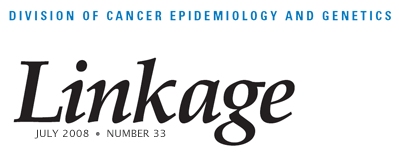Division Provides Specialized Training
Continuing its rich tradition of providing high-caliber training to its investigators and fellows, DCEG has presented a remarkable roster of unique offerings over the last year.
In January, the fourth biennial DCEG Molecular Epidemiology Course commenced, with 57 participants in attendance, including fellows, staff scientists, and tenure-track and senior investigators. Co-directed by Nathaniel Rothman, M.D., M.P.H., M.H.S., Occupational and Environmental Epidemiology Branch (OEEB), and Alice J. Sigurdson, Ph.D., Radiation Epidemiology Branch (REB), along with course coordination by Kristin Kiser, M.H.A., Office of Education (OE), this 14-session course provided students with the foundation for understanding key concepts of molecular epidemiology and incorporating biomarkers into analytic epidemiologic research.
The course focused on four broad content areas: exposure biomarkers, classic and emerging techniques for the analysis of intermediate end points, genotyping and related issues at DCEG, and preneoplastic and tumor markers. In addition to classroom sessions led by the Division’s course faculty (see sidebar), participants received a tour of the state-of-the-art NCI Central Repository at Wedgewood in Frederick facilitated by Karen E. Pitt, Ph.D., special assistant for biological resources in the DCEG Office of the Director, Dr. Kathleen Groover, Director of the repository, and Dr. Mark Cosentino, project manager of repository services.
Since the first Molecular Epidemiology Course in 2000, significant advances in methods and technology have occurred in the field. The curriculum has gradually expanded over the years to include additional lectures in genomic methods, while at the same time continuing to cover the core issues of study design and quality control.

Course Organizers: Alice Sigurdson, Nathaniel Rothman, Stephen Chanock, Meredith Yeager, and Jackie Lavigne.
Dr. Rothman stated, “Our goal is to provide the tools that will enable our investigators to rapidly develop proposals and carry out state-of-the-art molecular epidemiology research in DCEG and to provide them with the background to better understand emerging trends in the field.”
In a final exercise, students submitted proposals for molecular epidemiology projects, which were competitively scored and evaluated by a panel that included Christian C. Abnet, Ph.D., M.P.H., Nutritional Epidemiology Branch (NEB), Demetrius Albanes, M.D. (NEB), Patricia Hartge, Sc.D., Deputy Director of the Epidemiology and Biostatistics Program, Ruth M. Pfeiffer, Ph.D., Biostatistics Branch (BB), Charles S. Rabkin, M.D., Infections and Immunoepidemiology Branch (IIB), Preetha Rajaraman, Ph.D. (REB), Sharon A. Savage, M.D., Clinical Genetics Branch (CGB), Catherine Schairer, Ph.D. (BB), and Sholom Wacholder, Ph.D. (BB). Students who submitted the top proposals received $10,000 to carry out the project described.
Dr. Rothman stated, “Our goal is to provide the tools that will enable our investigators to rapidly develop proposals and carry out state-of-the-art molecular epidemiology research in DCEG and to provide them with the background to better understand emerging trends in the field.”
This year’s winners are Victoria M. Chia, Ph.D., Hormonal and Reproductive Epidemiology Branch (HREB), and Michael B. Cook, Ph.D. (HREB), for their proposal for a pilot study of methylation profiles in testicular germ cell tumors; Linda Dong, Ph.D. (OEEB), for her project on the role of chronic inflammation and oxidative stress on telomere length and risk of gastric cancer; Gretchen L. Gierach, Ph.D., M.P.H. (HREB), and Jennifer T. Loud, R.N., C.R.N.P., D.N.P. (CGB), for a pilot study of circulating estrogens and estrogens produced within the breast in relation to mammographic density among BRCA1/2 mutation–positive women from CGB’s Breast Imaging Study; H. Dean Hosgood, III, Ph.D. (OEEB), for his proposal on mitochondrial DNA copy number and lung cancer risk in nonsmoking females; and Jill Koshiol, Ph.D., Genetic Epidemiology Branch (GEB), for her project evaluating the role of Epstein-Barr virus miRNAs in lung cancer.
In conjunction with the Molecular Epidemiology Course, the Core Genotyping Facility (CGF) held its second Genetic Analysis Course at the NIH Neuroscience Building in February. A “must-see” for newcomers to the Division, this one-day course was intended for scientists who plan to integrate genotyping into epidemiologic projects. This popular training opportunity was developed and facilitated by instructors from DCEG and Harvard University. The course reviewed the basics of genomics and population genetics, progressing to the more complex topics of genotyping design and analysis. The course showcased custom-built tools to familiarize new investigators with the genotyping process.
The course also highlighted the molecular epidemiology infrastructure, study design, and strategies used in DCEG genome-wide association studies. Co-organized by Stephen J. Chanock, M.D., Director of CGF and Chief of the Laboratory of Translational Genomics (LTG), and Meredith Yeager, Ph.D., Scientific Director of CGF, the course featured faculty including Laufey Amundadottir, Ph.D. (LTG), Sonja I. Berndt, Pharm.D., Ph.D. (OEEB), Belynda Hicks, M.S. (CGF), Amy Hutchinson, M.S. (CGF), Kevin B. Jacobs (CGF), Nicholas Orr, Ph.D. (LTG), Nianqing (Nick) Xiao, Ph.D. (CGF), Dr. Pitt, and Dr. Peter Kraft from the Harvard School of Public Health.

Molecular Epidemiology Course Proposal Winners: (front) Jennifer Loud, H. Dean Hosgood, and Linda Dong; (back) Jill Koshiol, Gretchen Gierach, Victoria Chia, and Michael Cook.
In February, the Molecular Epidemiology Course participants practiced fundamental concepts in the first-ever Genetics Analysis Tutorial, which provided comprehensive, intensive instruction on statistical issues in genetic association studies. After an overview by Dr. Chanock, the class discussed various considerations in study design, led by Nilanjan Chatterjee, Ph.D. (Chief of BB), and Dr. Pfeiffer, who then co-chaired a session with Philip S. Rosenberg, Ph.D. (BB), on the conduct and analysis of association studies, which was followed by a session on population stratification led by Kai Yu, Ph.D. (BB). Dr. Chatterjee concluded the session with a lesson on statistical methods for the assessment of gene-gene and gene-environment interactions. David Check (BB) provided administrative and technical support to the tutorial.
In March, the NCI Cancer Prevention Fellowship Program (CPFP) hosted a “Grants and grantsmanship” workshop on the effective development of grant proposals. This informative workshop was organized by Jackie Lavigne, Ph.D., M.P.H., Chief of OE and former Associate Director of CPFP, and was facilitated by scientific staff in the extramural division. The workshop introduced fellows to various types of NIH and non-NIH grants as well as the scientific review process. The workshop concluded with a mock study section, led by Dr. Mac Stiles, a former scientific review administrator in the Epidemiology and Disease Control-2 Study Section. DCEG fellows prepared short grant proposals, which were reviewed and scored by the other workshop participants.
Other training highlights include the interdisciplinary seminar series on public health genomics, organized by Dr. Muin J. Khoury, Director of the National Office of Public Health Genomics at CDC, and sponsored by DCEG and the NCI Division of Cancer Control and Population Sciences; the DCEG Monthly Fellows’ Colloquia Series, a forum for the discussion of career development and scientific initiatives within the Division; and, as always, the DCEG weekly seminar series.
—Alyssa Minutillo, M.P.H.
Other Molecular Epidemiology Course Faculty
Christian C. Abnet, Ph.D., M.P.H. (BB)
Bharathi Anekella, Ph.D. (SeraCare Life Sciences Repository)
Neil E. Caporaso, M.D. (GEB)
Stephen J. Chanock, M.D. (CGF and LTG)
Eric A. Engels, M.D., M.P.H. (IIB)
Roni T. Falk, M.S. (HREB)
Montserrat García-Closas, M.D., Dr.P.H. (HREB)
Marianne K. Henderson, M.S. (Office of Division Operations and Analysis)
Qing Lan, M.D., Ph.D., M.P.H. (OEEB)
Lisa McShane, Ph.D. (NCI Division of Cancer Treatment and Diagnosis)
Lee E. Moore, Ph.D. (OEEB)
Ruth M. Pfeiffer, Ph.D. (BB)
Sharon A. Savage, M.D. (CGB)
Arthur Schatzkin, M.D., Dr.P.H. (NEB)
Mark Schiffman, M.D., M.P.H. (HREB)
Mark E. Sherman, M.D. (HREB)
Timothy Veenstra, Ph.D. (SAIC-Frederick, Inc.)
Sholom Wacholder, Ph.D. (BB)
Sophia S. Wang, Ph.D. (HREB)
THREE NEW CHIEFS APPOINTED

Allan Hildesheim
In 2008, DCEG appointed two new Branch Chiefs as well as a Chief for a new office within the Office of the Director (OD).
In February,
Allan Hildesheim, Ph.D., began serving as Chief of the Infections and Immunoepidemiology Branch (IIB), formerly known as the Viral
Epidemiology Branch. Dr. Hildesheim is an international leader in
research on DNA virus–related tumors, with an emphasis on host and
immunological factors associated with human papillomavirus (HPV)– and
Epstein-Barr virus–related tumors. Previously, Dr. Hildesheim was a
senior investigator in the Hormonal and Reproductive Epidemiology
Branch, where he led the HPV vaccine trial in Costa Rica and a research
program on nasopharyngeal carcinoma. He brings to IIB his expertise in
viral epidemiology as well as plans to increase research into the role
of immunity and inflammation in cancer etiology.
Dr. Hildesheim received a Ph.D. in epidemiology from the Johns Hopkins School of Hygiene and Public Health in 1991. He joined NCI in 1987 while still a student and progressed to become a tenure-track investigator in 1997 and a tenured senior investigator in 2001. He received an NIH Merit Award for prevention-oriented research to reduce cervical cancer incidence in 2004, and in 2005, he received the NIH Director’s Award for efforts to evaluate a novel prophylactic HPV vaccine.
FELLOWSHIP BROCHURE EARNS NIH PLAIN LANGUAGE AWARD
On April 15, the DCEG fellowship brochure was recognized with a bronze NIH Plain Language Award. Recipients included Joseph F. Fraumeni, Jr., M.D., Division Director, Demetrius Albanes, M.D., senior investigator in the Nutritional Epidemiology Branch and former Chief of the Office of Education (OE), Kristin Kiser, M.H.A., fellowship coordinator (OE), and Hong Vo, NIH Office of Research Services. The award is part of an NIH-wide initiative to promote the use of plain language in all documents written for the public or within the government. Criteria for the award include how clearly the document answers the readers’ questions and whether the language is appropriate for the intended audience. Plain language is characterized by the active voice; personal pronouns such as “we” and “you”; short sentences and paragraphs; and easy-to-understand tables, lists, and other design features.
For more information about the NIH Plain Language Initiative, visit: http://execsec.od.nih.gov/plainlang/.

Jackie Lavigne
Also in February, Jackie A. Lavigne, Ph.D., M.P.H., joined DCEG as the new Chief of the Office of Education. Dr. Lavigne received a Ph.D. in molecular toxicology and an M.P.H. with a concentration in epidemiology and biostatistics from the Johns Hopkins Bloomberg School of Public Health. She completed a postdoctoral fellowship in NCI’s Cancer Prevention Fellowship Program (CPFP), conducting research on the role of insulin-like growth factors in diet-related cancer. Subsequently, she joined NCI’s Center for Cancer Research (CCR) as a scientific program specialist and then became Associate Director of CPFP in 2006 while remaining an adjunct investigator in CCR’s Laboratory of Human Carcinogenesis.
During her tenure with CPFP, Dr. Lavigne developed and expanded a series of training courses, retreats, and symposia for fellows and junior scientists. Dr. Lavigne brings to DCEG her passion and experience in mentoring and training, so that all fellows have the highest quality professional training in epidemiology or biostatistics, preparing them for successful research careers.

