ISSN: 1080-6059
Volume 14, Number 9–September 2008
Dispatch
Mycobacterium haemophilum and Lymphadenitis in Immunocompetent Children, Israel
Yishai Haimi Cohen, ![]() Jacob Amir, Shai Ashkenazi, Tal
Eidlitz-Markus, Zmira Samra, Lea Kaufmann, and Avraham Zeharia
Jacob Amir, Shai Ashkenazi, Tal
Eidlitz-Markus, Zmira Samra, Lea Kaufmann, and Avraham Zeharia
Author affiliations: Schneider Children's Medical
Center of Israel, Petah Tiqwa, Israel (Y. Haimi Cohen, J. Amir, S. Ashkenazi, T. Eidlitz-Markus, A.
Zeharia); Rabin Medical Center, Petah Tiqwa (Z. Samra, L. Kaufmann); and
Tel Aviv University, Tel Aviv, Israel (Y. Haimi Cohen, J. Amir, S. Ashkenazi,
T. Eidlitz-Markus, Z. Samra, A. Zeharia)
Suggested citation for this article
Abstract
The database of a major microbiology laboratory in
Israel was searched to determine the prevalence of nontuberculous mycobacterial
lymphadenitis in immunocompetent children. We observed a 4-fold increase in
nontuberculous mycobacteria isolates during 1985–2006, which was attributable
mainly to increased detection of Mycobacterium haemophilum starting in 1996.
Nontuberculous mycobacteria (NTM) are a common cause of nonpyogenic craniofacial lymphadenitis in otherwise healthy children. Mycobacterium avium complex (MAC) is the main pathogen (1–3). M. haemophilum is traditionally considered a cause of NTM in immunocompromised patients (4–7), although a recent study from the Netherlands found that it is also common in immunocompetent children (8).
The Study
Prompted by the increasing number of M. haemophilum isolates identified at our tertiary medical center in Israel in the past decade, we investigated the current prevalence and clinical characteristics of NTM lymphadenitis in immunocompetent children. The database of our microbiology laboratory was searched for all NTM-positive cervical lymph node cultures of children during 1985–2006. In addition, we reviewed records of the Day Hospitalization Unit (DHU) for all patients with a diagnosis of NTM lymphadenitis from January 1996 (when M. haemophilum was first isolated in our laboratory) through December 2006. Data obtained were patient age and sex, Mycobacterium species, ethnic background (Jewish/Arab), medical history, duration of node enlargement until referral, site affected, number of infected sites, size of nodes at initial visit (measured by the clinician), discoloration of the skin overlying the lymph nodes, and maximal induration in response to purified protein derivative (PPD). Patients with M. haemophilum infection were compared with those with MAC infection.
All specimens were processed for direct Ziehl-Neelsen staining. From 1985 through 1995, specimens were placed on solid Lowenstein-Jensen (L-J) medium. Thereafter, liquid MB Redox broth and liquid Bactec 460 12B medium (Becton Dickinson Microbiology Systems, Cockeysville, MD, USA) were added to the L-J medium. Toward the end of 1999, Bactec medium was replaced with the liquid Mycobacteria Growth Indicator Tube system (Becton Dickinson Microbiology Systems). A hemin-containing paper strip (X-factor) was regularly placed into the 2 liquid media, which were incubated with the L-J medium at 37°C and 30°C.
Discrete variables were compared between groups by using a Pearson χ2 test or Fisher exact test, as appropriate. Continuous variables were compared with 1-way analysis of variance. A p value <0.05 was considered statistically significant. Data were analyzed by using BMDP software (www.statsol.ie/html/bmdp/bmdp_home.html). The study was reviewed and approved by the local ethics committee.
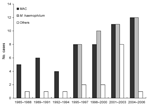 |
Figure. Distribution of nontuberculous mycobacteria species (Mycobacterium avium complex [MAC], M. haemophilum, and others) isolated from craniocervical lymph nodes of immunocompetent children in Israel, 1985–2006. |
The laboratory database contained 111 NTM isolates during 1985–2006, of which 77 (69%) were in samples from patients who visited the DHU from January 1996 through December 2006. Species distribution was as follows: MAC, 54; M. haemophilum, 41; others, 16. The Figure shows the increase in isolation rate of NTM and M. haemophilum since the initial isolation of M. haemophilum in March 1996.
