenicity in animals of clarithromycin.
[5] Vaccination may be offered to persons who have a CD4+ T-lymphocyte count <200 cells/µL, although the efficacy is likely to be diminished. Revaccination 5 years after the first dose or sooner if the initial immunization was given when the CD4+ count was <200 cells/µL and the CD4+ count has increased to >200 cells/µL on HAART is considered optional. Some authorities are concerned that immunizations might stimulate the replication of HIV.
[6] Although data demonstrating clinical benefit of these vaccines in HIV-infected persons are not available, it is logical to assume that those patients who develop antibody responses will derive some protection. Some authorities are concerned that immunizations might stimulate HIV replication, although for influenza vaccination, a large observational study of HIV-infected persons in clinical care showed no adverse effect of this vaccine, including multiple doses, on patient survival (J. Ward, CDC, personal communication). Also, this concern may be less relevant in the setting of HAART. However, because of the theoretical concern that increases in HIV plasma RNA following vaccination during pregnancy might increase the risk of perinatal transmission of HIV, providers may wish to defer vaccination for such patients until after HAART is initiated.
[7] Hepatitis B vaccine has been recommended for all children and adolescents and for all adults with risk factors for hepatitis B virus (HBV). For persons requiring vaccination against both hepatitis A and hepatitis B, a combination vaccine is now available. For additional information regarding vaccination against hepatitis A and B, see CDC. Hepatitis B virus: a comprehensive strategy for eliminating transmission in the United States through universal childhood vaccination. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 1991;40 (No RR13).
[8] Oseltamivir is appropriate during outbreaks of either influenza A or influenza B. Rimantadine or amantadine are appropriate during outbreaks of influenza A (although neither rimantadine nor amantadine is recommended during pregnancy). Dosage reduction for antiviral chemoprophylaxis against influenza might be indicated for decreased renal or hepatic function, and for persons with seizure disorders. Physicians should consult the drug package inserts and the annual CDC influenza guidelines for more specific information about adverse effects and dosage adjustments. For additional information about vaccination, antiviral chemoprophylaxis and therapy against influenza, see: CDC. Prevention and Control of Influenza: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2001;50(No. RR-4).
[9] In a few unusual occupational or other circumstances, prophylaxis should be considered; consult a specialist.
[10] Acyclovir is not protective against CMV. Valacyclovir is not recommended because of an unexplained trend toward increased mortality observed in persons with AIDS who were being administered this drug for prevention of CMV disease.
Source: USPHS/IDSA, 2001. Available at http://www.aidsinfo.nih.gov. B. PRESENTATION AND MANAGEMENT OF THE MOST COMMON COMPLICATIONS OF ADVANCED HIV DISEASE (AIDS)
Summaries are presented below. However, specific agents and dosing regimens for acute conditions and secondary opportunistic infection prophylaxis, respectively, can be found at http://www.aidsinfo.nih.gov, or in the 2003 Medical Management of HIV Infection by John Bartlett.
Pneumocystis Carinii Pneumonia
The diagnosis of PCP can be challenging and requires a heightened index of suspicion. Although there are “classic” symptoms, findings on exam, and chest X-ray manifestations, the presentation of PCP can be subtle and nonspecific. The classic triad of fever, exertional dyspnea, and nonproductive cough occurs in only half of cases, although almost all have at least two of the following: fever, cough, dyspnea, lactate dehydrogenase greater than 460 U/L or an arterial partial pressure of oxygen (PaO2) less than 75 mm Hg. A careful history may reveal longstanding exertional dyspnea that has worsened incrementally over weeks to months. Physical exam findings are also nonspecific. Fine, dry “cellophane” rales may be heard or auscultation may be entirely normal. In 2–6% of patients, PCP may present as spontaneous pneumothorax. The classic X-ray findings are diffuse interstitial or perihilar infiltrates, but a wide range of X-ray abnormalities is possible and radiography is normal in over one third of cases. Extrapulmonary pneumocystosis is uncommon. PCP is suggested by oxygen desaturation with exercise, easily measured in the office or clinic with a pulse oximeter. This is particularly useful when symptoms are minimal, the patient does not appear acutely ill, and the chest X-ray is unimpressive. Severity of illness is indicated by hypoxemia or a widened alveolar to arterial oxygen difference (A-aDO2) on blood gas analysis.
Many diseases may have a similar presentation, including mycobacterial, fungal, viral, or bacterial pneumonias, heart failure, pulmonary KS, and pulmonary embolus. The definitive diagnostic test requires bronchoalveolar lavage of affected lung segments that is then concentrated and stained for P. carinii organisms. Experienced sites can make a histologic diagnosis from an induced sputum sample that is concentrated and stained, but this less invasive, cheaper diagnostic test should not be attempted where expertise in both obtaining and interpreting the smear is lacking.
Trimethoprim-sulfamethoxazole (TMP-SMX) is the mainstay of treatment for PCP; intravenous or oral administration depends on the severity of the episode. There are a number of alternative regimens for patients who experience treatment-limiting toxicity or who fail to respond to TMP-SMX. PCP should be treated for 21 days. After completing acute therapy, the patient should begin routine daily PCP prophylaxis to prevent recurrence. Patients with PaO2 less than 70 mm Hg or with an A-aDO2 greater than 35 on room air should receive adjunctive steroids, which have been shown to decrease the incidence of ventilatory failure and death. A 21-day course of prednisone (40 mg twice daily for 5 days, then 20 mg twice daily for 5 days, followed by 20 mg once daily for 11 days) is the most popular and cost-effective approach. No additional taper is required.
Candidiasis
The appearance of mucosal candidiasis is often the first clinical indication of impaired T-cell immunity in HIV-infected individuals. Whereas oral and vaginal thrush are almost ubiquitous and Candida esophagitis is the second most common OI after PCP, candidemia and tissue-invasive disease are rare. Pharyngitis may be asymptomatic or may cause dysphagia. White plaques can be easily scraped from the pharynx or buccal mucosa; severe cases will involve the tongue, gums, and lips. Vaginitis causes a thick white discharge, pruritus, and sometimes dyspareunia, and has a similar appearance on speculum exam. Intense erythema may be the most prominent finding in some patients with either pharyngitis or vaginitis. Scrapings will be KOH-positive by microscopic exam and will grow readily in culture. These forms of candidiasis may be treated with topical or oral antifungals; topical agents are more cost-effective and avoid the risk of systemic side effects or drug interactions.