Office of Communications and Special Initiatives: Alyssa Minutillo, Catherine McClave, Samantha Nhan, and Jennifer Loukissas.
Catherine B. McClave, M.S., has been appointed Chief of the Office of Communications and Special Initiatives (OCSI) within the OD. This newly established office will consolidate a wide variety of activities within the OD, including development of scientific narratives for the Congressional Justification, Bypass Budget, NIH Roadmap, and other reports for the NCI or NIH budget and planning offices. The Office is responsible for preparing responses to congressional inquiries and Freedom of Information Act requests, coordinating DCEG-related activities in conjunction with the NCI Office of Media Relations and Office of Communications and Education, and managing communication efforts for high-visibility publications and initiatives. This work often requires rapid response and close coordination across NCI and NIH and with several other agencies. Ms. McClave’s position requires frequent interactions with cancer research community. OCSI congressional staff, cancer advocacy assists with quadrennial site visit groups, the public, the media, and the reviews by the NCI Board of Scientific Counselors (BSC) and with reports to the BSC and the National Cancer Advisory Board. The Office also provides Executive Secretarial functions for the DCEG Senior Advisory Group and the Promotion and Tenure Review Panel and produces the Division newsletter, Linkage. OCSI includes Jennifer Loukissas, M.P.P., the DCEG communications manager; Alyssa Minutillo, M.P.H., the Division’s scientific technical writer; Samantha Nhan, the assistant for special projects; and Michael Donovan, Ph.D., a Presidential Management Fellow.
—Alyssa Minutillo, M.P.H.
RADIOLOGY CONFERENCE PROMOTES LOWER DOSES FOR CHILDREN
DCEG and the NIH Office of Rare Diseases co-sponsored the Society for Pediatric Radiology (SPR) meeting on pediatric oncology imaging held in Scottsdale, Arizona, in May. The conference, titled The ALARA (As Low As Reasonably Achievable) Concept in Pediatric Imaging: Oncology, was designed to develop strategies for safe and high-quality imaging of young cancer patients. These patients often undergo a number of imaging procedures, such as computed tomography (CT) scans, during and after cancer treatments, each of which exposes them to radiation. The ALARA principle aims to reduce excess radiation exposure for situations in which some exposure is necessary.
To provide background about the etiology of childhood cancer, Martha S. Linet, M.D., M.P.H., Chief of the Radiation Epidemiology Branch (REB), opened the conference with a presentation titled “Epidemiology of childhood cancer: Descriptive features and radiation as a risk factor.” Following Dr. Linet’s remarks, Ruth A. Kleinerman, M.P.H., a staff scientist in REB, spoke on “Radiation-sensitive genetically susceptible pediatric sub-populations,” and Dr. Leslie Robison from St. Jude Children’s Research Hospital described radiation-induced second malignant neoplasms in the Childhood Cancer Survivor Study cohort.
Other speakers at the conference focused on when to use various types of radiation imaging (e.g., chest x-rays, CT scans, FDG positron emission tomography [PET], PET/CT scans) and alternatives, such as ultrasonography and magnetic resonance imaging, for managing treatment response and monitoring cancer recurrence in children. The meeting also provided a forum for discussion of the risks and benefits of radiation imaging in pediatric cancer patients. The participants agreed that SPR needs to communicate these concerns to pediatric radiologists and encourage the use of alternative imaging strategies when appropriate.
REB has worked closely with SPR to develop an educational brochure on radiation risks to children from CT scans. The brochure was recently updated and is available at www.cancer.gov/cancertopics/causes/radiation-risks-pediatric-CT.
CHILDHOOD CANCER EXPERT LESLIE ROBISON VISITS DIVISION

Leslie Robison (second) with Joseph Fraumeni, Martha Linet, and Demetrius Albanes,
former Chief of the
DCEG Office of Education.
In January, DCEG welcomed Dr. Leslie Robison, the first Visiting Scholar in 2008. Dr. Robison is the Associate Director for Cancer Prevention and Control and founding chair of the Department of Epidemiology and Cancer Control at St. Jude Children’s Research Hospital in Memphis, Tennessee. Dr. Robison’s visit was hosted by Martha S. Linet, M.D., M.P.H., Chief of the Radiation Epidemiology Branch (REB), and sponsored by the DCEG Office of Education.
Dr. Robison is an internationally recognized pediatric cancer epidemiologist specializing in survivorship. While working on his master’s and doctoral degrees in epidemiology at the University of Minnesota, he began his research focus on pediatric cancers through involvement in a multicenter trial of children with acute lymphoblastic leukemia. He remained at the University of Minnesota for 23 years in dual appointments in the Schools of Medicine and Public Health and eventually became Associate Director for Population Sciences at the University of Minnesota Comprehensive Cancer Center. He moved to St. Jude Children’s Research Hospital in 2005.
Throughout his career, Dr. Robison has led several major studies of childhood leukemia and other malignancies. He is also known for spearheading the development of much-needed registries and databases of childhood cancer patients and long-term survivors. In 1994, Dr. Robison initiated the landmark Childhood Cancer Survivor Study (CCSS), a large multi-institutional interdisciplinary cohort study designed to assess health outcomes in five-year survivors of childhood cancers. The study, which Dr. Robison still directs, serves as a unique resource for evaluating late effects in this growing population. Dr. Robison serves on the Board of Directors and as North American Continental President of the International Society of Paediatric Oncology.
Dr. Robison devoted this Visiting Scholar seminar to describing CCSS in his presentation, titled “Pediatric cancer survivorship: Unique opportunities for research.” With 29 institutions now participating around the United States and Canada, the study provides a wealth of data on more than 14,000 childhood cancer survivors, nearly half of all pediatric cancer patients diagnosed between 1970 and 1986 in the United States. Participants respond to questionnaires and surveys approximately every two years, and they have supplied the biospecimen repository with buccal cell DNA and biopsied tumor tissue from second malignancies. Additionally, a random sample of 5,857 patient siblings was assembled into a “control” sub-cohort as a comparison group used in assessing various health and quality-of-life factors.
Data from the Childhood Cancer Survivor Study have already been the source of 75 publications, advancing our understanding of second cancers and other late effects of cancer treatment on children as well as the determinants of survival.
Dr. Robison highlighted several analyses from the study, particularly those related to the effects of smoking and obesity on long-term health among study participants. He also discussed findings about the psychosocial well-being and quality of life among survivors and their siblings. These data have already been the source of 75 publications, advancing our understanding of second cancers and other late effects of cancer treatment on children as well as the determinants of survival. These studies have revealed that both the type of treatment (radiotherapy, chemotherapy, other treatments) and genetics are important determinants of risk for late effects. Risk may increase further when these factors interact. The CCSS cohort is being expanded with the addition of childhood cancer survivors diagnosed between 1987 and 1999.
After the seminar, Joseph F. Fraumeni, Jr., M.D., Division Director, presented Dr. Robison with the DCEG Visiting Scholar Award in recognition of Dr. Robison’s vision and leadership in the epidemiology of childhood cancer. Dr. Robison then met with NCI investigators to discuss the consortial approach for studying childhood cancers in a lunchtime session moderated by Margaret A. Tucker, M.D., Director of the Human Genetics Program and Chief of the Genetic Epidemiology Branch, and Dr. Carol Kasten, Division of Cancer Control and Population Sciences (DCCPS).
Later, Dr. Robison met with DCEG fellows and tenure-track investigators in a two-part session to discuss career development and effective communication strategies for sharing epidemiologic research findings. Such findings are a particularly sensitive topic in communities in which links between environmental exposures and childhood cancer are suspected. This popular session was moderated by Elizabeth C. Bluhm, M.D., M.P.H. (REB), Lee E. Moore, Ph.D., Occupational and Environmental Epidemiology Branch (OEEB), and Jennifer Loukissas, M.P.P., Office of Communications and Special Initiatives. Dr. Robison’s first day ended with a scientific session moderated by Mary H. Ward, Ph.D. (OEEB), and Sharon A. Savage, M.D., Clinical Genetics Branch, discussing epidemiologic and genetic research into childhood cancer etiology.
On the second day, the DCEG Women Scientist Advisors hosted a breakfast with Dr. Robison to discuss strategies for successful career development at various stages. The session was moderated by Ann W. Hsing, Ph.D., and Montserrat García-Closas, M.D., Dr.P.H., both of the Hormonal and Reproductive Epidemiology Branch. Peter D. Inskip, Sc.D. (REB), and Dr. Julia Rowland, Director of the Office of Cancer Survivorship in DCCPS, then moderated a session on cancer survivorship research at NCI with presentations by Dr. Inskip, Ruth A. Kleinerman, M.P.H. (REB), and Dr. Keith Bellizzi of DCCPS. A later session on future directions of CCSS featured a presentation by Dr. Linet, Preetha Rajaraman, Ph.D. (REB), and Alice J. Sigurdson, Ph.D. (REB).
Dr. Robison completed his successful visit by participating in several one-on-one meetings with DCEG investigators to discuss various topics of interest. On leaving, he noted, “I am always happy to visit DCEG; I come away with so many new ideas.”
—Alyssa Minutillo, M.P.H.

CGB Team: (front) Larissa Korde and June Peters; (back) Christine Mueller and Phuong Mai. (Not shown: Mark Greene)
BEST CASE REPORT
A Clinical Genetics Branch (CGB) team led by Phuong Mai, M.D., was honored with the inaugural Award for Best Case Report in 2007 from the new Journal of Medical Case Reports (JMCR). Other members of the team included Larissa A. Korde, M.D., M.P.H., June A. Peters, M.S., C.G.C., Christine M. Mueller, D.O., and Mark H. Greene, M.D. (Chief of CGB), who worked with investigators from NCI’s Center for Cancer Research, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and Westat, Inc.
The winning case, “A possible new syndrome with growth-hormone secreting pituitary adenoma, colonic polyposis, lipomatosis, lentigines and renal carcinoma in association with familial testicular germ cell malignancy,” has become one of the most frequently accessed articles published by JMCR during the past year. Professor Michael Kidd, editor-in-chief of JMCR, noted that the paper received multiple nominations and strong support from the judges. The paper illustrates CGB’s success in combining astute clinical observation with cutting-edge genetic technology to elucidate known genetic syndromes and to identify possible new syndromes.
The award was announced at BioMed Central’s Research Awards ceremony at the Royal Society of Medicine in London in March.
To listen to Dr. Kidd’s award introduction and Dr. Mai’s acceptance speech, visit http://blogs.openaccesscentral.com/blogs/jmcrblog/entry/jmcr_award_winner_announced.
OLA LANDGREN INVESTIGATES MULTIPLE MYELOMA

Ola Landgren
Multiple myeloma is a clonal plasma cell malignancy. After non-Hodgkin lymphoma, it is the second most common hematologic neoplasm, reflected in about 20,000 new cases diagnosed each year in the United States. The disease is twice as common in African Americans as in whites, and it is slightly more common in males than in females. Multiple myeloma usually evolves from an asymptomatic premalignant condition termed “monoclonal gammopathy of undetermined significance” (MGUS).
These observations have intrigued Ola Landgren, M.D., Ph.D., Genetic Epidemiology Branch (GEB), for years. “I was always fascinated by the fact that multiple myeloma is so common but there’s so little known about it,” Dr. Landgren said. Traditionally, multiple myeloma is known to be an incurable cancer without identifiable causes and with poor survival, which has made the disease difficult to study. However, in recent years, the field has exploded. The genetics of multiple myeloma has been increasingly explored, leading to the definition of biologically distinct and clinically relevant molecular subtypes of multiple myeloma and enhancing science’s understanding of pathogenic mechanisms in etiology and outcomes. Anti-myeloma treatment has also improved significantly. Based on novel molecular insights, several targeted agents have demonstrated exciting preclinical activity and are presently being tested in early phase I/II clinical trials. Despite these advances, researchers still lack an understanding of the risk factors for MGUS and predictors for progression from MGUS to multiple myeloma.
“A major focus of my research is to improve our understanding of these candidates in order to facilitate the development of strategies to prevent progression of MGUS to multiple myeloma,” Dr. Landgren said. “Overall, the future for multiple myeloma research looks very promising.”
Dr. Landgren grew up in Lund, Sweden, where he attended college. After earning graduate and medical degrees from the Karolinska Institute in Stockholm, he worked as an attending physician at the Hematology Center at Karolinska University Hospital.
“A major focus of my research is to improve our understanding of these candidates in order to facilitate the development of strategies to prevent progression of MGUS to multiple myeloma,” Dr. Landgren said. “Overall, the future for multiple myeloma research looks very promising.”
“I was always interested in the natural sciences, but I also was interested in humanistic aspects,” he said of his career path. “I thought being an engineer was too mechanical and I wanted to make a difference in people’s lives—so medicine was it.” Dr. Landgren earned an M.D. in 1995 and obtained a Ph.D. in 2002, focusing on Hodgkin lymphoma. He worked at the Karolinska Institute as a hematologist before joining DCEG in 2004 as a GEB fellow. He became a tenure-track investigator in 2006.
At NCI, Dr. Landgren had an opportunity to focus his research further on the role of race in the etiology and progression of MGUS and multiple myeloma. “I had two possible explanations for the higher rates of myeloma among African Americans than whites,” he said. “One, African Americans must have more MGUS than whites—if it’s true that MGUS precedes every case of myeloma—and two, African Americans have the same rate of MGUS but are more likely to progress from MGUS to cancer.” Using data from the U.S. Department of Veterans Affairs, Dr. Landgren found some answers. He observed that African American veterans were more likely to have MGUS than whites and that the progression rate from MGUS to myeloma was the same in both groups. The study was published in Blood in 2006.
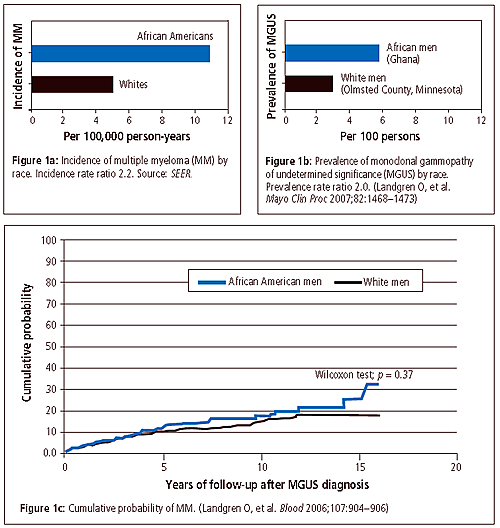
Dr. Landgren then decided to test his results among men in Africa. He initiated a collaborative study with Ann W. Hsing, Ph.D., a senior investigator in the Hormonal and Reproductive Epidemiology Branch, who is conducting a survey of prostate cancer in West African men, and with colleagues at the Mayo Clinic and the University of Ghana. At the Protein Immunology Laboratory at the Mayo Clinic, “we screened for MGUS in nearly 1,000 serum samples from mature (50–74 years old) Ghanaian men,” he said. “Then we compared the results with previously collected data on 8,000 white men from Minnesota.”
The study, published in Mayo Clinic Proceedings in December 2007, showed that Ghanaian men have twice the rate of MGUS that U.S. white men do. Also, among blacks, the prevalence of MGUS was found to be stable, about 6 percent, independent of age. This is in contrast to whites, for whom MGUS is known to become more common at older ages. “There is some evidence to suggest that myeloma is diagnosed 5 to 10 years earlier in blacks than in whites, so it’s possible MGUS starts earlier, too,” he said. The youngest men in the study were 50, so Dr. Landgren suggested that younger men should be included in further studies to see if MGUS has an earlier onset in black men.
Dr. Landgren is now trying to define the association between MGUS and myeloma, using data from NCI’s Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial. After screening the records of more than 75,000 subjects, he has identified about 100 cases of myeloma. “We have blood samples from the cases before they were diagnosed and are looking at them for evidence of MGUS,” he said.“I want to know if every myeloma patient has a preceding MGUS state. This is an important, missing piece of information.”
Dr. Landgren believes there’s a genetic component to MGUS but that environmental factors are also at work, particularly through immune-related and inflammatory mechanisms. “In our Ghana study, we had information about medical history,” he said. “Eighty percent of the men had had malaria, and most had a sexually transmitted disease. We have not been able to explain the difference in MGUS by the differences in selected infections, but that doesn’t rule out a role.”
Dr. Landgren is now planning a U.S.-wide study with Mayo researchers to study risk factors for MGUS using National Health and Nutrition Examination Survey (NHANES) data on African Americans, whites, and Hispanics. He is also collaborating with Vanderbilt University and the Mayo Clinic to look at risk factors for MGUS among low-income African Americans and whites.
In addition to these projects, Dr. Landgren is the lead investigator of a large study in Sweden that focuses on familial clustering of MGUS. Through linked registries, the group has identified close to 5,000 MGUS cases and has found an increased risk of the condition among family members, reinforcing the idea of a genetic component. Dr. Landgren is investigating the opportunities to map genes for MGUS in African Americans using “mapping by admixture linkage disequilibrium” markers.
When he has time outside the lab, Dr. Landgren plays tennis and racquetball with his daughters and attends their ballet recitals. He and his family take advantage of the area’s many outdoor activities, museums, and other cultural activities. And they try to make it back to Sweden every summer for a visit.
—Nancy Volkers
AMANDA CROSS INVESTIGATES RED MEAT AND CANCER RISK