The 77 patients managed at our DHU for NTM lymphadenitis included 38 boys (49%) and 39 girls 8 months to 15.5 years of age (median 2.4 years). M. haemophilum was isolated from 39 children and MAC from 29. The demographic and clinical features of these children are shown in the Table. The patients in the M. haemophilum group were significantly older than those in the MAC group (mean 4.7 years vs. 2.3 years; p<0.001); 9 patients with M. haemophilum infection (23%) were >7 years of age compared with none with MAC infection. Mean time to referral was significantly longer in the M. haemophilum group (1.5 months vs. 1.1 months; p = 0.045). No statistically significant differences were noted for the other parameters studied.
Conclusions
We speculate that the nearly 4-fold increase in the recovery rate of NTM from lymph nodes of immunocompetent children in the past 22 years at our center was attributable to the emergence of M. haemophilum as a major pathogen of craniocervical lymphadenitis starting in 1996. This assumption is supported by the only slight increase in the other NTM pathogens during the study period. The increased prevalence of NTM cervical lymphadenitis may be explained by conversion of our hospital to a tertiary pediatric center in 1991, concomitant with the growing awareness of NTM as a cause of craniocervical lymphadenitis among its physicians. Furthermore, the rate of isolation of M. haemophilum in this study was 51%. Another study from the Netherlands reported a similarly high prevalence rate (44%) of M. haemophilum lymphadenitis in immunocompetent children (8).
Although the distribution of NTM species may depend on local ecologic factors (9), given the wide geographic range of M. haemophilum infection in immunocompromised children (4–7) we would have expected to see reports of increased infection in immunocompetent children from >2 countries. We suspect that the change in our laboratory's processing procedure in 1996 to include broth with an iron supplement and incubation of the samples at 30°C in addition to 37°C (10) contributed to the high isolation rate. Our failure to use these conditions before 1996 could have led to an underdiagnosis of M. haemophilum infection; this may also be true for other laboratories (11).
These findings suggest that a failure to isolate a pathogen in children with suspected mycocbacterial craniocervical lymphadenitis, especially those >7 years of age, should prompt a targeted laboratory search for M. haemophilum by using proper culture conditions or molecular techniques (10–12). Identification of NTM infection has serious clinical implications because it can spare these patients, who often have a positive PPD response and cytologic results compatible with tuberculosis, unwarranted, prolonged antituberculosis therapy. The mean PPD response in our patients (>14.5 mm) confirms the lack of value of PPD in distinguishing NTM infection from tuberculosis (13).
The higher mean age of the children with M. haemophilum infection in our series compared with that of children in the MAC group is consistent with findings of a study in the Netherlands (8). Our finding may be explained by the younger age at which children are exposed to playgrounds, which are presumably linked to MAC infection, than to swimming pools, which are presumably linked to M. haemophilum infection (8). However, the mean age of our patients was lower by >1 year than the age of the Dutch children (8), perhaps the result of warmer climate and of the younger age of daycare attendees in Israel, both of which are associated with longer and earlier exposure to sandpits and swimming pools.
In contrast to the results of the Dutch study (8), ethnicity was not a risk factor; the rates of affected Jewish and Arab children matched the distribution of these ethnicities in the general population of Israel. This finding may reflect the similar environmental conditions to which these ethnic groups are exposed.
Despite the wide variability in the interval from onset of lymph node swelling to patient referral for investigation, the mean time of ≈5 weeks is consistent with that in previous studies (1–3). The difference of ≈10 days between the 2 groups in our study, although statistically significant, was not of clinical importance. The longer interval in the M. haemophilum group might have been caused by less attention parents tend to pay to physical changes in older children than in infants. In contrast to the findings in the Dutch study (8), we noted no predilection to multisite infection in either group, and all 4 patients with extranodal (cheek) involvement were infected with MAC.
In conclusion, M. haemophilum is an emerging pathogen in nonpyogenic craniocervical lymphadenitis in immunocompetent children in Israel. M. haemophilum infection usually affects older children (>7 years of age) but is otherwise clinically similar to MAC infection.
Dr Haimi Cohen is an attending physician in pediatric infectious diseases in the day hospitalization unit of Schneider Children's Medical Center of Israel. His primary research interest is nontuberculous mycobacterial infections in pediatric patients.