Candida esophagitis is a more serious infection that may result in significant weight loss because of odynophagia. Esophagitis should be considered when the patient describes midline substernal chest discomfort with swallowing instead of pain limited to the throat. It may occur in the absence of oropharyngeal thrush, and can be diagnosed by endoscopy or by barium swallow. Topical agents should not be used for esophagitis. Oral fluconazole, 200 mg once daily for 10 days, is the treatment of choice.
Prolonged usage of oral azoles such as fluconazole can result in resistant candidiasis, so it is important to avoid chronic use. Most experts try to use topical antifungals or intermittent courses of azole drugs whenever possible. Prophylaxis for vaginal candidiasis with topical antifungals should be considered when systemic antibiotics are given. Some patients with fluconazole-resistant esophagitis may respond to itraconazole, especially the cyclodextrin solution, oral voriconazole, or to oral amphotericin B solution. However, most patients with resistant infection will ultimately require intravenous (IV) amphotericin for relief. Cryptococcal Meningitis
Cryptococcal meningitis may present as nothing more than the worst headache of the patient’s life. Fever is common but meningismus may be minimal or absent. Altered mental status and elevated intracranial pressure above 180 mm of water portend a poorer prognosis. Therefore, it is important for patient management to obtain an opening pressure when performing the diagnostic lumbar puncture. Cranial nerve deficits and seizures are only seen in patients who present very late in the course of their infection and are often antemortem events. The diagnosis is made by detection of cryptococcal capsular antigen in the cerebrospinal fluid (CSF); relying upon a positive India ink stain that demonstrates the organism’s thick capsule is too insensitive. Cryptococcus neoformans may also be cultured from blood and CSF. Computed tomography (CT) or magnetic resonance imaging (MRI) scans may reveal basilar inflammation, and in patients with intracranial hypertension, the ventricles may be enlarged. Very mild cases may be treated from the outset with oral fluconazole, 400 mg once daily for 10 wk, followed by chronic suppressive therapy (200 mg once daily). Most experts prefer using intravenous amphotericin B at a dose of 0.7–1.0 mg/kg per day for the first 2 wk, with or without flucytosine, and then switching to fluconazole as described above if the patient is responding. Intracranial hypertension can be managed with frequent lumbar punctures to remove large volumes of CSF (20–30 mL at a time). Serum cryptococcal antigen may occasionally be positive before the onset of headache. It may also be detectable when extrameningeal infection occurs, and in the evaluation of a fever of unknown origin. In these situations, oral fluconazole is appropriate therapy.
Toxoplasmosis
Toxoplasmosis manifests almost exclusively as an encephalitis in AIDS patients. The patient presents with a neurologic deficit, and classically one or more ring-enhancing space-occupying lesions can be seen on CT or MRI scan. However, the radiographic appearance of the lesions is not pathognomonic and may mimic other processes such as primary CNS lymphoma. Because serology may be negative and because it is often difficult to obtain a brain biopsy for a definitive diagnosis, the standard approach is a diagnostic trial of antitoxoplasma therapy with pyrimethamine and sulfadiazine for at least 2 wk. Both clinical and radiographic improvement should be evident in response to therapy if the patient has toxoplasmic encephalitis (TE). Clindamycin may be substituted for sulfidiazine if the latter is poorly tolerated. Although TE in AIDS patients results from reactivation of latent infection, a baseline negative IgG test for Toxoplasma gondii does not exclude the diagnosis, and seronegative patients will routinely receive a trial of therapy regardless of their serostatus. For this reason, and because PCP prophylaxis with TMP-SMX will also prevent TE, obtaining a Toxoplasma gondii IgG may not be very cost-effective. A situation where knowledge of Toxoplasma gondii serostatus is helpful is when a patient cannot tolerate TMP-SMX prophylaxis; in this case pyrimethamine should be added to second-line PCP prophylaxis with dapsone to provide protection from TE as well.
Herpes Simplex Virus
HIV-infected individuals may have recurrent genital HSV that can be suppressed with oral antiviral drugs such as acyclovir, valacyclovir, and famciclovir. Both treatment and prophylaxis of HSV may require higher doses and, in the case of treatment, longer administration than is required in the management of HIV-negative patients; this is particularly the case in women with more advanced immunosuppression (see Chapter VI on Gynecologic Problems). Definitive diagnosis is usually made by culturing HSV from the base of the lesion(s), although experienced clinicians will often rely on typical appearance, distribution, and symptoms. When patients develop severe mucocutaneous lesions or ulcers that persist for more than 4 wk, this unusually persistent form of HSV is considered an AIDS-defining illness. Similar to fluconazole-resistant candidiasis, injudicious chronic use of antiherpes drugs may result in drug-resistant infection, which then requires treatment with intravenous foscarnet. Varicella-zoster virus, a related member of the herpesvirus family, causes shingles, which responds to higher doses of antiherpes drugs than those needed for HSV. Shingles can be exquisitely painful and patients may have prolonged postherpetic neuralgia. Secondary bacterial infection may occur, so it is important to keep the lesions clean and to use topical or systemic antibiotics as needed. Control of pruritus and pain is essential for patient comfort. Drug-resistant varicella-zoster virus has been reported and is also treated with IV foscarnet.