Amanda Cross and Atlantis at the
American Eventing National
Championships in Raeford,
North Carolina.
When Amanda J. Cross, Ph.D., arrived at NCI in 2003 as a postdoctoral fellow to work with Rashmi Sinha, Ph.D., Nutritional Epidemiology Branch (NEB), on the association of red meat intake with cancer, she had another concern. The tenure-track investigator in NEB was sure she had found an ally in her research, but she still needed something else. “I’ve ridden horses since I was a little girl, so I needed to find somewhere to ride,” she said. Throughout her time at NCI, she has combined first-class research with a blue-ribbon riding avocation.
Dr. Cross’s undergraduate work in applied biology and nutrition at Newcastle University, Newcastle upon Tyne, United Kingdom, culminated in a six-month study of diet and colon cancer using a mouse model. “I begged to do that project because I thought it would yield information that could actually make a difference in preventing colon cancer,” Dr. Cross recalled. The project led to doctoral work at the University of Cambridge with Dr. Sheila Bingham, investigating the endogenous formation of N-nitroso compounds in the human gastrointestinal tract. By manipulating the amounts of meat, protein, and iron in subjects’ diets, Dr. Cross showed that neither vegetable protein nor inorganic iron increased nitrosation, but red meat consumption was linked to DNA damage in the colon. At a meeting in France, she met Arthur Schatzkin, M.D., Dr.P.H., Chief of NEB, leading to a postdoctoral fellowship at DCEG.
Dr. Cross’s work on red meat fit naturally with that of Dr. Sinha on the role of heterocyclic amines (HCAs) and polycyclic aromatic hydrocarbons (PAHs) from cooking meat at high temperatures. Dr. Sinha had developed a database on 5 HCAs and 12 PAHs associated with different meats cooked to varying doneness. “Her database is used by researchers around the world,” Dr. Cross said. “She has been amazingly supportive as we have worked together to investigate this component of the diet.”
In work published last December, Dr. Cross found significantly increased risk associated with high red meat intake for lung, colorectal, esophageal, and liver tumors in the NIH-AARP Diet and Health Study, a large prospective sample of approximately 500,000 U.S. adults aged 50 to 71 years.
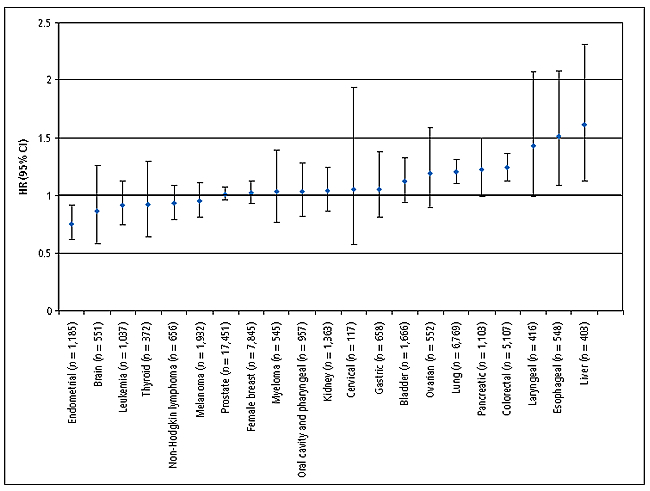
Hazard ratios (HRs) and confidence intervals (CIs) for the fifth versus first quintile of red meat intake and cancer risk for both sexes combined. (Cross AJ, et al. PloS Med 2007;4:e325)
In her latest work, she used the NIH-AARP study to examine meat and fat intake in relation to small intestine cancer. “The exciting thing is that almost nothing has been done on the small intestine, an uncommon site of cancer, yet 90 percent of nutrient absorption occurs there,” she said. “Previous research was limited to case-control studies, but using this large cohort from eight U.S. states, we were able to conduct the first prospective study,” she said.
Dr. Cross noted that the association between meat and colorectal cancer is now recognized as “convincing” in the World Cancer Research Fund’s 2007 Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective.
Dr. Cross found no clear association with processed meats, a slightly increased risk with red meats, and a markedly elevated risk with saturated fats—both meat and dairy. “I wanted to see if the red meat finding held up when we adjusted for saturated fats, and it did not,” Dr. Cross said. “I then needed to demonstrate that the risk associated with saturated fat was independent of red meat.” She admits she did not think it was. But even after adjusting for red meat intake, the elevated risk from saturated fat was significant for small intestine cancer.
Dr. Cross noted that the association between meat and colorectal cancer is now recognized as “convincing” in the World Cancer Research Fund’s 2007 Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective.
In her downtime, Dr. Cross found a competition barn where she could exercise horses. One day a horse came in, “a bit of a delinquent” named Atlantis, “and they asked me to teach him to jump so he could be sold.” Atlantis did learn to jump, and they did sell him—“I bought him,” Dr. Cross explained.
“If I’m not in my office, I am in the barn.” She and Atlantis compete in eventing, which is “like a triathlon for horse and rider,” she explained, including dressage, arena show jumping, and cross-country jumping. With pride, she reported that at the end of 2007, they were ranked number 8 in the country. “This horse had never been in an arena or jumped before. From there to the national championships, and to end the year ranked 8th, he’s my baby!”
—Terry Taylor, M.A.
Breast Cancer Association Consortium Holds Semiannual Meeting

Participants in the Breast Cancer Association Consortium meeting.
Asemiannual meeting of the Breast Cancer Association Consortium (BCAC) was held in January at the Center for Research in Environmental Epidemiology in Barcelona. The primary aim of the BCAC is to identify genetic markers associated with breast cancer risk.
Organized by Montserrat García-Closas, M.D., Dr.P.H., a senior investigator in the Hormonal and Reproductive Epidemiology Branch (HREB), the meeting focused on efforts to follow up on the associations between breast cancer risk and candidate genetic loci identified through genome-wide association studies. The BCAC includes more than 35 studies from around the world, and the meeting was attended by more than 67 researchers from 15 countries. Other participants from DCEG included Mark E. Sherman, M.D., a pathologist in HREB, Rose Yang, Ph.D., M.P.H., a tenure-track investigator in the Genetic Epidemiology Branch (GEB), and Kelly Bolton (HREB) and Elizabeth Azzato, M.P.H. (GEB), from the NIH/Oxford/Cambridge Scholars Program.
Participants discussed projects aimed at identifying genetic and environmental risk factors by tumor subtypes. Dr. García-Closas presented findings from a pooled analysis of more than 7,000 cases from 10 studies designed to identify novel breast cancer susceptibility loci by molecular subtypes of breast cancer. These data were presented at the 2008 AACR Annual Meeting in San Diego.
To learn more about the BCAC, visit www.srl.cam.ac.uk/consortia/bcac.
DCEG ANNUAL TOWN MEETING

Outstanding Research Paper by a Fellow Awards: Farin Kamangar and Jiyoung Ahn with Joseph Fraumeni
and John Niederhuber. (Not shown: Anil Chaturvedi and Unhee Lim)

Shelia Zahm opening the town meeting with a
year-in-review slideshow.

Exemplary Service Award: Sholom Wacholder with
Joseph Fraumeni and John Niederhuber.

Mentoring Award: Alice Sigurdson with Joseph
Fraumeni and John Niederhuber.

Mentoring Award: Philip Taylor with Joseph
Fraumeni and John Niederhuber.