References
- Wolinsky E. Mycobacterial lymphadenitis in children: a prospective study of 105 nontuberculous cases with long term follow-up. Clin Infect Dis. 1995;20:954–63.
- Mushtaq I, Martin HC. Atypical mycobacterial disease in children: a personal series. Pediatr Surg Int. 2002;18:707–11.
- Stewart MG, Starke JR, Coker NJ. Nontuberculous mycobacterial infections of the head and neck. Arch Otolaryngol Head Neck Surg. 1994;120:873–6.
- Saubolle MA, Kiehn TE, White MH, Rudinsky MF, Armstrong D. Mycobacteium haemophilum: microbiology and expanding clinical and geographic spectra of disease in humans. Clin Microbiol Rev. 1996;9:435–47.
- Shah MK, Sebti A, Kiehn TE, Massarella SA, Sepkowitz KA. Mycobacterium haemophilum in immunocompromised patients. Clin Infect Dis. 2001;33:330–7. PubMed DOI
- Tan HH, Tan A, Theng C, Ng SK. Cutaneous Mycobacterium haemophilum infections in immunocompromised patients in a dermatology clinic in Singapore. Ann Acad Med Singapore. 2004;33:532–6.
- Sampaio JL, Alves VA, Leão SC, de Magalhães VD, Martino MD, Mendes CM, et al. Mycobacterium haemophilum: emerging or underdiagnosed in Brazil? Emerg Infect Dis. 2002;8:1359–60.
- Lindeboom JA, Prins JM, Bruijnestein van Coppenraet ES, Lindeboom R, Kuijper EJ. Cervicofacial lymphadenitis in children caused by Mycobacterium haemophilum. Clin Infect Dis. 2005;41:1569–75. PubMed DOI
- O'Brien DP, Currie BJ, Krause VL. Nontuberculous mycobacterial disease in northern Australia: a case series and review of the literature. Clin Infect Dis. 2000;31:958–68. PubMed DOI
- Samra Z, Kaufmann L, Zeharia A, Ashkenazi S, Amir A, Bahar J, et al. Optimal detection and identification of Mycobacterium haemophilum in specimens from pediatric patients with cervical lymphadenopathy. J Clin Microbiol. 1999;37:832–4.
- Bruijnesteijn van Coppenraet ES, Kuijper EJ, Lindeboom JA, Prins JM, Claas EC. Mycobacterium haemophilum and lymphadenitis in children. Emerg Infect Dis. 2005;11:62–8.
- Wang SX, Sng LH, Leong HN, Tan BH. Direct identification of Mycobacterium haemophilum in skin lesions of immunocompromised patients by PCR-restriction endonuclease analysis. J Clin Microbiol. 2004;42:3336–8. PubMed DOI
- Haimi-Cohen Y, Zeharia A, Mimouni M, Soukhaman M, Amir J. Skin indurations in response to tuberculin testing in patients with nontuberculous mycobacterial lymphadenitis. Clin Infect Dis. 2001;33:1786–8. PubMed DOI
Figure
Table
Suggested Citation for this Article
Haimi Cohen Y, Amir J, Ashkenazi S, Eidlitz-Markus T, Samra Z, Kaufmann L, et al. Mycobacterium haemophilum and lymphadenitis in immunocompetent children, Israel. Emerg Infect Dis [serial on the Internet]. 2008 Sep [date cited]. Available from http://www.cdc.gov/EID/content/14/9/1437.htm
DOI: 10.3201/eid1409.070917
Please use the form below to submit correspondence to the authors or contact them at the following address:
Yishai Haimi Cohen, Day Hospitalization Unit, Schneider Children's Medical Center of Israel, 14 Kaplan St, Petah Tiqwa 49202, Israel; email: yhaimi@bezeqint.net
Please contact the EID Editors at eideditor@cdc.gov
The opinions expressed by authors contributing to this journal do not necessarily reflect the opinions of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.
This page posted August 26, 2008
Centers for Disease Control and Prevention, 1600 Clifton Rd, Atlanta, GA 30333, U.S.A
Tel: (404) 639-3311 / Public Inquiries: (404) 639-3534 / (800) 311-3435