Cytomegalovirus
Cytomegalovirus (CMV) causes retinitis in 80–85% of AIDS patients with end-organ CMV disease. Gastrointestinal disease anywhere from the mouth to the anus is diagnosed in another 12–15%. Other diagnoses, such as encephalitis and pneumonitis, are uncommon (~1%). CMV retinitis can cause visual loss, and untreated, progresses inexorably to blindness. Because retinitis is a necrotizing process, with effective antiviral treatment the lesions become quiescent and atrophic, but the affected areas do not regain function. Retinitis near critical structures such as the macula or optic nerve may cause catastrophic visual loss even when the total infected area is small. Patients may be completely asymptomatic, or may complain of floaters (due to inflammatory debris), diminished acuity, or visual field defects when the lesion(s) is(are) in the periphery. Diagnosis is made by visual inspection of the entire retina by an experienced ophthalmologist using dilated indirect ophthalmoscopy. Extensive disease may lead to retinal detachment, which may require surgical repair. Treatment is usually begun with oral valganciclovir or with intravenous ganciclovir, foscarnet, or cidofovir for 2–3 wk, followed by chronic suppression with either less frequent IV doses or oral valganciclovir or ganciclovir. Chronic use of these intravenous agents requires the placement of an indwelling catheter for ease of administration, or IV therapy can be used briefly until an intraocular device can be inserted surgically that slowly releases small amounts of ganciclovir directly into the vitreous. Because CMV is a systemic infection with viremia, patients who receive the ganciclovir implant also need chronic suppressive therapy with oral valganciclovir to prevent the development of extraocular CMV disease. CMV can become resistant to antivirals. Refractory disease is often treated with intraocular injections, which, like the ganciclovir implant, deliver high concentrations of drug to the site of active viral replication. End-organ disease at nonocular sites is treated with 2–3 wk of intravenous induction therapy; oral valganciclovir has not been studied for monocular disease but should be effective. There is no clear agreement that CMV disease at sites outside the eye requires chronic maintenance therapy, but with the availability of oral valganciclovir it seems reasonable to provide continued anti-CMV treatment in patients whose immune systems remain badly damaged (CD4 cells <100/mm3).
Disseminated Mycobacterium Avium Comples (MAC)
Like CMV, disseminated MAC is one of the OIs that appears at end-stage disease, when the total CD4 cell count is extremely low. It presents nonspecifically with fever, weight loss, diarrhea, anemia, and sometimes abdominal discomfort due to organomegaly and impressive intra-abdominal lymphadenopathy. Mycobacterial blood culture provides a definitive diagnosis; culture of sputum or other specimens is not helpful. Combination oral antimicrobial therapy is required and should include, at a minimum, an azalide (azithromycin or clarithromycin) and ethambutol, 15–25 mg/kg per day. Other drugs, such as ciprofloxacin and amikacin, have been used but do not routinely provide much additional benefit; clofazimine has been shown to have an adverse effect on survival and should not be used.
Tuberculosis
There is a bidirectional interaction between Mycobacterium tuberculosis and HIV; each facilitates acquisition of the other, so it is critical to assess all HIV-infected patients for active tuberculosis (TB), and to test all patients with active TB for HIV. Because TB is virulent enough to cause disease in patients with intact immune systems, it may occur in HIV-infected individuals who still have high CD4 cell counts. TB is especially virulent in HIV seropositive individuals. Aspects of this virulence include the high frequency of positive blood cultures and of disseminated (miliary) infection. However, standard combination antimicrobial therapy is effective as long as the patient is adherent and the acquired strain is not multidrug resistant. It is essential to provide directly observed therapy to ensure an adequate course of treatment and conversion of positive sputum cultures to negative. Until susceptibilities are known, all HIV-infected patients should be treated initially with at least four drugs expected to be active according to local susceptibility patterns; for susceptible TB, typically INH, rifampin or rifabutin, pyrazinamide and ethambutol or streptomycin are given for 2 months, followed by INH and rifampin or rifabutin for 4 months. Subsequently, when the results of susceptibility testing are available, therapy for drug-sensitive infection can usually be narrowed to two agents. Patients with advanced HIV disease or a delayed response to TB treatment (symptomatic and/or culture-positive after 2 months of treatment) should receive a total course of 9 months. Clinicians should work closely with their local health department to ensure that patients receive directly observed therapy, and to track and limit the spread of TB, especially resistant strains. All close contacts — especially young children — must be evaluated for TB so they may be treated promptly for active disease or given prophylaxis as indicated.
Cryptosporidiosis And Microsporidiosis
These enteric protozoa can cause debilitating diarrhea and weight loss in patients with advanced HIV disease. Diagnosis is made by special stool stains. Unfortunately there is no effective therapy (except for Septata intestinalis, which may respond to albendazole), so care is supportive. Every effort should be made to optimize the patient’s antiretroviral therapy because there are reported cases of clinical resolution (and even clearing of the organism from stool) with potent combination antiretroviral therapy. Patients may develop severe dehydration due to voluminous watery diarrhea. In addition to volume repletion, attempts at slowing the diarrhea should be made as follows by adding (not substituting) each additional agent in a stepwise manner: 1) diphenoxylate or loperamide, increased to their maximum dose, plus 2) tincture of opium or paregoric, with the dose titrated gradually until the desired effect is achieved, and, if additional control is needed, 3) parenteral somatostatin, which is very expensive.
Peripheral Neuropathy
Distal, symmetrical polyneuropathy, typically affecting the feet more than the hands, may result from use of the neurotoxic dideoxy nucleoside analogues (didanosine, stavudine, zalcitabine) and much less commonly from dapsone, or may be a consequence of advanced HIV disease itself. Most patients present with paresthesias and/or numbness, but some experience pain that can be disabling. Examination reveals slow or absent ankle jerks, diminished vibratory and proprioceptive responses in both feet, and in patients whose primary complaint is pain, discomfort sometimes even with light touch. If drug toxicity is suspected, the offending agent(s) should be discontinued immediately and replaced. If this is accomplished quickly enough after the onset of symptoms, the syndrome may resolve entirely. When the nerve damage is not attributable to anti-HIV therapy or does not resolve after drug discontinuation, supportive care may be offered. Nonsteroidal antiinflammatory drugs; agents useful in chronic pain syndromes such as amitryptiline, phenytoin, or carbamazepine; the neurotransmitter inhibitor gabapentin; mexilitene, lamotrigine; and, in refractory cases, long-acting narcotics, all have a role in the management of dysesthesias and pain due to peripheral neuropathy.