Outstanding Research Paper by a Staff Scientist
Award: Ruth Kleinerman with Joseph Fraumeni and
John Niederhuber.
This April, DCEG held its annual town meeting to recognize the accomplishments of Division members over the past year. Joseph F. Fraumeni, Jr., M.D., DCEG Director, welcomed the staff and introduced John E. Niederhuber, M.D., NCI Director and returning featured speaker. Shelia Hoar Zahm, Sc.D., DCEG Deputy Director, opened the meeting with a year-in-review slideshow highlighting significant scientific accomplishments; outside recognition received by DCEG members; new tenured and tenure-track investigators; new Branch and Office Chiefs; creation of the Laboratory of Translational Genomics; fellowship programs; completed doctoral dissertations; the Visiting Scholars Program and Distinguished Lecturer events; international workshops to shape future research directions; and interactions with the media, Congress, and the public.
In his presentation, Dr. Niederhuber shared his perspectives on the national cancer program, including recognition of Dr. Fraumeni’s leadership and the Division’s contributions to cancer epidemiology and genetics. Dr. Niederhuber described many of the Division’s groundbreaking findings in genomics as well as exciting new opportunities in research on the tumor microenvironment and imaging, insights from physicists and others that may contribute to new conceptual approaches to complex problems of biology, and the importance of maintaining a vibrant intramural research program. He predicted that rapidly advancing technologies will transform the conduct of cancer research by providing new opportunities for prevention, diagnosis, and highly personalized therapies.
Dr. Zahm then led the annual awards ceremony recognizing scientific contributions and outstanding service during the past year. DCEG was commended for its extraordinary support of volunteerism and generosity through the Combined Federal Campaign (CFC), which benefits nonprofit charities in the local area and around the world. In 2007, the Division received its 10th consecutive CFC Presidential Award for meeting 107% of its participation goal and 121% of its dollar goal. The division’s coordinator for the CFC, Elyse Wiszneauckas, Office of Division Operations and Analysis (ODOA), and Branch keyworkers Cherise Banks, Nutritional Epidemiology Branch (NEB), Patricia Chandler (ODOA), David Check, Biostatistics Branch (BB), Jennifer Connor, formerly Hormonal and Reproductive Epidemiology Branch (HREB), Sadie Holmes-Lillie, Genetic Epidemiology Branch (GEB), An-tsun (Angela) Huang, Ph.D., Occupational and Environmental Epidemiology Branch (OEEB), Jenna Nober, Radiation Epidemiology Branch (REB), and Rashida Williams, DCEG Administrative Resource Center, were recognized for their efforts on the successful campaign.
Four papers published in 2007 were selected for Outstanding Research Paper by a Fellow Awards. The competition was judged by the Division’s Senior Advisory Group based on each paper’s impact, innovation, and clarity of thought and language. Awards were presented to Jiyoung Ahn, Ph.D. (NEB), for her paper on “Adiposity, adult weight change, and postmenopausal breast cancer risk,” published in the Archives of Internal Medicine; Anil K. Chaturvedi, Ph.D., Infections and Immunoepidemiology Branch (IIB), for his paper on “Second cancers among survivors of cervical cancer: Evaluation of long-term risk,” published in the Journal of the National Cancer Institute; Farin Kamangar, M.D., Ph.D. (NEB), for his paper on “Helicobacter pylori and esophageal and gastric cancers in a prospective study in China,” published in the British Journal of Cancer; and Unhee Lim, Ph.D. (NEB), for her paper on “Genomic methylation of leukocyte DNA in relation to colorectal adenoma among asymptomatic women,” which was published in Gastroenterology.
The award for Outstanding Research Paper by a Staff Scientist or Staff Clinician was given to Ruth A. Kleinerman, M.P.H. (REB), for her paper titled “Risk of soft tissue sarcomas by individual subtype in survivors of hereditary retinoblastoma,” which was published in the Journal of the National Cancer Institute.
Fellowship Achievement Awards honor fellows who have excelled during their time at DCEG, and provide a two-step stipend increase at their next appointment renewal. Awards were given to Michael B. Cook, Ph.D. (HREB), Qizhai (James) Li, Ph.D. (BB), Steven C. Moore, Ph.D. (NEB), and Evgenia Ostroumova, M.D., Ph.D. (REB).
DCEG Special Appreciation and Recognition Awards were given to Michael B. Beerman, Core Genotyping Facility (CGF), for his outstanding work on the laboratory information management system that facilitates high-throughput genotyping; William (Bill) Branson, NIH Office of the Director, for his talented photography at the DCEG town meetings and other events; Catherine B. McClave, M.S., Chief of the Office of Communications and Special Initiatives (OCSI), for her analysis, scientific planning, writing, and management of critical operations within the DCEG Office of the Director and across the Division; Marianne Rivera-Silva (CGF) for her superb project management and work on the Data Access Committee; Timothy Sheehy, SAIC-Frederick, for his contributions to improving operations at the DNA Extraction Laboratory; and Dr. Deborah Winn, NCI Division of Cancer Control and Population Sciences, for her vision and collaborative efforts coordinating extramural-intramural partnerships.
DCEG Special Achievement Awards were given to outgoing Branch Chiefs Mitchell H. Gail, M.D., Ph.D. (BB), and James J. Goedert, M.D. (IIB), in recognition of their scientific leadership and service to BB and the former Viral Epidemiology Branch, respectively.
Alice J. Sigurdson, Ph.D. (REB), and Philip R. Taylor, M.D., Sc.D. (GEB), received DCEG Outstanding Mentor Awards in recognition of their exceptional commitment to the growth and productivity of junior scientists. Dr. Sigurdson was described as having a genuine enthusiasm for research that excites fellows and others who work with her, tirelessly coordinating research teams and making resources available so fellows can succeed, and preparing fellows for the next step in their careers. Dr. Taylor was commended for providing practical and impartial advice, encouragement, guidance on obtaining funding, and opportunities for hands-on experiences in the Division and in the field.
The 2008 DCEG Exemplary Service Award was given to Sholom Wacholder, Ph.D. (BB), for his sustained research accomplishments and outstanding service to NCI. Dr. Wacholder plays a key role across DCEG, collaborating and consulting on many important studies, including the Breast and Prostate Cancer Cohort Consortium, the InterLymph consortium of case-control studies of non-Hodgkin lymphoma, and the Cancer Genetic Markers of Susceptibility project. In addition, he plays a critical role in the success of the DCEG research program on human papillomavirus (HPV) and serves as the lead statistician on the data safety monitoring board for the HPV Vaccine Trial. He has been an international leader in the development of biostatistical methods for epidemiologic research, including his recent false-positive report probability approach, and he excels at mentoring and training junior investigators.
The 2007 winners of DCEG Intramural Research Awards, which were announced earlier this year, also received plaques at the town meeting.
The event was coordinated by Samantha Nhan (OCSI) with assistance from Phyllis Nimeroff (OEEB).
FRENCH DELEGATION VISITS DIVISION

DCEG-French delegation meeting participants.
In January, DCEG welcomed a delegation of 12 senior French scientists led by Professor Dominique Maraninchi, President of the Board of Directors at the Institut National du Cancer (INCa), and Professor Fabien Calvo, Director of Scientific Programs at INCa.
During their two-day visit, the French delegation met with senior leadership from NCI and across NIH to explore opportunities for collaborative projects between the institutions and to learn how to develop and maintain large-scale epidemiology studies. The French agency plans to launch new initiatives, including biological sample collections linked to high-quality epidemiologic studies with application of genomic technologies. In particular, INCa seeks to establish new epidemiologic directions using genome-wide scans, RNA profiling, high-throughput sequencing, and other molecular techniques to investigate cancer etiology and outcomes. Spearheaded by Gilles F. Thomas, M.D., Ph.D., a senior scientist in the Laboratory of Translational Genomics (LTG) and co-leader of the Cancer Genetic Markers of Susceptibility (CGEMS) initiative, the meeting with DCEG scientists set the stage for cutting-edge collaborative studies between NCI and INCa.
Joseph F. Fraumeni, Jr., M.D., Division Director, opened a two-hour session at DCEG by welcoming the French delegation and providing an overview of activities, with a special focus on research into genetic susceptibility. Following his introduction, Robert N. Hoover, M.D., Sc.D., Director of the Epidemiology and Biostatistics Program, and Margaret A. Tucker, M.D., Director of the Human Genetics Program and Chief of the Genetic Epidemiology Branch, gave an overview of the Division’s organizational structure, mission, and accomplishments. Stephen J. Chanock, M.D., Director of the Core Genotyping Facility and Chief of LTG, chaired a roundtable to discuss the design and conduct of molecular epidemiology studies in cancer, with a focus on genome-wide association studies.
After the meeting, the French scientists
and their American hosts gathered for a
reception at the residence of the French
Ambassador in Washington, DC.
 Chinese Delegation Visits DCEG: (front) Joseph Fraumeni and Li Zhu; (back) Hao Ling, Martha Linet,
Li Song, Terry Dwyer, Li Zhiwen, and Zhu Jianghui.
Chinese Delegation Visits DCEG: (front) Joseph Fraumeni and Li Zhu; (back) Hao Ling, Martha Linet,
Li Song, Terry Dwyer, Li Zhiwen, and Zhu Jianghui.
NEW TEAM OF RADIATION RESEARCHERS

Amy Berrington de Gonzalez and Lindsay Morton
The Radiation Epidemiology Branch (REB) welcomed two new tenure-track investigators in February: one new to NCI and the other a former research fellow in DCEG.
Amy Berrington de Gonzalez, D.Phil., grew up in Oxford, England. After earning degrees in mathematics and applied statistics, she worked as a biostatistician for a British pharmaceutical company. While considering whether to apply to a Ph.D. program, she met statistician Sir David Cox, renowned for the Cox proportional hazards model, at a meeting. Their conversation resulted in a visit to the Cancer Epidemiology Unit at the University of Oxford, where Dr. Berrington de Gonzalez ultimately earned her doctoral degree and conducted postdoctoral research. She joined the Johns Hopkins Bloomberg School of Public Health in 2005 before coming to REB.
Dr. Berrington de Gonzalez studies the risks of second cancers from medical radiation exposures, both from treatment and from diagnostic imaging, including computed tomography (CT) scanning. “CT scans are the most rapidly increasing source of radiation exposure to the general population, and there’s growing concern that there may be overuse, particularly here in the United States,” she said.
Dr. Berrington de Gonzalez’s interest in radiation may be innate: her mother is a nuclear physicist-turned-biostatistician (they have attended meetings together), and her grandfather designed nuclear power plants. One of his prized possessions was an unused nuclear fuel rod casing, which he kept in his garage.
She is thrilled to be at NCI. “As a scientist, you do a certain amount of work on your own, but I like interacting with people interested in the same subject. And there’s more expertise in my field here than anywhere else.”
Dr. Berrington de Gonzalez lives in Bethesda with her husband and their toddler son.
Lindsay M. Morton, Ph.D., joined DCEG’s Hormonal and Reproductive Epidemiology Branch as a postdoctoral fellow in 2004, became a research fellow in 2005, and was promoted to tenure-track investigator in 2008.
Dr. Morton grew up outside Chicago. At Dartmouth College, she became interested in epidemiology, though she didn’t realize it at first. She was doing research with Dr. Thomas B. Roos, a Dartmouth biology professor, and discussed career possibilities with him. “I said I wasn’t sure what I wanted to do. I knew that I loved research, biology, math, and medicine, but I didn’t want to be a biology or math professor or a physician, and he said, ‘Have you ever heard of epidemiology? Because you just defined it!’”
Still, for Dr. Morton’s senior honors thesis, she flew to New Zealand to study the mating behavior of dusky dolphins. Part of her felt she should be delving into epidemiology research, but Dr. Roos gave her some sage advice: “He said that if I knew I wanted to do epidemiology, I’d be doing that for many years, and I should go study dolphins now.”
At Yale, where Dr. Morton earned her Ph.D., she started working on the epidemiology of non-Hodgkin lymphoma and never looked back. “I’m intrigued by the complex biology of cancer, and I love immunology,” she said. Dr. Morton is interested in the etiology of the different lymphoma subtypes. “There’s compelling evidence that some risk factors are the same, but some are different,” she said. “I also want to find out whether our current definitions of subtypes are relevant in terms of cancer etiology. For example, HIV infection increases the risk for both diffuse large B-cell lymphoma and Burkitt lymphoma. So should these cancers be grouped together by some common characteristic? We don’t know.”
In her new role in REB, Dr. Morton is also studying risk factors, including radiotherapy and chemotherapy, for second cancers, as well as shared risk factors for multiple primary malignancies. She has a lead role in an international, multicenter study whose goal is to provide new information on the dose-response relationship of radiotherapy and chemotherapy to second gastrointestinal cancers.
Dr. Morton lives in Washington, DC, with her husband and two-year-old daughter. When not in the office, she enjoys hiking, playing outside as much as possible, and visiting the Smithsonian museums with her family.
—Nancy Volkers
DCEG MAKES SCIENTIFIC IMPACT AT AACR

Robert Hoover presenting AACR-ACS
Award Lecture
for Research
Excellence in Cancer Epidemiology and
Prevention. (Photograph Credit:
©©2008 AACR/Todd
Buchanan)

Jasmine Lew speaking on alcohol
and breast cancer
risk.
(Photograph Credit: ©2008 AACR/
Todd Buchanan)
In April, DCEG members participated in the 2008 Annual Meeting of the American Association for Cancer Research (AACR) in San Diego.
Robert N. Hoover, M.D., Sc.D., Director of the Epidemiology and Biostatistics Program, presented the 17th Annual AACR-American Cancer Society (ACS) Award Lecture for Research Excellence in Cancer Epidemiology and Prevention. He received the award for his pioneering research in identifying environmental and genetic determinants of cancer, most notably bladder and breast cancer. AACR and ACS established the award in 1992 to honor outstanding research accomplishments in the fields of cancer epidemiology, biomarkers, and prevention.
In his presentation, titled “Breast cancer: Epidemiologic insights into etiologic mechanisms,” Dr. Hoover described the decades-long history of research into the etiologic role of hormones in breast cancer, highlighting the impact of population-based studies in elucidating the endocrine-related risk factors associated with this disease.
Tram Kim Lam, Ph.D., Genetic Epidemiology Branch (GEB), and Quan Lan (Jasmine) Lew, M.S., Nutritional Epidemiology Branch, represented the Division in a minisymposium on dietary patterns and cancer risk. Dr. Lam presented her research on the association between meat consumption and lung cancer from the Environment and Genetics in Lung Cancer Etiology (EAGLE) Study. Ms. Lew, a fourth-year medical student and Howard Hughes Medical Institute-NIH Research Scholar, showed that among post-menopausal women in the NIH-AARP Diet and Health Study, consuming even modest amounts of alcohol increased the risk of estrogen and progesterone receptor–positive breast tumors. Her work was also highlighted during a press conference at the meeting.
Dr. Hoover described the decades-long history of research into the etiologic role of hormones in breast cancer, highlighting the impact of population-based studies in elucidating the endocrine-related risk factors associated with this disease.
Montserrat García-Closas, M.D., Dr.P.H., Hormonal and Reproductive Epidemiology Branch, and Sonja I. Berndt, Pharm.D., Ph.D., Occupational and Environmental Epidemiology Branch (OEEB), presented their work on cancer susceptibility loci for breast and colorectal cancers during a special session on genetics and genomics.
A number of DCEG staff participated in poster sessions over the course of the five-day meeting. Three fellows were recognized for their meritorious abstracts: H. Dean Hosgood, III, Ph.D. (OEEB), Jill Koshiol, Ph.D. (GEB), and Kyoung-Mu Lee, Ph.D. (OEEB), received AACR Scholar-in-Training Awards, which are travel grants intended to enhance the education and training of young scientists. Other DCEG senior investigators served as featured speakers or session moderators, including Stephen J. Chanock, M.D., Director of the Core Genotyping Facility and Chief of the Laboratory of Translational Genomics, Charles S. Rabkin, M.D., Infections and Immunoepidemiology Branch, and Nathaniel Rothman, M.D., M.P.H., M.H.S. (OEEB).
—Alyssa Minutillo, M.P.H.
Scientific Highlights
ALL-CAUSE MORTALITY
Effects of Physical Activity
Whether physical activity at recommended levels was related to decreased mortality was examined among 252,925 women and men aged 50 to 71 years in the NIH-AARP Diet and Health Study. Compared with inactivity, moderate activity (at least 30 minutes on most days of the week) or vigorous exercise (at least 20 minutes, three times/week) was associated with a 27% and 32% decreased mortality risk, respectively. Physical activity that met both recommendations was related to substantially decreased mortality overall and among smokers (relative risk [RR] = 0.48) and nonsmokers (RR = 0.54), normal weight (RR = 0.45) and overweight or obese individuals (RR = 0.48), and those with up to two hours/day (RR = 0.53) and more than two hours/day of television or video watching (RR = 0.50). Engaging in some physical activity but less than the recommended level was also related to reduced mortality (RR = 0.81). (Leitzmann MF, Park Y, Blair A, Ballard-Barbash R, Mouw T, Hollenbeck AR, Schatzkin A. Physical activity recommendations and decreased risk of mortality. Arch Intern Med 2007;167:2453–2460)
Mediterranean Diet in a U.S. Population
Within the NIH-AARP study, the Mediterranean dietary pattern was evaluated in relation to mortality among 214,284 male and 166,012 female participants. During follow-up (1995–2005), 27,799 deaths were documented. A nine-point scoring was used to assess conformity with the Mediterranean dietary pattern, based on intake of vegetables, legumes, fruits, nuts, whole grains, fish, alcohol, and meat, as well as the ratio of monounsaturated fat to saturated fat. The Mediterranean diet was associated with reduced all-cause and cause-specific mortality. In men, the multivariate hazard ratios (HRs) comparing high to low conformity for all-cause, cardiovascular disease, and cancer mortality were 0.79, 0.78, and 0.83, respectively. In women, these HRs were 0.80, 0.81, and 0.88, respectively. Among never smokers, associations with all-cause mortality persisted. (Mitrou PN, Kipnis V, Thiebaut AC, Reedy J, Subar AF, Wirfalt E, Flood A, Mouw T, Hollenbeck AR, Leitzmann MF, Schatzkin A. Mediterranean dietary pattern and prediction of all-cause mortality in a U.S. population: Results from the NIH-AARP Diet and Health Study. Arch Intern Med 2007;167:2461–2468)
BILIARY TRACT CANCER
Serum Lipid Levels
The associations of serum lipid levels with biliary stones and cancers were examined in a population-based case-control study conducted in Shanghai, where incidence rates of these conditions are rising sharply. The study included 460 biliary cancer patients (264 gallbladder, 141 extrahepatic bile duct, and 55 ampulla of Vater), 981 biliary stone patients, and 858 healthy controls. Participants completed an in-person interview and gave overnight fasting blood samples. Participants in the highest quintile of triglycerides had a 1.4-fold risk of biliary stones, a 1.9-fold risk of gallbladder cancer, and a 4.8-fold risk of bile duct cancer, compared to a reference group. Participants in the lowest quintile of high-density lipoprotein had a 4.2-fold risk of biliary stones, an 11.6-fold risk of gallbladder cancer, and a 16.8-fold risk of bile duct cancer, relative to a reference group. Total cholesterol, low-density lipoprotein (LDL), and apolipoprotein (apo) A were inversely associated with biliary stones, whereas low and high levels of total cholesterol, LDL, apo A, and apo B were associated with excess risks of biliary tract cancers. Findings support a role for serum lipids in gallstone development and biliary carcinogenesis. (Andreotti G, Chen J, Gao YT, Rashid A, Chang SC, Shen MC, Wang BS, Han TQ, Zhang BH, Danforth KN, Althuis MD, Hsing AW. Serum lipid levels and the risk of biliary tract cancers and biliary stones: A population-based study in China. Int J Cancer 2008;122:2322–2329)
Lipid Metabolism Genes
Associations of 12 single nucleotide
polymorphisms (SNPs) of five genes
in the lipid metabolism pathway with
the risks of biliary cancers and stones
were examined in the Shanghai case-control
study. Men carrying the G allele
of APOE IVS1+69 had a 1.7-fold risk
of stones, a 1.8-fold risk of gallbladder
cancer, a 3.7-fold risk of bile duct cancer,
and a 4-fold risk of ampullary cancer.
Male carriers of the T allele of APOB IVS6+360C![]() T had a 2-fold risk of
bile duct cancer. The APOB T-T haplotype
(APOB IVS6+360C
T had a 2-fold risk of
bile duct cancer. The APOB T-T haplotype
(APOB IVS6+360C![]() T, EX4+
56C
T, EX4+
56C![]() T) was associated with a 1.6-fold
risk of bile duct cancer, and male and
female carriers of the T allele of LDLR IVS9-30C
T) was associated with a 1.6-fold
risk of bile duct cancer, and male and
female carriers of the T allele of LDLR IVS9-30C![]() T (rs1003723) had a 1.5-fold
risk of bile duct cancer. Findings suggest
that gene variants in the lipid metabolism
pathway contribute to the risk of
biliary tract stones and cancers, particularly
of the bile duct. (Andreotti G, Chen J,
Gao YT, Rashid A, Chen BE, Rosenberg P, Sakoda
LC, Deng J, Shen MC, Wang BS, Han TQ, Zhang
BH, Yeager M, Welch R, Chanock S, Fraumeni
JF Jr, Hsing AW. Polymorphisms of genes in
the lipid metabolism pathway and risk of biliary
tract cancers and stones: A population-based
case-control study in Shanghai, China. Cancer
Epidemiol Biomarkers Prev 2008;17:525–534)
T (rs1003723) had a 1.5-fold
risk of bile duct cancer. Findings suggest
that gene variants in the lipid metabolism
pathway contribute to the risk of
biliary tract stones and cancers, particularly
of the bile duct. (Andreotti G, Chen J,
Gao YT, Rashid A, Chen BE, Rosenberg P, Sakoda
LC, Deng J, Shen MC, Wang BS, Han TQ, Zhang
BH, Yeager M, Welch R, Chanock S, Fraumeni
JF Jr, Hsing AW. Polymorphisms of genes in
the lipid metabolism pathway and risk of biliary
tract cancers and stones: A population-based
case-control study in Shanghai, China. Cancer
Epidemiol Biomarkers Prev 2008;17:525–534)
BLADDER CANCER
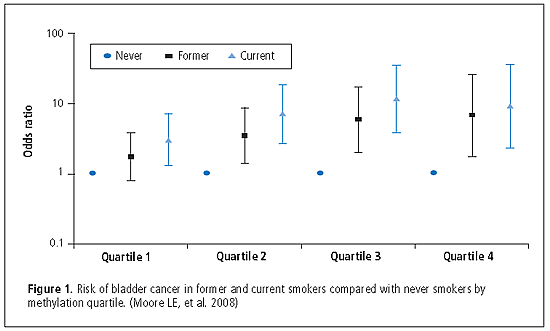
DNA Hypomethylation
To assess whether DNA hypomethylation was associated with increased risk of bladder cancer, cytosine methylation (5-mC) content in genomic DNA from participants enrolled in a large case-control study in Spain between 1998 and 2001 was measured. Relative 5-mC content was expressed as a percentage (%5-mC) with respect to the sum of methylated and non-methylated cytosines, as measured in leukocyte DNA available from 775 cases and 397 controls. Median %5-mC DNA was significantly lower in cases (3.03%) than in controls (3.19%; p = 0.0002). The odds ratios (ORs) for decreasing methylation quartiles were 2.05 for Q3, 1.62 for Q2, and 2.67 for Q1 compared with Q4 (p < 0.0001). By comparison with never smokers in the highest methylation quartile, current smokers in the lowest methylation quartile had the highest risk of bladder cancer (Q1: OR = 25.51; p for interaction = 0.06). In analyses stratified by smoking, hypomethylation was a strong risk factor in never smokers (OR = 6.39). Amount of methylation in controls was not associated with baseline characteristics, micronutrients, or selected genotypes in folate metabolism pathways. (see Figure 1) (Moore LE, Pfeiffer RM, Poscablo C, Real FX, Kogevinas M, Silverman D, García-Closas R, Chanock S, Tardón A, Serra C, Carrato A, Dosemeci M, García-Closas M, Esteller M, Fraga M, Rothman N, Malats N. Genomic DNA hypomethylation as a biomarker for bladder cancer susceptibility in the Spanish Bladder Cancer Study: A case-control study. Lancet Oncol 2008;9:359–366)
Fluid Consumption and Disinfection Byproducts
In an analysis of 397 cases and 664 controls within the Spanish Bladder Cancer Study, the association between total fluid and water consumption and bladder cancer risk, and the interaction between water intake and exposure to trihalomethane (THM), a byproduct of water disinfection, were examined. Total fluid intake was associated with a significant decrease in bladder cancer risk (OR = 0.62 for highest vs. lowest quintile comparison). A significant inverse association was observed for water intake (for > 1,399 vs. < 400 ml/ day, OR = 0.47; p for trend < 0.0001), but not for other individual beverages. The inverse association between water intake and bladder cancer persisted within each level of THM exposure. (Michaud DS, Kogevinas M, Cantor KP, Villanueva CM, García-Closas M, Rothman N, Malats N, Real FX, Serra C, García-Closas R, Tardón A, Carrato A, Dosemeci M, Silverman DT. Total fluid and water consumption and the joint effect of exposure to disinfection by-products on risk of bladder cancer. Environ Health Perspect 2007;115:1569–1572)
BRAIN CANCER
Pesticide Exposure
The risk of brain cancer associated with occupational exposure to insecticides and herbicides was evaluated in a case-control study of 462 glioma and 195 meningioma patients diagnosed between 1994 and 1998 in three U.S. hospitals and 765 controls admitted to the same hospitals for nonmalignant conditions. Exposure to pesticides was estimated from questionnaire data on occupation, combined with pesticide measurement data abstracted from published sources. There was no association between glioma and insecticides or herbicides among men or women. Women who reported ever using herbicides had a significantly increased risk for meningioma compared with nonusers (OR = 2.4), and risk increased with years of herbicide exposure and cumulative exposure. There was no association between meningioma and herbicide or insecticide exposure among men. (Samanic CM, De Roos AJ, Stewart PA, Rajaraman P, Waters MA, Inskip PD. Occupational exposure to pesticides and risk of adult brain tumors. Am J Epidemiol 2008;167:976–985)
BREAST CANCER
Adult Physical Activity
Data on physical activity patterns for 2,176 incident breast cancer cases and 2,326 controls in a population-based study conducted in Poland between 2000 and 2003 were analyzed. Total adult lifetime activity significantly reduced risk of breast cancer; individuals in the highest quartile had an OR of 0.80 compared with the lowest quartile. Reduced risks were most consistent for the highest quartiles of moderate-to-vigorous activities: moderate/vigorous recreational activities (OR = 0.74), outdoor activities (OR = 0.81), heavy physical work (OR = 0.60), and combined high-intensity (metabolic equivalent > 6.0) activities (OR = 0.75). Reductions in risk with moderate/vigorous recreational activities were stronger for larger tumors and those with nodal involvement. Women who increased their recreational activity in their 50s had significantly reduced risk. Women in the highest tertile of change showed a 27% lower risk. (Peplonska B, Lissowska J, Hartman TJ, Szeszenia-Dabrowska N, Blair A, Zatonski W, Sherman ME, García-Closas M, Brinton LA. Adulthood lifetime physical activity and breast cancer. Epidemiology 2008;19:226–236)
Hormonal Markers
The investigators evaluated the expression patterns of 17 hormone-related markers by immunohistochemistry and their relationships with pathologic characteristics and epidemiologic risk factors among 842 breast carcinoma patients in the Polish case-control study. Based on marker correlations, factor analysis identified four major coexpression patterns (factors): nuclear receptor, estrogen metabolism/ER-β, HER2, and proliferation (see Figure 2). Three of these factors corresponded to molecular subtypes previously defined by expression profiling; however, the estrogen metabolism/ER-β factor seemed distinctive. High scores for this factor were significantly associated with high tumor grade, younger age at menarche, lower current body mass index (BMI) among premenopausal women, and older age at menopause. High scores for the proliferation factor were also significantly associated with early menarche and, in contrast to the estrogen metabolism/ER-β factor, with higher current BMI among premenopausal women. The analysis confirmed several molecular subtypes and suggested additional relationships for ER-β and enzymes involved in hormone metabolism. (Yang XR, Pfeiffer RM, García-Closas M, Rimm DL, Lissowska J, Brinton LA, Peplonska B, Hewitt SM, Cartun RW, Mandich D, Sasano H, Evans DB, Sutter TR, Sherman ME. Hormonal markers in breast cancer: Coexpression, relationship with pathologic characteristics, and risk factor associations in a population-based study. Cancer Res 2007;67:10608–10617)
CERVICAL CANCER
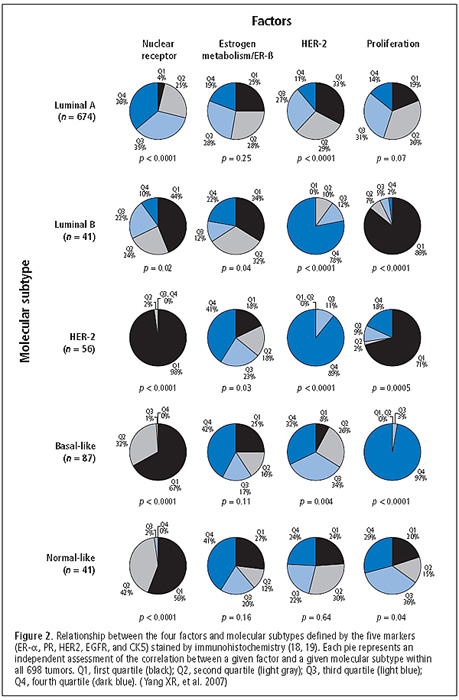
Human Papillomavirus (HPV) Clearance and Persistent Infection
To better understand the natural history of cervical HPV infections, researchers investigated the outcomes of 800 carcinogenic HPV infections detected among 599 women at enrollment in a population-based cohort in Guanacaste, Costa Rica. The proportions of three outcomes (viral clearance, persistence without cervical intraepithelial neoplasia grade 2 or worse [CIN2+], or persistence with new diagnosis of CIN2+) were calculated at successive 6-month time points for the first 30 months of follow-up (see Figure 3). Infections typically cleared rapidly—67% cleared by 12 months. However, among infections that persisted for at least 12 months, the risk of CIN2+ by 30 months was 21%. The risk of CIN2+ was highest among women under 30 years old who had HPV-16 infections that persisted for at least 12 months (53%). Findings suggest that the medical community should emphasize persistence of cervical HPV infection, not single-time detection of HPV, in management strategies and health messages. (Rodriguez AC, Schiffman M, Herrero R, Wacholder S, Hildesheim A, Castle PE, Solomon D, Burk R, Proyecto Epidemiologico Guanacaste Group. Rapid clearance of human papillomavirus and implications for clinical focus on persistent infections. J Natl Cancer Inst 2008;100:513–517)
Novel Methylation Markers
Aberrant methylation of CpG islands, genomic regions that contain a high frequency of cytosine/guanine (CG) dinucleotides, in gene promoters often represents an early clonal event in carcinogenesis. Defining methylation profiles may be useful for developing marker panels for early detection or predicting cancer risk. Methylation profiling of 20 cervical cancers was conducted using NotI-based restriction landmark genomic scanning (RLGS). Of 2,172 RLGS fragments analyzed (average = 1,753 CpG islands per patient), 186 RLGS fragments were lost in at least one tumor, and 40 were lost in three or more. Methylation was identified in 19 of 20 tumor samples compared with normal DNA. Bisulfite sequencing was conducted to confirm RLGS results. Among the confirmed markers frequently methylated were two corresponding genes, nucleolar protein 4 (NOL4), and lipoma HMGIC fusion partner–like protein 4 (LHFPL4), which were methylated in 85% and 55% of cancers, respectively. Using Methylight assays, the authors further confirmed frequent CpG island methylation in the original cancers and in another independent series of 15 cervical cancers. Methylation was reduced in a set of carefully reviewed cytology specimens, demonstrating cells exfoliated from cancer precursor lesions. Thus, NOL4 and LHFPL4 were identified for the first time as novel methylation targets specific for cervical cancer and should be included in methylation panels being evaluated for early detection, risk prediction, and etiologic research on cervical cancer. (Wang SS, Smiraglia DJ, Wu YZ, Ghosh S, Rader JS, Cho KR, Bonfiglio TA, Nayar R, Plass C, Sherman ME. Identification of novel methylation markers in cervical cancer using restriction landmark genomic scanning. Cancer Res 2008;68:2489–2497)
DIETHYLSTILBESTROL
Offspring of Exposed Women