AIDS Dementia Complex (ADC)/HIV Encephalopathy
In the pre-HAART era, frank dementia was the AIDS-defining illness in up to 10% of patients. The initial manifestations may be subtle, and can be uncovered by questioning the patient carefully about short-term memory loss and difficulty concentrating. Useful questions about the latter include the ability to balance a checkbook or to make change. In some patients, a depressed affect may be a prominent finding, and in others, unexplained seizures may bring the patient to medical attention. Psychomotor retardation — slowing of the impulses that match actions to thoughts and intentions — is another hallmark of AIDS dementia complex (ADC). CT and MRI scans show diffuse cortical loss with prominent sulci (“walnut sign”). A good sense of the patient’s level of intellectual functioning can often be obtained at the bedside. In subtle or difficult cases, especially when there is a prior history of depression or subnormal IQ, the patient can be referred for a battery of neuropsychologic tests that may clearly demonstrate the losses characteristic of ADC. There is no specific treatment for ADC other than effective antiretroviral therapy, although symptoms may respond in part to methylphenidate or selegiline. Patients may demonstrate a remarkable degree of recovery with antiretroviral therapy even when they present with advanced dementia, so it is valuable to attempt treatment of all patients, even those initially referred for nursing home care. It may be particularly useful to include agents that achieve good CSF levels.
Wasting Syndrome (“Slim Disease”)
Weight loss is common in HIV disease, especially in its advanced stages, but the CDC surveillance definition of wasting syndrome specifically refers to involuntary weight loss that exceeds 10% of the patient’s baseline weight in the presence of diarrhea ( 2 loose stools per day) or chronic weakness and documented fever (intermittent or constant) for at least 30 days that is not attributable to a condition other than HIV itself. Typically wasting syndrome is accompanied by loss of muscle mass, for example in the temporal areas, and complaints of generalized fatigue and modest weakness. In severe cases the serum albumin level will be very low. Wasting can accompany any of the typical end-stage illnesses, such as disseminated MAC, or may occur by itself in the absence of any evident concomitant illness. Loss of weight, and, especially, of lean body mass, portends poorer survival. Appetite stimulants, such as the progestin megestrol acetate or the marijuana derivative dronabinol, may be used although weight gain with these agents typically consists of fat and water, rather than an increase in lean body mass. However, the psychologic benefit of an improved appetite and some weight gain cannot be underestimated, even if the gain is primarily fat. Recombinant human growth hormone has been used with some short-term success for improvement in lean body mass, but it is very expensive and must be given parenterally. Other approaches include enteral and parenteral feedings, anabolic steroids such as nandrolone or oxandrolone, and thalidomide or pentoxifylline for cytokine suppression. Men with symptoms of hypogonadism often respond to testosterone replacement, but this approach has not been evaluated in women. 2 loose stools per day) or chronic weakness and documented fever (intermittent or constant) for at least 30 days that is not attributable to a condition other than HIV itself. Typically wasting syndrome is accompanied by loss of muscle mass, for example in the temporal areas, and complaints of generalized fatigue and modest weakness. In severe cases the serum albumin level will be very low. Wasting can accompany any of the typical end-stage illnesses, such as disseminated MAC, or may occur by itself in the absence of any evident concomitant illness. Loss of weight, and, especially, of lean body mass, portends poorer survival. Appetite stimulants, such as the progestin megestrol acetate or the marijuana derivative dronabinol, may be used although weight gain with these agents typically consists of fat and water, rather than an increase in lean body mass. However, the psychologic benefit of an improved appetite and some weight gain cannot be underestimated, even if the gain is primarily fat. Recombinant human growth hormone has been used with some short-term success for improvement in lean body mass, but it is very expensive and must be given parenterally. Other approaches include enteral and parenteral feedings, anabolic steroids such as nandrolone or oxandrolone, and thalidomide or pentoxifylline for cytokine suppression. Men with symptoms of hypogonadism often respond to testosterone replacement, but this approach has not been evaluated in women.
Kaposi's Sarcoma
Kaposi’s sarcoma (KS) is an endothelial cell tumor that, along with PCP, was the harbinger of the AIDS epidemic. It primarily affects gay and bisexual men, and is fairly uncommon among injecting drug users and women. It is caused by human herpesvirus-8. KS can occur at a range of total CD4 cell counts, but prognosis is poorer at lower values. Most commonly it is limited to mucocutaneous surfaces, where it is a cosmetic problem but not a significant threat to health. KS of the gastrointestinal mucosa is very vascular and may lead to slow, chronic blood loss. KS may also involve the lymphatic system, and may invade the viscera, especially lung parenchyma. Experienced clinicians can generally diagnose mucocutaneous KS by inspection, but a punch biopsy showing typical spindle-shaped cells is easy to obtain and is definitive. Visceral KS, which may occur in the absence of mucocutaneous disease, requires a tissue diagnosis.
Mucocutaneous KS may be treated with a number of local modalities including intralesional vincristine or vinblastine, radiation, and topical retinoids. Gastrointestinal lesions can be cauterized endoscopically. Visceral disease requires systemic chemotherapy. More recently, KS has regressed in patients begun on potent combination antiretroviral therapy alone.
Systemic Lymphoma
Several different types of lymphoma occur at increased frequency among HIV-infected individuals. These too may occur at any CD4 cell count although once again prognosis is worse at lower absolute numbers of CD4 cells. HIV seropositive patients may develop Hodgkin’s disease, immunoblastic lymphoma, and Burkitt’s lymphoma as well as less common forms, but the most common type is an aggressive non-Hodgkin’s B cell lymphoma. There is a marked tendency for extranodal presentations (Stage 1E), and AIDS patients have been described with non-Hodgkin’s lymphoma at a range of unusual sites. AIDS-associated lymphoma is diagnosed and staged in the same manner as in seronegative patients, and the same types of combination chemotherapy are used. However, HIV-infected patients typically require lower doses or aggressive support with granulocyte colony-stimulating factor because of their baseline bone marrow fragility.
Central Nervous System Lymphoma
Central nervous system lymphoma occurs at total CD4 cell counts well under 100 cells and is a typical end-stage complication. Definitive diagnosis is made by brain biopsy or CSF cytology in the presence of a space-occupying lesion(s) on CT or MRI scan. A presumptive diagnosis may sometimes be made by nuclear SPECT scan. Because brain biopsy may be difficult to obtain, patients who fail a trial of therapy for toxoplasmosis are often assumed to have CNS lymphoma. There is no effective cytotoxic chemotherapy for this disease, and irradiation is considered palliative. Survival after a diagnosis of CNS lymphoma is usually limited, on the order of a few months.