In a study examining risks for benign or malignant pathology in offspring of prenatal diethylstilbestrol (DES)–exposed women, data from mothers’ reports of cancers in 8,216 sons and daughters and pathology-confirmed cancers and benign diagnoses self-reported by a subset of 793 daughters were analyzed. Although statistical power is limited, data are consistent with no overall increased cancer risk in the offspring. Based on pathology-confirmed diagnoses reported by the daughters, no association between DES and benign breast disease or reproductive tract conditions was observed. Based on three cases, the incidence of ovarian cancer was higher than expected in the daughters. Although it is preliminary, this finding supports continued monitoring of daughters of women who were exposed to DES in utero. (Titus-Ernstoff L, Troisi R, Hatch EE, Hyer M, Wise LA, Palmer JR, Kaufman R, Adam E, Noller K, Herbst AL, Strohsnitter W, Cole BF, Hartge P, Hoover RN. Offspring of women exposed in utero to diethylstilbestrol: A preliminary report of benign and malignant pathology in the third generation. Epidemiology 2008;19:251–257)
LUNG CANCER
Gene Expression Signature
To identify genes that are altered by smoking and that have a role in cancer development and survival, gene expression analysis was performed on 135 fresh-frozen tissue samples of adenocarcinoma and paired uninvolved lung tissue from current, former, and never smokers. A gene expression signature characteristic of smoking that includes cell-cycle genes, particularly those involved in the mitotic spindle formation (e.g., NEK2, TTK, PRC1) was identified. Expression of these genes strongly differentiated smokers from nonsmokers in lung tumors and early-stage tumor tissue from non-tumor tissue (p < 0.001 and fold-change > 1.5 for each comparison), consistent with a role for this pathway in smoking-induced lung carcinogenesis. These changes persisted many years after smoking cessation. NEK2 and TTK expression in the uninvolved lung tissue was also associated with a 3-fold increased risk of mortality from lung adenocarcinoma in smokers. This work provides insight into the smoking-related mechanisms of lung neoplasia. These genes are candidate targets for chemoprevention and treatment of lung cancer in smokers. (Landi MT, Dracheva T, Rotunno M, Figueroa JD, Liu H, Dasgupta A, Mann FE, Fukuoka J, Hames M, Bergen AW, Murphy SE, Yang P, Pesatori AC, Consonni D, Bertazzi PA, Wacholder S, Shih JH, Caporaso NE, Jen J. Gene expression signature of cigarette smoking and its role in lung adenocarcinoma development and survival. PLoS ONE 2008;3:e1651)
LYMPHOMA
One-carbon Metabolism Nutrients
The associations between nutrients related to one-carbon metabolism and risk of non-Hodgkin lymphoma (NHL) were examined in a population-based case-control study of Connecticut women diagnosed between 1996 and 2000. A total of 594 cases and 710 controls completed a food frequency questionnaire, which was used to determine their intakes of folate; vitamins B2, B6, and B12; and methionine. Comparing the highest quartile of intake with the lowest, lower risks of all NHL were associated with increasing intakes of folate and methionine. Analysis by NHL subtype indicated significantly lower risks of diffuse large B-cell lymphoma (highest vs. lowest quartile: OR = 0.54, p for trend = 0.02) and marginal zone lymphoma (highest vs. lowest quartile: OR = 0.08; p for trend < 0.0001) associated with folate intake. Vitamin B6 intake was also associated with lower risk of NHL overall (p for trend = 0.03) and of marginal zone lymphoma (highest vs. lowest quartile: OR = 0.23; p for trend = 0.002). Findings suggest that these nutrients may be important in susceptibility to NHL. (Koutros S, Zhang Y, Zhu Y, Mayne ST, Zahm SH, Holford TR, Leaderer BP, Boyle P, Zheng T. Nutrients contributing to one-carbon metabolism and risk of non-Hodgkin lymphoma subtypes. Am J Epidemiol 2008;167:287–294)
MELANOMA
CDKN2A Mutations in Iceland
Because germline CDKN2A mutations have been observed in 20% to 40% of families prone to melanoma, researchers resequenced the gene, including the p14ARF variant and promoter regions, in approximately 700 registry-ascertained melanoma cases and 690 population-based controls from Iceland, where the incidence of melanoma has increased rapidly. A novel germline variant, G89D, was identified, which was strongly associated with increased melanoma risk and appeared to be an Icelandic founder mutation. The G89D variant was present in about 2% of Icelandic invasive cutaneous malignant melanoma cases. Relatives of affected G89D carriers were at significantly increased risk of melanoma, head and neck cancers, and pancreatic carcinoma compared to relatives of other melanoma patients. Nineteen other germline variants were identified, but none conferred an unequivocal risk of melanoma. The frequency of disease-related CDKN2A mutant alleles ranged between 0.7% and 1.0%. In contrast to North America and Australia, where a broad spectrum of mutations was observed, in Iceland, functional CDKN2A mutations consisted of only one or two variants. Additional genetic and/or environmental factors are likely critical for explaining the high incidence rates for melanoma in Iceland. (Goldstein AM, Stacey SN, Olafsson JH, Jonsson GF, Helgason A, Sulem P, Sigurgeirsson B, Benediktsdottir KR, Thorisdottir K, Ragnarsson R, Kjartansson J, Kostic J, Masson G, Kristjansson K, Gulcher JR, Kong A, Thorsteinsdottir U, Rafnar T, Tucker MA, Stefansson K. CDKN2A mutations and melanoma risk in the Icelandic population. J Med Genet 2008;45:284–289)
MULTIPLE MYELOMA
Prior Medical Disorders
The roles of prior autoimmune, infectious, inflammatory, and allergic disorders in the etiology of multiple myeloma (MM) and monoclonal gammopathy of undetermined significance (MGUS) were explored in a cohort of more than 4 million U.S. veterans. Patients were selected from inpatient discharge records at U.S. Department of Veterans Affairs hospitals. The analysis included 4,641 patients (3,040 white, 1,601 black) diagnosed with MM and 2,046 patients (1,312 white, 734 black) with MGUS. MM was significantly associated with broad categories of autoimmune (RR = 1.15), infectious (RR = 1.29), and inflammatory (RR = 1.18) disorders, as well as specific autoimmune (polymyositis/dermatomyositis, systemic sclerosis, autoimmune hemolytic anemia, pernicious anemia, ankylosing spondylitis), infectious (pneumonia, hepatitis, meningitis, septicemia, herpes zoster, poliomyelitis), and inflammatory (glomerulonephritis, nephrotic syndrome, osteoarthritis) conditions. Risks for MGUS were of similar magnitude. Results suggest that immune-mediated conditions may act as triggers for MM/MGUS development. (Brown LM, Gridley G, Check D, Landgren O. Risk of multiple myeloma and monoclonal gammopathy of undetermined significance among white and black male United States veterans with prior autoimmune, infectious, inflammatory, and allergic disorders. Blood 2008;111:3388–3394)
ORAL CANCER
Incidence Trends in HPV-related and -unrelated Cancers
To investigate the impact of HPV on the epidemiology of oral squamous cell carcinomas (OSCCs) in the United States, the researchers assessed differences in patient characteristics, incidence, and survival between HPV-related and -unrelated OSCC sites using data from nine Surveillance, Epidemiology, and End Results program registries. HPV-related OSCCs (n = 17,625) were diagnosed at younger ages than HPV-unrelated OSCCs (n = 28,144; mean ages = 61.0 and 63.8 years, respectively; p < 0.001). Incidence increased significantly for HPV-related OSCC from 1973 to 2004 (annual percentage change [APC] = 0.80), particularly among white men and at younger ages. By contrast, the incidence of HPV-unrelated OSCC did not significantly change through 1982 (APC = 0.82; p = 0.186) and declined from 1983 to 2004 (APC = –1.85; p < 0.001). When patients were treated with radiation, improvements in two-year survival across calendar periods were more pronounced for HPV-related OSCCs (absolute increase in survival from 1973–1982 to 1993–2004 for localized, regional, and distant stages = 9.9%, 23.1%, and 18.6%, respectively) than HPV-unrelated OSCCs (5.6%, 3.1%, and 9.9%). During 1993–2004, for all stages treated with radiation, patients with HPV-related OSCCs had significantly higher survival rates than those with HPV-unrelated OSCCs. The proportion of OSCCs that are potentially HPV-related increased from 1973 to 2004, perhaps due to changing sexual behaviors. Recent improvements in survival with radiotherapy may be due in part to a shift in OSCC etiology. (Chaturvedi AK, Engels EA, Anderson WF, Gillison ML. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol 2008;26:612–619)
PANCREATIC CANCER
Adiposity and Physical Activity
Using data from a self-administered baseline questionnaire (1995–1996), associations between adiposity and physical activity with pancreatic cancer among 495,035 participants in the NIH-AARP Diet and Health Study, aged 50 to 71 years, were investigated. A subcohort (n = 302,060) completed a second questionnaire with information about physical activity and waist and hip circumference. During follow-up though 2000, 654 pancreatic cancer cases were identified. Compared with those with a BMI of 18.5 to almost 25, those with a BMI of 35 or greater had a 45% greater pancreatic cancer risk. Significant positive associations for BMI were observed among nonsmokers (BMI of 35 or greater: HR = 1.70; p for trend = 0.004) but not recent smokers (p for interaction = 0.08). Waist circumference was positively associated with pancreatic cancer (fourth vs. first quartile: HR = 2.53; p for trend = 0.04) in women but not men. There was no association with physical activity. (Stolzenberg-Solomon RZ, Adams K, Leitzmann M, Schairer C, Michaud DS, Hollenbeck A, Schatzkin A, Silverman DT. Adiposity, physical activity, and pancreatic cancer in the National Institutes of Health-AARP Diet and Health Cohort. Am J Epidemiol 2008;167:586–597)
PROSTATE CANCER
Genome-wide Association Study (GWAS)
The authors followed their initial GWAS of 527,869 SNPs in 1,172 individuals with prostate cancer and 1,157 controls from the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer study by testing 26,958 SNPs in four independent studies (3,941 cases and 3,964 controls). In the combined joint analysis, they confirmed three previously reported loci (two independent SNPs at 8q24 and one in HNF1B [formerly known as TCF2 on 17q]; p < 10-10). In addition, loci on chromosomes 7, 10 (two loci), and 11 were highly significant (between p < 7.31 x 10-13 and p < 2.14 x 10-6). Loci on chromosome 10 include MSMB, which encodes β-microseminoprotein, a primary constituent of semen and a proposed prostate cancer biomarker, and CTBP2, a gene with antiapoptotic activity; the locus on chromosome 7 is at JAZF1, a transcriptional repressor that is fused by chromosome translocation to SUZ12 in endometrial cancer. Of the nine loci that showed highly suggestive associations (p < 2.5 x 10-5), four best fit a recessive model and included candidate susceptibility genes CPNE3, IL16, and CDH13. Findings point to multiple loci with moderate effects associated with susceptibility to prostate cancer that, taken together, may predict high risk in select individuals. (Thomas G, Jacobs KB, Yeager M, Kraft P, Wacholder S, Orr N, Yu K, Chatterjee N, Welch R, Hutchinson A, Crenshaw A, Cancel-Tassin G, Staats BJ, Wang Z, Gonzalez-Bosquet J, Fang J, Deng X, Berndt SI, Calle EE, Feigelson HS, Thun MJ, Rodriguez C, Albanes D, Virtamo J, Weinstein S, Schumacher FR, Giovannucci E, Willett WC, Cussenot O, Valeri A, Andriole GL, Crawford ED, Tucker M, Gerhard DS, Fraumeni JF Jr, Hoover R, Hayes RB, Hunter DJ, Chanock SJ. Multiple loci identified in a genome-wide association study of prostate cancer. Nature Genet 2008;40:310–315)
SECOND CANCERS
Mortality and Second Cancers in NHL Survivors
Mortality and second malignant neoplasms (SMNs) were assessed among 1,082 five-year survivors of NHL in the Childhood Cancer Survivor Study, a multi-institutional North American retrospective cohort study of cancer survivors diagnosed between 1970 and 1986. There were 87 deaths (standardized mortality ratio = 4.2) with elevated rates of death from solid tumors, leukemia, cardiac disease, and pneumonia. Risk for death remained elevated more than 20 years after NHL. Risk factors for death from causes other than NHL included female sex (RR = 3.4) and cardiac radiation therapy (RT) exposure (RR = 1.9). There were 27 solid tumor SMNs (standardized incidence ratio = 3.9) with 3% cumulative incidence between 5 and 20 years after NHL diagnosis. Risk factors were female sex (RR = 3.1), mediastinal NHL disease (RR = 5.2), and breast irradiation (RR = 4.3). Survivors of childhood NHL, particularly those treated with chest RT, are at continued increased risk of early mortality and solid tumor SMNs. (Bluhm EC, Ronckers C, Hayashi RJ, Neglia JP, Mertens AC, Stovall M, Meadows AT, Mitby PA, Whitton JA, Hammond S, Barker JD, Donaldson SS, Robison LL, Inskip PD. Cause-specific mortality and second cancer incidence after non-Hodgkin lymphoma: A report from the Childhood Cancer Survivor Study. Blood 2008;111:4014–4021)
TESTICULAR CANCER
Genetic Variation
Gene-knockout studies in mice suggest that INHA, encoding a subunit of gonadotropin-regulating proteins known as inhibins, is a tumor suppressor for testicular stromal cell tumors. To address whether genetic variation in the inhibin pathway also influences susceptibility to testicular germ cell tumors (TGCTs), a case-control analysis (577 cases and 707 controls) of SNPs in inhibin pathway genes among participants in the U.S. Servicemen’s Testicular Tumor Environmental and Endocrine Determinants (STEED) Study. Thirty-eight tagging SNPs in six genes (INHA, INHBA, INHBB, INHBC, INHBE, and SMAD4) were genotyped. An elevated risk was observed for carriers of the T allele of the INHA variant rs2059693 (CT genotype: OR = 1.33; TT: OR = 1.60; p for trend = 0.008). The association with rs2059693 was stronger for nonseminomas and for teratomas and teratocarcinomas in particular (N = 58; CT: OR = 1.63; TT: OR = 4.54; p for trend = 0.0008). The authors found no evidence of association with variants in the other investigated genes. (Purdue MP, Graubard BI, Chanock SJ, Rubertone MV, Erickson RL, McGlynn KA. Genetic variation in the inhibin pathway and risk of testicular germ cell tumors. Cancer Res 2008;68:3043–3048)
Organochlorine Pesticides
Exposure to endocrine-disrupting chemicals, such as persistent organochlorine pesticides (POP), has been suggested to increase the risk of TGCTs. To study the relationship of POP exposure to TGCT risk, prediagnostic serum samples from 754 cases and 928 controls enrolled in the STEED Study were analyzed. Risk was significantly associated with higher plasma levels of p,p'-dichlorodiphenyldichloroethylene (p,p'-DDE; highest quartile vs. lowest quartile: OR = 1.71; p for trend = 0.0002) and of two chlordane components, cis-nonachlor (OR = 1.56; p for trend = 0.009) and trans-nonachlor (OR = 1.46; p for trend = .026). Seminoma risk was significantly associated with p,p'-DDE (OR = 1.91; p for trend = 0.0008), cis-nonachlor (OR = 1.93; p for trend = 0.0045), trans-nonachlor (OR = 1.72; p for trend = 0.033), and a chlordane metabolite, oxychlordane (OR = 1.64; p for trend = 0.048), whereas nonseminoma risk showed a significant association with p,p'-DDE only (OR = 1.63; p for trend = 0.0044). Because evidence suggests that TGCTs are initiated in very early life, it is possible that POP exposure during fetal life or via breastfeeding may increase the risk of TGCTs in young men. (McGlynn KA, Quraishi SM, Graubard BI, Weber JP, Rubertone MV, Erickson RL. Persistent organochlorine pesticides and risk of testicular germ cell tumors. J Natl Cancer Inst 2008;100:663–671)
TOBACCO
Intensity of Smoking and Cancer
Relative risks for lung and bladder cancers by smoking intensity level off at more than 15–20 cigarettes per day. A three-parameter excess RR model in pack-years and intensity has been developed to quantify this leveling. Above 15–20 cigarettes per day, smoking at a lower intensity for a longer duration was more harmful than smoking at a higher intensity for a shorter duration. After adjustment for pack-years, intensity effects were similar across studies of lung, bladder, oral cavity, pancreatic, and esophageal cancers. In this report, new analyses were conducted examining intensity patterns for bladder, esophageal, kidney, larynx, liver, lung, oropharynx, and pancreatic cancers using data from the Alpha-tocopherol, Beta-carotene Cancer Prevention Study. At more than 10 cigarettes per day, there was an inverse exposure rate pattern for each cancer site. The intensity effects were similar across the diverse cancer sites and homogeneous with the prior analysis of multiple studies. Consistency of intensity patterns suggested a general phenomenon and may provide clues to the molecular basis of smoking-related cancer risk. (Lubin JH, Virtamo J, Weinstein SJ, Albanes D. Cigarette smoking and cancer: Intensity patterns in the Alpha-tocopherol, Betacarotene Cancer Prevention Study in Finnish men. Am J Epidemiol 2008;167:970–975)
DCEG PEOPLE IN THE NEWS
In March, Christian C. Abnet, Ph.D., M.P.H., and Sanford M. Dawsey, M.D., both of the Nutritional Epidemiology Branch (NEB), gave invited lectures at the Second International Workshop on Cancer of the Esophagus in Porto Alegre, Brazil. Dr. Abnet spoke on “Cancer of the esophagus: Is the world epidemiology changing?” and “Polycyclic aromatic hydrocarbon exposure: A global cause of esophageal squamous cell carcinoma?” Dr. Dawsey talked about “Early diagnosis of esophageal squamous cell carcinoma: The NIH experience in China” and “Does adenocarcinoma of the esophagogastric junction originate in the esophagus or the stomach? Does it matter?”
Blanche P. Alter, M.D., M.P.H., Clinical Genetics Branch (CGB), spoke on “Diagnosis, genetics, and management of inherited bone marrow failure syndromes” at the education sessions of the 49th American Society of Hematology Annual Meeting in Atlanta in December.
In February, Nilanjan Chatterjee, Ph.D., Chief of the Biostatistics Branch (BB), spoke at the National Institute of Environmental Health Sciences in Research Triangle Park, North Carolina about “Analysis of case-control studies in genetic epidemiology: Beyond logistic regression.” In May, he was selected as a Fellow of the American Statistical Association.
Mitchell H. Gail, M.D., Ph.D. (BB), delivered a lecture on “A model for breast cancer risk that includes mammographic density” at a Da Costa Foundation meeting in San Antonio in December. He also gave an invited talk titled “Absolute risk: Clinical applications and controversies” at the University of Texas M.D. Anderson Cancer Center in February. In March, Dr. Gail presented “The probability of detecting disease-associated SNPs in genome-wide association studies” at the National Heart, Lung, and Blood Institute and the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Later in March, he spoke on “Cause-specific risk models for estimating absolute risk” at a regional meeting of the International Biometric Society in Arlington, Virginia.

Annelie Landgren
In March, Annelie M. Landgren, M.P.H., Radiation Epidemiology Branch (REB), received an M.P.H. from the Mittuniversitetet in Sweden. Her thesis was on “Autoimmune disease and subsequent solid tumor risk among 4.5 million U.S. male veterans.” Her mentors were Lindsay M. Morton, Ph.D., Martha S. Linet, M.D., M.P.H. (Chief of REB), and Dr. Katja Gillander-Gådin at Mittuniversitetet.
Qizhai “James” Li, Ph.D. (BB), gave a talk on “An efficient method to approximate the family-wide p-value: A GWAS application” at the Chinese Academy of Science in Beijing in December.

Jennifer Loud
Jennifer T. Loud, R.N., C.R.N.P., D.N.P. (CGB), was recently in the first graduating class of the Doctor of Nursing Practice Program of the School of Nursing at the University of Maryland, Baltimore. She was awarded a scholarship to travel to the Southern Nursing Research Conference in Birmingham in February. She also finished a two-year term as the Coordinator, Cancer Genetics Special Interest Group, of the Oncology Nursing Society.
In November, Jay H. Lubin, Ph.D. (BB), delivered a talk titled “Analysis of environmental and occupational variables: Toward a more informative approach for total exposure, exposure rate, and duration” at the Department of Epidemiology of the University of North Carolina at Chapel Hill. In February, he spoke at the University of Texas M.D. Anderson Cancer Center on “Analyzing total exposure and exposure rate for smoking and alcohol for head and neck cancer based on pooled data from the INHANCE consortium.”
In December, Yikyung Park, Sc.D. (NEB), led a seminar titled “Diet, lifestyle, and colorectal cancer: The NIH-AARP Diet and Health Study” at the University of Maryland School of Public Health.
Ruth M. Pfeiffer, Ph.D. (BB), led a seminar on “Probability of detecting disease-associated SNPs in genome-wide association studies” at the Department of Mathematics of the University of Maryland, College Park in November. She also delivered a talk at the National Institute of Allergy and Infectious Diseases on “Probability of detecting disease-associated single nucleotide polymorphisms in single-stage or multistage case-control genome-wide association studies” in January.