Progressive Multifocal Leukoencephalopathy (PML)
Progressive multifocal leukoencephalopathy is another end-stage complication of HIV disease, usually presenting as a focal neurologic deficit(s). It is caused by the JC virus, which can be detected by PCR performed on CSF. MRI scan of the brain demonstrates involvement of the white matter that can be focal or fairly diffuse, but is not usually associated with either mass effect or surrounding edema. Most commonly it affects areas adjacent to the cortex, but lesions can be located anywhere. Definitive diagnosis is made by brain biopsy or positive PCR, which is highly specific in the appropriate clinical context. Where these diagnostic modalities are unavailable, the typical MRI picture usually suffices. There is no specific proven therapy for this condition, although a number of case reports describe clinical remission in patients begun on potent combination antiretroviral therapy. In the pre-HAART era, survival was very limited, but now there are patients alive more than a few years after diagnosis.
Figure 4-5: Guidelines for Use of Erythropoietin in the Anemic HIV Patient
Goals of Therapy
- Resolution of Anemia: Hgb
 12g/dL or Hct 12g/dL or Hct  36% 36%
- Increased energy, activity, and overall quality of life for patients, prolonged survival
- Reduced need for transfusion
![Figure 4-5: Guidelines for Use of Erythopoietin in Anemic HIV Patient. Flow diagram which begins at the top with a block labeled: Patient Candidate; Anemic HIV patient, Hgb<11g/dL or Hct <33%. Next block down is “Exclude Other Causes of Anemia,” which includes: Bleeding (guaiac stools), Hemolysis (smear), Iron deficiency (serum iron, transferrin, % saturation, ferritin), and B12, folate deficiency (serum B12, rbc folate if macrocytic). If the answer to any of these conditions is “Yes,” flow points to “Correct underlying cause” and the process stops. If the answer is “No,” flow leads to next block which is “Start EPO 40,000 units sc/wk. Consider iron supplementation.” Next step is “Monitor Response”: Full response will generally not be seen for at least 4 wk. Three options for next step (left to right): “At wk 4, if Hgb increases >1g/dL, continue at this dose.” Second option (which appears at bottom of page because there is a second path to this option): “When Hgb approaches 13/gdL, decrease EPO by 10,000 units/wk*. Titrate to maintain desired hemoglobin.[ † ]” Third option: “At 4 wk, if Hgb increases <1g/dL, increase dose to 60,000 units/wk.” From this third option, the flow branches two ways. The first leads to an end point box: “At 8 wk. if Hgb increases <1g/dL, check iron, folate and B12 levels. If adequate, discontinue EPO.” The second branch leads down to “At 8 wk, if Hgb increases >1g/dL, continue at this dose.” From this box, the flow leads back to the second option mentioned earlier, that is “When Hgb approaches 13g/dL, decrease EPO by 10,000 units/wk*. Titrate to maintain desired hemoglobin.[ † ]”](images/WG05fig4_5.gif)
* If Hgb  15g/dL at any point, hold erythropoietin (EPO) and restart when Hgb < 12 g/dL, using dose reduced by 10,000/wk. 15g/dL at any point, hold erythropoietin (EPO) and restart when Hgb < 12 g/dL, using dose reduced by 10,000/wk.
† During dose adjustment phase, hemoglobin should be monitored every 2-4 wk. Allow at least 4 wk to assess response to dose changes.
Source: John Bartlett, 2003. Reprinted with permission. Chronic Hepatitis B And C
Many of the same behaviors that put women at risk of acquiring HIV also result in hepatitis B and/or C infection. Up to 90% of HIV-infected individuals have evidence of prior exposure to hepatitis B (HBV) and up to 10% have chronic HBV infection (Homann, 1991; Bodsworth, 1991). Risk of developing chronic infection is increased in the presence of HIV co-infection (Bodsworth, 1991; Houssett,1992); limited data also suggest that HIV-HBV coinfection is associated with higher HBV DNA levels and worse prognosis in terms of liver-related morbiditiy and mortality (Thio, 2001; Colin,1999).Persistence of HbsAg for longer than 6 months is indicative of chronic infection; these patients should be tested for HbeAg and anti-Hbe and have periodic assessment (e.g., every 6 months) of transaminases, albumin, prothrombin time, bilirubin, complete blood count, and platelet count. Patients with chronic HBV infection are at increased risk of cirrhosis and end-stage liver disease and hepatocellular carcinoma. Periodic screening every 6–12 months with alfa fetoprotein (AFP) and/or liver ultrasound should be considered in patients with chronic HBV and other high risk characteristics (age >45 years, cirrhosis, family history of liver cancer). Indications for HBV treatment include: HbsAg positive > 6 months, evidence of active viral replication (HbeAg + HBV DNA positive), and moderate or severe liver inflammation on biopsy. The goal of treatment is to prevent or delay the progression of liver disease; eradication of the virus is not possible with current therapies. Agents used to treat HBV include interferon, lamivudine (3TC), tenofovir, adefovir, and emtricitabine; 3TC, tenofovir, and emtricitabine should only be used as part of or in conjunction with a fully suppressive antiretroviral regimen.
A recent cross-sectional analysis of a large heterogeneous group of HIV-infected individuals found that 16% had HCV coinfection (Sherman, 2002). Hepatitis C (HCV) becomes a chronic infection in about 85% of cases. Data from a recent meta-analysis indicate that HIV-HCV coinfected patients have a 2.9 times higher risk of progressive liver disease than those infected with HCV alone (Graham, 2001). There is some evidence that HCV may accelerate progression of HIV disease as well (Piroth, 2000). Screening for HCV is recommended using a 2nd or 3rd generation HCV enzyme-linked immunosorbent assay with confirmation using HCV RNA testing. Hepatitis C viral loads do not correlate with severity of liver damage on biopsy. Serum transaminases should be monitored, especially ALT, which is more specific for hepatocellular injury. ALT levels may wax and wane, and may be normal or only modestly elevated. Screening for hepatocellular carcinoma with AFP and/or liver ultrasound should be considered, particularly in patients with cirrhosis. Indications for HCV treatment include: detectable HCV RNA, persistently elevated ALT levels, and liver biopsy with portal or bridging fibrosis and at least moderate inflammation and necrosis. The goals of treatment include eradication of HCV infection and prevention or delay of progressive liver disease. Recommended treatment for chronic HCV is pegylated interferon plus ribavirin; preliminary data suggest that HCV virologic response correlates with CD4 cell count in HIV-HCV coinfected patients (Soriano, 1996).