Karen Pitt
In May, Karen E. Pitt, Ph.D., Office of the Director, received a Special Service Award from the International Society for Biological and Environmental Repositories for her outstanding work as editor-in-chief of the second edition of Best Practices for Repositories: Collection, Storage, Retrieval, and Distribution of Biological Materials for Research.
In February, Arthur Schatzkin, M.D., Dr.P.H. (Chief of NEB), spoke on “Energy balance and cancer: What role for surrogate end points?” at the American Association for Cancer Research (AACR)–NCI Conference, Energy Balance and Cancer: Mediators and Mechanisms, in Lansdowne, Virginia. In March, he gave a talk on “Mendelian randomization: Can it help us establish causality for nutrition vs. cancer?” at the American Society of Preventive Oncology meeting in Bethesda.
In March, Mark E. Sherman, M.D., Hormonal and Reproductive Epidemiology Branch (HREB), gave a lecture in Orlando on “Human papillomavirus and the HPV vaccine” as part of Molecular Medicine: Applying Current and Emerging Technologies, a course sponsored by the University of Florida.
In March, Alice J. Sigurdson, Ph.D. (REB), reported on the U.S. Radiologic Technologists Cohort Study in an invited talk titled “Polymorphisms and radiation interactions in breast cancer risk” at Columbia University.
In March, Rachael Stolzenberg-Solomon, M.P.H., Ph.D. (NEB), gave a talk at the University of Texas M.D. Anderson Cancer Center on “The epidemiology and etiology of pancreatic cancer.”
mark schiffman wins achievement award

Mark Schiffman with Lifetime Achievement Award
In March, Mark Schiffman, M.D., M.P.H. (HREB), was awarded the American Society for Colposcopy and Cervical Pathology’s Lifetime Achievement Award at the biennial meeting in Orlando for his role in translating new knowledge regarding the natural history and pathogenesis of cervical cancer into clinical practice.
At the Third Environmental Cancer Risk, Nutrition, and Individual Susceptibility Annual Meeting held in Barcelona in March, Philip R. Taylor, M.D., Sc.D., Genetic Epidemiology Branch (GEB), gave a talk, “Design and outcomes of long-term selenium anticancer trials,” as part of a workshop on the Protective Effects of Selenium on Cancer.
In January, Jorge R. Toro, M.D. (GEB), spoke at grand rounds for the Department of Dermatology at Johns Hopkins University School of Medicine on “Genodermatoses with an increased risk of kidney cancer.”
At the AACR International Conference on Advances in Cancer Research: From the Laboratory to the Clinic, held in Jordan in March, Margaret A. Tucker, M.D., Director of the Human Genetics Program and Chief of GEB, gave two presentations. She spoke at an educational symposium on Scientific Writing and the Publication Process and at a scientific workshop on Cancer Epidemiology and Prevention.
In February, Sholom Wacholder, Ph.D. (BB), gave an invited talk titled “Screening for rare disease and other problems of searching for the few among the many” as part of a Scholar Seminar Series at the Mel and Enid Zuckerman College of Public Health at the University of Arizona.
Kai Yu, Ph.D. (BB), spoke at Duke University on “Population substructure and control selection in genome-wide association studies” in February. In March, he delivered a talk on “A partially linear tree-based regression model for multivariate outcomes” at George Mason University in Fairfax, Virginia.

Stella Koutros, H. Dean Hosgood, and Anjoeka Pronk.
THREE NEW PH.D.S
IIn March, three predoctoral fellows in the Occupational and Environmental Epidemiology Branch (OEEB) successfully defended their doctoral dissertations. H. Dean Hosgood, III, Ph.D., received his degree from Yale University, where he examined the environmental and genetic risk factors for lung cancer in Xuanwei, China. His mentors were Qing Lan, M.D., Ph.D., M.P.H., and Dr. Tongzhang Zheng at Yale. Stella Koutros, Ph.D., also studied at Yale, investigating the risk factors for prostate cancer in the Agricultural Health Study under the mentorship of Michael C.R. Alavanja, Dr.P.H., and Dr. Zheng. Anjoeka Pronk, Ph.D., completed her work on isocyanate exposure and respiratory health effects in the spray painting industry at the Medical Faculty of Utrecht University in The Netherlands under the guidance of Professors Dick Heederik and Liesbeth Preller.
COMINGS . . . GOINGS

Sonya Heltshe
After seven months in the Radiation Epidemiology Branch (REB), Michael Abend, M.D., has returned to the Institute of Radiobiology of the German Armed Forces in Munich.
Kenneth Adams, Ph.D., has left the Nutritional Epidemiology Branch (NEB) to join the Health Partners Research Foundation in Minneapolis, where he will investigate the metabolic and physiologic consequences of obesity in children and adults, ways to improve care for hypertension, and the efficacy of colorectal cancer screening.
Elizabeth C. Bluhm, M.D., M.P.H., an NCI Cancer Prevention Fellow since 2003, has left REB to take a faculty position at Washington Hospital Center in the District of Columbia.

Briseis Kilfoy
Sonya Heltshe, Ph.D., joined the Biostatistics Branch (BB) as a postdoctoral fellow. She recently completed a Ph.D. in biostatistics at the University of Colorado Denver Health Sciences Center. She has worked as a biostatistician at the Rocky Mountain Poison and Drug Center, the Burn Model Systems Project, and the Cystic Fibrosis Center. With mentors Barry I. Graubard, Ph.D., and Jay H. Lubin, Ph.D., she is working on several projects, including study design in the U.S. Radiologic Technologists Study, evaluation of molecular marker data, exposure assessment and analysis of multilevel environmental/census data in the Agricultural Health Study and other cohorts, and analysis of admixture genome scans.
Jose Jeronimo, M.D., has left the Hormonal and Reproductive Epidemiology Branch (HREB) for a position with PATH (Program for Appropriate Technology in Health) in Seattle.

Laura Reimers
Briseis Kilfoy, M.P.H., joined the Occupational and Environmental Epidemiology Branch as a predoctoral fellow in the Yale University-NIH Partnership Training Program. She received an M.P.H. from Yale and a B.A. from the University of Chicago. Under the mentorship of Mary H. Ward, Ph.D., Ms. Kilfoy is investigating the relationship of drinking water and dietary nitrate exposure to thyroid cancer and other thyroid abnormalities. She is also conducting descriptive analyses of thyroid cancer with William F. Anderson, M.D., M.P.H., and Susan S. Devesa, Ph.D.

Guillermo Seratti
Unhee Lim, Ph.D., left NEB to become an assistant researcher and professor in the Epidemiology Program of the Cancer Research Center at the University of Hawaii.
Laura Reimers has joined BB as a Cancer Research Training Award Fellow while studying at the George Washington University School of Public Health and Health Services. She is working with Dr. Anderson to use descriptive techniques and biomathematical models to compare squamous cell carcinoma and adenocarcinoma of the cervix for her M.P.H. thesis. She holds a B.S. in biochemistry and political science from Syracuse University.

Nataša Tasevska
Ana Cecilia Rodriguez, M.D., has left HREB and returned to Costa Rica, where she will resume her work as a principal investigator on the Guanacaste human papillomavirus (HPV) and cervical cancer natural history study, and the Costa Rica HPV vaccine trial.
Guillermo Seratti, M.D., has joined the Clinical Genetics Branch. He received an M.D. from Universidad del Salvador Medical School in Buenos Aires, Argentina, completed a residency in pediatrics at the University of Illinois at Chicago, and is a fellow in genetics at the National Human Genome Research Institute. As part of his genetics fellowship during the next year, Dr. Seratti is working with Sharon A. Savage, M.D., to study dyskeratosis congenita and other cancer predisposition syndromes. His focus will be on genetic risk factors for osteosarcoma.
Nataša Tasevska, M.D., Ph.D., joined NEB as a postdoctoral fellow. She received an M.D. from the Ss. Cyril and Methodius University in the Republic of Macedonia, an M.Sc. in human nutrition and metabolism from the University of Aberdeen, United Kingdom, and a Ph.D. in nutritional epidemiology from the University of Cambridge. She is working with Amanda J. Cross, Ph.D., and Rashmi Sinha, Ph.D., on developing biomarkers to improve the assessment of meat-related exposure and on meat intake as a cancer risk factor.

Huei-Ting Tsai
Huei-Ting Tsai, Ph.D., has joined the Genetic Epidemiology Branch as a visiting fellow. A native of Taiwan, she received a doctorate in epidemiology from the University of North Carolina at Chapel Hill. For her dissertation, she studied the association between tardive dyskinesia and dopamine receptor genes among patients with chronic schizophrenia. She is interested in pursuing studies in pharmacoepidemiology and pharmacogenetics and is working with Ola Landgren, M.D., Ph.D., and Neil E. Caporaso, M.D., on the etiology of hematologic and lymphoproliferative diseases.

Lene Veiga
Lene Veiga, Ph.D., joined REB as a visiting fellow from the Institute of Radioprotection and Dosimetry in Brazil. Since 1987, she has worked on environmental impact assessment projects. After conducting the first Brazilian cohort study of miners exposed to radon, she received her Ph.D. in cancer epidemiology at the National School of Public Health/Oswaldo Cruz Foundation in 2004. She has served as a consultant to the Brazilian Nuclear Energy Commission and represented Brazil on the risk assessment group of the WHO International Radon Project. At DCEG she is working on radiation-associated thyroid cancer with Elaine Ron, Ph.D., M.P.H., and Jay H. Lubin, Ph.D. (BB).

Geoffrey Howe
GEOFFREY HOWE MEMORIAL SYMPOSIUM
In February, a symposium to honor the late Dr. Geoffrey R. Howe was held at Columbia University, where Dr. Howe was a faculty member. The theme was “The Chornobyl project: Summarizing a decade of research.” Several REB scientists who collaborated with Dr. Howe spoke at the symposium, including Andre Bouville, Ph.D., who discussed dose estimation, Alina V. Brenner, M.D., Ph.D., who spoke on autoimmune thyroiditis, Maureen C. Hatch, Ph.D., who presented findings on thyroid disease among children exposed in utero, Ihor J. Masnyk, Ph.D., who described the history of the project, and Elaine Ron, Ph.D., M.P.H., whose presentation covered thyroid cancer risk.

Robert Hoover presents DCEG Special Recognition Award to Mustafa Dosemeci.
MUSTAFA DOSEMECI RETIRES
In April, Mustafa Dosemeci, Ph.D., retired from OEEB. During his 22-year career at NCI, he played a pioneering role in the development of exposure assessment methods in occupational cancer research and became an internationally recognized expert in the field. He received multiple awards from NIH and other agencies during his career, including the Charles C. Shepard Science Award from CDC’s Agency for Toxic Substances and Disease Registry.
Dr. Dosemeci joined DCEG in 1986 after completing his Ph.D. in occupational health at Hacettepe University, Faculty of Medicine in Ankara, Turkey, and following a position as a visiting scientist at the London School of Hygiene and Tropical Medicine. He made critical contributions to the exposure assessment components of several OEEB cohort studies, including the Agricultural Health Study, the China Benzene Cohort Study, the Silica Cohort Study, and many case-control studies carried out in the United States and other countries. He applied retrospective exposure assessment methods to the study of numerous cancers, including biliary, bladder, brain, breast, kidney, laryngeal, lung, salivary, stomach, and hematological malignancies. He also led important studies of cancer risk associated with physical activity in the workplace and carried out methodologic work on the impact of occupational exposure misclassification. He developed a series of software programs to assist in retrospective occupational exposure assessment that became widely used by investigators at NCI and around the world. In addition, Dr. Dosemeci initiated and was a lead investigator in the Spanish Bladder Cancer Study, one of the largest interdisciplinary studies of bladder cancer to date.
A TRIBUTE TO ROBERT WELCH

Robert Welch
Robert Allan Welch, M.S., Director of Operations at the NCI Core Genotyping Facility (CGF), died February 23 at his home in Martinsburg, West Virginia.
Mr. Welch played a key role in developing and managing CGF, which carries out large-scale studies of common genetic variations and cancer. His skill, dedication, and hard work were major contributions to CGF’s success, and his commitment and leadership inspired his colleagues at CGF, NCI, and elsewhere.
Mr. Welch was born in Cheverly, Maryland, and received his undergraduate degree from the University of Scranton in 1995 and a master’s degree in scientific management from the University of Maryland in 1999.
Prior to joining CGF in 2002, Mr. Welch held positions at the Beltsville Agricultural Research Center, the University of Scranton, BioServe Biotechnologies, Ltd., and Biognosis U.S., Inc.
Mr. Welch coauthored more than 75 papers and book chapters. He received an SAIC-Frederick Special Achievement Award for advances in human genetics research, including the Cancer Genetic Markers of Susceptibility (CGEMS) initiative, a three-year collaborative genome-wide association study for prostate and breast cancer. He also received an SAIC Customer Relations Award and a Best Poster Presentation Award from the Association of Biomedical Research Facilities.
Survivors include his wife of seven years, Patricia Welch; three stepchildren, Kristopher, John, and Lisa Canning; his mother, Rosemary Welch; his father and stepmother, Robert and Peggy Welch; a sister, Rachel Altemose; and a stepbrother, Russell Alion.
A memorial service held on February 29 was widely attended by staff from NCI and SAIC-Frederick. At the request of his wife, DCEG has established the Robert A. Welch Fellowship, a pre- and postdoctoral training program in molecular epidemiology with special emphasis on the application of emerging genetic and genomic technology. Donations to support the fellowship may be made through the Foundation for the National Institutes of Health at www.fnih.org.
Details about the Robert A. Welch Fellowship can be found at http://dceg.cancer.gov/fellowships/information/welch_fellowship.
NIH Publication No. 08-6051
Printed July 2008