Patients with either HBV or HCV infection should avoid alcohol consumption, which may increase risk of progressive liver disease. Transplantation of coinfected patients with end–stage liver disease has been successful and is being evaluated further.
Anemia
Modest anemia ( 9–10 g/dL) is a hallmark of chronic HIV infection and may be complicated by menstrual losses in women of childbearing age. Severe anemia ( 9–10 g/dL) is a hallmark of chronic HIV infection and may be complicated by menstrual losses in women of childbearing age. Severe anemia ( 9 g/dL) may occur as part of certain opportunistic diseases, especially MAC, disseminated histoplasmosis, and lymphoma, and may also be the result of drug toxicity. Although severe anemia has been shown to be associated with a poorer prognosis for survival in a number of studies, diagnosis and treatment of the opportunistic process is often sufficient to improve anemia in these cases. 9 g/dL) may occur as part of certain opportunistic diseases, especially MAC, disseminated histoplasmosis, and lymphoma, and may also be the result of drug toxicity. Although severe anemia has been shown to be associated with a poorer prognosis for survival in a number of studies, diagnosis and treatment of the opportunistic process is often sufficient to improve anemia in these cases.
Patients who are symptomatic with exertional dyspnea and dizziness can be transfused acutely. Most HIV-infected patients become anemic gradually, and unconsciously limit their activities to control symptoms. These individuals can be managed with changes of antiretroviral or OI therapies known to be toxic to red blood cells, such as AZT (zidovudine) and TMP-SMX. In patients refractory to conservative management, red blood cell production can be stimulated by using recombinant erythropoietin along with sufficient iron replacement to stimulate production of new red cells (see Figure 4-5).
C. OPPORTUNISTIC DISEASE IN THE HAART ERA
The impact of highly active antiretroviral therapy on the natural history of opportunistic diseases has been profound, and the clinician must be familiar with at least the broad outline of these changes. There may be sufficient immune restoration that even patients with end-stage disease become capable of mounting an inflammatory response to opportunistic pathogens. This can result in worsening of the clinical manifestations of an OI that has been under treatment or atypical presentation of a new acute OI, generally within the first couple of months after initiating potent anitretroviral therapy, after CD4 cell counts have begun to improve. For example, in the case of MAC lymphadenitis, there may be the acute focal development of a tender, enlarged lymph node with negative blood cultures, whereas in the pre-HAART era the typical presentation would have been diffuse, with widespread nontender adenopathy and high-grade mycobacteremia. This seemingly paradoxical development of an OI with rising CD4 cell counts is likely due to an inflammatory response to an OI that was subclinical when HAART was begun. Similarly, immune reconstitution syndromes have been described for TB, PCP, toxoplasmosis, cryptococcal infection, and PML, when these infections have been under treatment. Management includes continuation of antiretroviral therapy while making sure there has been no recrudescence of the underlying OI, plus addition of NSAIDs or corticosteroids to alleviate the inflammatory reaction.
Patients who recover pathogen-specific immunity in addition to the overall increase in CD4 cells may be able to discontinue chronic suppressive (maintenance) therapy, because the patient’s immune system is now capable of containing the infection. Thus far this has been best demonstrated for discontinuing chronic suppression for CMV retinitis. Similar phenomena have been described for other OIs, such as disseminated MAC, and there is no reason to think that other OIs will behave differently. Last, patients with previously untreatable opportunistic processes, such as PML or cryptosporidiosis, have had clinical remissions after initiating HAART.
A number of studies have shown that patients receiving primary prophylaxis for PCP and MAC are at very low risk of developing these OIs if prophylaxis is withdrawn after total CD4 cell counts have improved above the threshold levels for risk of each specific OI for at least 3–6 mo. Most of these studies have been performed among patients with reasonably well controlled HIV viral loads, with the majority undetectable or at most, less than 10,000 copies. At this point, it is clear that specific prophylaxis can be safely stopped for any OI when CD4 cell counts have increased above the threshold of risk  3 months. The 2002 revision of the USPHS guidelines on OI prophylaxis describes the data and rationale for discontinuing suppressive therapy and prophylaxis in the appropriate patient. These guidelines are revised periodically just as the ones for treatment and HIV are, so it is wise to check www.aidsinfo.nih.gov. 3 months. The 2002 revision of the USPHS guidelines on OI prophylaxis describes the data and rationale for discontinuing suppressive therapy and prophylaxis in the appropriate patient. These guidelines are revised periodically just as the ones for treatment and HIV are, so it is wise to check www.aidsinfo.nih.gov.
VI. Algorithms for Diagnosis and Management of Symptoms
TOP
Figure 4-6: Fever of Unknown Origin in Patients with AIDS
Figure 4-7: Acute Diarrhea in Patients with AIDS
Figure 4-8: Chronic Diarrhea (CD4 Count <300/mm3)
Figure 4-9: Cough, Fever, Dyspnea
Figure 4-10: Headache in Patients with AIDS
Figure 4-11: Advanced HIV Infection Plus Altered Status, New Seizures, Headache (Severe or Persistent), or Focal Neurologic Deficits
Source: Adapted from Bartlett, 2001. Reprinted with permission.
Figure 4-12: Sensory Neuropathies in Patients with AIDS
Source: Bartlett, 2003. Reprinted with permission.
Figure 4-13: Odynophagia in Patients with AIDS
Evaluation
- Medication or food related
- Gastroesophageal reflux disease: (heartburn ± regurgitation and dysphagia)
- Opportunistic infection or tumor
Common: Candida sp.
Less Common: HSV, CMV, idiopathic (aphthous)
Rare: TB, M. avium, histoplasmosis, PCP, cryptosporidia, Kaposi's sarcoma, lymphoma

Source: Bartlett, 2003. Reprinted with permission.
VII. References TOP
Adult Treatment Panel. Executive summary of the 3rd report of the National Cholesterol Education Program (NCEP) Expert panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA.
2001;285:2486–2497.
Bartlett JG and Gallant JE. 2001-2002 Medical Management of HIV Infection. Baltimore, MD: Johns Hopkins University; 2001.
Bartlett JG and Gallant JE. 2003 Medical Management of HIV Infection. Baltimore,
MD: Johns Hopkins University; 2003.
Baxter J, Mayers D, Wentworth D, et al. A pilot study of the short-term effects of antiretroviral management based on plasma genotypic antiretroviral resistance testing in patients failing antiretroviral treatment.
6th Conference on Retroviruses and Opportunistic Infections; January 30–February 4, 1999; Chicago, IL. Abstract LB8.
Bersoff-Matcha SJ, Miller WC, Aberg JA et al. Sex difference in nevirapine rash. Clin Infect Dis. 2001;32:124–129. Bodsworth NJ, Cooper DA, Donovan B. The influence of human immunodeficency
virus type 1 infection on the development of the hepatitis B carrier state. J Infect Dis.
1991;163:1138–1140.
Boehringer-Ingelheim. Viramune package insert. January, 2004.
Bourezane Y, Salard D, Hoen B et al. DRESS (drug rash with eosinophilia and systemic symptoms) syndrome associated with nevirapine therapy. Clin Infect Dis.
1998;27:1321–1322.
CDC. Advancing HIV prevention: new strategies for a changing epidemic– United States, 2003. MMWR. 2003;52:329–332.
CDC. Hepatitis B virus: a comprehensive strategy for eliminating transmission
in the United States through universal childhood vaccination. Recommendations
of the Advisory Committee on Immunization Practices (ACIP). MMWR. 40(RR-13), 1991.
CDC. Prevention of hepatitis A through active or passive immunization. Recommendations
of the Advisory Committee on Immunization Practices (ACIP). MMWR. 45(RR-15), 1996.
CDC. Prevention and control of influenza. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR. 48(RR-4), 1999.
Carr A, Samaras K, Burton S, et al. Syndrome of peripheral lipodystrophy, hyperlipidemia and insulin resistance in patients receiving HIV protease
inhibitors. AIDS. 1998; 12:F51–58.
Colin JF, Cazals-Hatem D, Loriot MA, Martinot-Peignoux M, Pham BN, Auperin A et al. Influence of human immunodeficiency virus infection
on chronic hepatitis B in homosexual men. Hepatology. 1999;29:1306–1310.
D’Acquila, RT, Schapiro JM, Brun-Vezinet F, et al. Drug resistance mutations in HIV-1. Topics in HIV Medicine 2002;10:21-25.
Deeks SG, Wrin T, Liegler T et al. Virologic and immunologic consequence of discontinuing combination antiretroviral-drug therapy in HIV-infected patients with detectable viremia. N Engl J Med. 2001;344:472–480.
DHHS (US Department of Health and Human Services). Guidelines for the Use of Antiretroviral Agents in HIV-Infected Adults and Adolescents. Rockville,
MD: Department of Health and Human Services. 2004. Available at: http://www.aidsinfo.nih.gov. Accessed March 21, 2004.
Dube MP, Sprecher D, Henry WK. Preliminary guidelines for the evaluation and management of dyslipidemia in adults infected with human immunodeficiency
virus and receiving antiretroviral therapy: recommendations
of the Adult AIDS Clinical Trials Group Cardiovascular Disease Focus Group. Clin Infect Dis. 2000;31:1216–1224.
Durant J, Clevenbergh P, Halfon P, et al. Drug-resistance genotyping in HIV-1 therapy: the VIRADAPT randomized controlled trial. Lancet. 1999;353: 2195–2199.
Fauci A, Pantaleo G, Stanley S, Weismann D. Immunopathogenic mechanisms
of HIV infection. Ann Intern Med. 1996;124:654–663.
Graham CS, Baden LR, Yu E et al. Influence of human immunodeficiency virus infection on the course of hepatitis C virus infection: a Meta-analysis.
Clin Infect Dis.
2001;33:562–569.
Ho D, Neumann A, Perelson A, et al. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature. 1995;373:123–126.
Homann C, Krogsgaard K, Pedersen C, Andersson P, Nielsen JO. High incidence
of hepatitis B infection and evolution of chronic hepatitis B infection
in patients with advanced HIV infection. J Acquir Immune Defic Syndr. 1991;4:416–420.
Houssett C, Pol S, Carnot F et al. Interactions between human immunodeficiency
virus-1, hepatitis delta virus and hepatitis B virus infections in 260 chronic carriers of hepatitis B virus. Hepatology. 1992;15:578–583.
Imam N, Carpenter CC, Mayer KH, Fisher A, Stein M, Danforth SB. Hierarchical
pattern of mucosal candida infections in HIV-seropositive women. Am J Med.
1990;89:142–146.
Joffe BI, Panz VR, Raal FJ. From lipodystrophy syndromes to diabetes mellitus.
Lancet. 2001;35:1379–1381.
Kitahata MM, Koepsell TD, Deyo RA, Maxwell CL, Dodge WT, Wagner EH. Physicians’ experience with the acquired immunodeficiency syndrome as a factor in patients’ survival. N Engl J Med. 1996;334:701–706.
Lawrence J, Mayers D, Huppler Hullsiek K et al. CPCRA 064: a randomized trial examining structured treatment interruption for patients failing therapy with multi-drug resistant HIV. 10th Conference on Retroviruses
and Opportunistic Infections; February
10–14, 2003; Boston MA. Abstract 67.
Mallal S, John M, Moore C et al. Protease inhibitors and nucleoside analogue
reverse transcriptase inhibitors interact to cause subcutaneous fat wasting in patients with HIV infection. 1st International Workshop of Adverse Drug Reactions and Lipodystrophy in HIV; June 26–28, 1999; San Diego, CA. Abstract 019.
Martinez E, Blanco JL, Arnaiz JA et al. Hepatotoxicity in HIV-1-infected aptients receiving nevirapine-containing antiretroviral therapy. AIDS. 2001;15:1261–1268.
McNaghten AD, Hanson DL, Jones JL, Dworkin MS, Ward JW, and the Adult/Adolescent Spectrum of Disease Group. Effects of antiretroviral therapy and opportunistic illness primary chemoprophylaxis on survival
after AIDS diagnosis. AIDS.
1999;13:1687–1695.
Mellors JW, Rinaldo CR, Gupta P, White RM, Todd JA, Kingsley LA. Prognosis in
HIV-1 infection predicted by the quantity of virus in plasma. Science.
1996;272:1167–1170.
Mellors JW, Munoz A, Gigorgi JV, et al. Plasma viral load and CD+ lymphocytes
as prognostic markers of HIV-infection. Ann Intern Med 1997;126:946-954.
Miller KD, Jones E, Yanovski JA et al. Visceral abdominal fat accumulation associated with use of indinavir. Lancet. 1998;351:871–875.
Phair JP, Mellors JW, Detels R, Margalick JB, Muñoz A. Virologic and immunologic
values allowing safe deferral of antiretroviral therapy. AIDS. 2002;16:2455–2459.
Piroth L, Grappin M, Cuzin L et al. Hepatitis C virus co-infection is a negative
prognostic factor for clinical evolution in human immunodeficiency
virus-positive patients. J Viral Hepatol. 2000;7:302–308.
Quinn TC, Mawer MJ, Sewankambo N, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. N Engl J Med. 2000;342:921–929.
Raboud J, Montaner J, Conway B, et al. Suppression of plasma viral load below 20 copies/ml is required to achieve a long term response to therapy.
AIDS.
1998;12:1619–1624.
Rich JD, Merriman NA, Mylonakis SE, Greenough T, Flanigan T, Mady B, Carpenter CCJ. Misdiagnosis of HIV infection by HIV-1 plasma viral load testing: a case series. Ann Intern Med. 1999;130:37–39.
Romeu J, Sirera G, Rego MJ et al. Cumulative risk for developing hyperlipidemia
in HIV-infected patients treated with protease inhibitors. 39th Interscience Conference on Antimicrobial Agents and Chemotherapy; Sept 26–29, 1999; San Francisco, CA. Abstract 1293.
Scribner AN, Troia-Cancio PV, Cox BA, et al. Osteonecrosis in HIV: a casecontrol
study. J Acquir Immune Defic Syndr Hum Retrovirol. 2000;25:19–25.
Sherman KE, Rouster SD, Chung RT, Rajicic N. Hepatitis C virus prevalence among patients infected with human immunodeficiency virus: a crosssectional
analysis of the US adult AIDS Clinical Trials Group. Clin Infect Dis. 2002;34:831–837.
Soriano V, Garcia-Samaniego J, Bravo R et al. Interferon alpha for the treatment
of chronic hepatitis C in patients infected with human immunodificiency
virus. Hepatitis-HIV Spanish Study Group. Clin Infect Dis. 1996;23:585–591.
Tebas P, Powderly WG, Claxton S et al. Accelerated bone mineral loss in HIV-infected patients receiving potent antiretroviral therapy. AIDS. 2000;14:F63–67.
Thiebaut R, Daucourt V, Mercie P et al. Lipodystrophy, metabolic disorders,
and human immunodeficiency virus infection: Aquitaine Cohort, France, 1999. Group d’Epidemiologie Clinique du Syndrome d’Immunodeficience Acquise en Aquitaine.
Clin Infect Dis. 2000;31:1482–1487.
Thio CL, Seaberg E, Skolasky RL, Kingsley LA, Phair J, Visscher B et al. HIV infection increases liver-related mortality in HBV-infected individuals. Hepatology. 2001;34:469. USPHS/ISDA. Guidelines for the prevention of opportunistic infections in persons infected with human immunodeficiency virus. November 28, 2001. Available at: http://www.aidsinfo.nih.gov. Accessed March 21, 2004.
Zolopa AR, Shafer RW, Warford A, Montoya JG, Hsu P, Katzenstein D, Merigan
TC, Efron B. HIV-1 genotypic resistance patients predict response to saquinavir–ritonavir therapy in patients in whom previous protease inhibitor failed. Ann Intern Med.
1999;131:813–821.
|






![]()



![Figure 4-5: Guidelines for Use of Erythopoietin in Anemic HIV Patient. Flow diagram which begins at the top with a block labeled: Patient Candidate; Anemic HIV patient, Hgb<11g/dL or Hct <33%. Next block down is “Exclude Other Causes of Anemia,” which includes: Bleeding (guaiac stools), Hemolysis (smear), Iron deficiency (serum iron, transferrin, % saturation, ferritin), and B12, folate deficiency (serum B12, rbc folate if macrocytic). If the answer to any of these conditions is “Yes,” flow points to “Correct underlying cause” and the process stops. If the answer is “No,” flow leads to next block which is “Start EPO 40,000 units sc/wk. Consider iron supplementation.” Next step is “Monitor Response”: Full response will generally not be seen for at least 4 wk. Three options for next step (left to right): “At wk 4, if Hgb increases >1g/dL, continue at this dose.” Second option (which appears at bottom of page because there is a second path to this option): “When Hgb approaches 13/gdL, decrease EPO by 10,000 units/wk*. Titrate to maintain desired hemoglobin.[ † ]” Third option: “At 4 wk, if Hgb increases <1g/dL, increase dose to 60,000 units/wk.” From this third option, the flow branches two ways. The first leads to an end point box: “At 8 wk. if Hgb increases <1g/dL, check iron, folate and B12 levels. If adequate, discontinue EPO.” The second branch leads down to “At 8 wk, if Hgb increases >1g/dL, continue at this dose.” From this box, the flow leads back to the second option mentioned earlier, that is “When Hgb approaches 13g/dL, decrease EPO by 10,000 units/wk*. Titrate to maintain desired hemoglobin.[ † ]”](images/WG05fig4_5.gif)


