In 1997, the National Advisory Committee on Microbiological Criteria for Foods (NACMCF/the Committee) was asked to review the current literature on sprout-associated outbreaks; identify the organisms and production practices of greatest public health concern; prioritize research needs; and provide recommendations on intervention and prevention strategies. In response to this charge, the Fresh Produce Work Group (FPWG) documented the relevant epidemiology and microbial ecology of sprout-associated outbreaks and reviewed current industry practices and initiatives related to the growing of seed and the production of sprouts.
Sprouts have been identified as a special problem because of the potential for pathogen growth during the sprouting process. If pathogens are present on or in the seed, sprouting conditions may favor their proliferation. There is no inherent step in the production of raw sprouts to reduce or eliminate pathogens. Contaminated seed is the likely source for most reported sprout-associated outbreaks. Research has been initiated on methods to reduce or eliminate pathogenic bacteria on seeds and sprouts and some treatments show promise. However, to date, no single treatment has been shown to completely eliminate pathogens under experimental conditions used.
Finally, the Committee found that, at the time of the charge, there was a lack of fundamental food safety knowledge along the continuum from seed production through sprout consumption. More recently, many have become aware of the potential for this food to be a vehicle for foodborne illness and the need for appropriate controls; however, such awareness is not universal. Although seed appears to be the most likely source of contamination in sprout associated outbreaks, practices and conditions at the sprouting facility may also impact on the safety of the finished product. In recent sprout-associated outbreak investigations, facilities associated with outbreaks did not consistently apply seed disinfection treatments prior to sprouting. Conversely, facilities that used seed from the same lot as an implicated facility, but had not been associated with reported illnesses, appear to have been consistently using seed disinfection treatments, such as 20,000 ppm calcium hypochlorite, to disinfect seed prior to sprouting.
The Committee has developed a number of specific recommendations, including:
1) The knowledge of all interested parties pertaining to the microbiological safety of sprouted seeds must be enhanced; government and industry should develop education programs for seed and sprout producers on basic principles for microbiological food safety, good agricultural practices, good manufacturing practices, and hazard analysis and critical control point (HACCP) systems,
2) Good agricultural practices should be systematically implemented to reduce the potential for microbial contamination of seeds for sprout production,
3) Seed cleaning, storage, and handling practices that minimize the potential for microbial contamination should be developed and implemented,
4) Seeds should be treated with one or more treatments that have been shown to reduce pathogenic bacteria that may be present. Intervention strategies that deliver less than a given reduction (at this time, 5-log) in levels of Salmonella spp. and enterohemorrhagic Escherichia coli O157 should be coupled with a microbiological testing program,
5) Establish good manufacturing practices and food safety systems, including regulatory oversight, microbial testing, adoption of HACCP, and improved traceback, that systematically look for means to prevent seeds from serving as the vehicle for foodborne disease, and
6) Conduct research related to the microbiological safety of sprouted seeds, particularly in the areas of pathogen reduction or elimination, sources of contamination and its prevention, and preventing or retarding pathogen growth during sprouting.
In 1995, the National Advisory Committee on Microbiological Criteria for Foods (NACMCF/the Committee) was asked to evaluate the association between foodborne illness and fresh produce. The Committee was also asked to make recommendations that could be used to reduce the risk of foodborne illness outbreaks associated with these commodities. NACMCF established a Fresh Produce Work Group (FPWG) to address this issue and in 1998, published a report entitled "Microbiological Safety Evaluations and Recommendations on Fresh Produce" (NACMCF, 1998).
Within that document, sprouted seeds were identified as a special problem because of the potential for pathogen growth during production. Sprouts are produced by first soaking viable seed in water and then placing the seed in a warm, humid environment for an average of 3 to 7 days to foster germination and sprout growth. These conditions also provide nearly ideal conditions for the exponential growth of bacteria. If pathogens are present on or in the seed, sprouting conditions may allow for their proliferation.
Since 1995, sprouts have been increasingly implicated in foodborne outbreaks. In 1997, the Centers for Disease Control and Prevention submitted a request to the Food and Drug Administration/Center for Food Safety and Applied Nutrition (FDA/CFSAN) suggesting that there be an expert consultation regarding the recent foodborne illness outbreaks associated with sprouts. Subsequently, the FDA asked NACMCF to review the current literature on sprout-associated outbreaks; identify the organisms and production practices of greatest public health concern; prioritize research needs; and provide recommendations on intervention and prevention strategies. In response to this charge, the FPWG documented the relevant epidemiology and microbial ecology of sprout-associated outbreaks and reviewed current industry practices and initiatives related to the growing of seed and the production of sprouts. The FPWG toured seed mills and sprout manufacturing facilities in Northern California to better understand current industry practices. Producers were very helpful in working with the FPWG to evaluate potential microbial hazards associated with sprouts. The Work Group also participated in a public meeting held September 28 and 29, 1998, to discuss the current state of science and technology in relation to the control of pathogenic bacteria on sprouts.
This paper contains an overview of sprout-associated outbreaks, microbial ecology of bacterial pathogens associated with sprout outbreaks, current industry practices and recent efforts to improve the safety of sprouts, prevention and intervention strategies, research needs and recommendations for improving the microbial safety of sprouted seeds.
Microbiological surveys have shown the presence of a variety of foodborne pathogens in sprouts. Salmonella spp., Listeria monocytogenes, Staphylococcus aureus, Bacillus cereus and Aeromonas hydrophila have been isolated from sprouted seeds, including alfalfa, mung bean, cress, soybean, and mustard (Beuchat, 1996). Escherichia coli O157, various serotypes of Salmonella, and Bacillus cereus have been the causative agents of documented outbreaks of foodborne illness associated with sprouts (Jackson, 1998). This overview will focus on Salmonella and Escherichia coli O157 sprout-associated outbreaks in the United States from 1995 through the spring of 1999.
During this time frame, there were nine reported commercial sprout outbreaks in the U.S., seven of which were due to different Salmonella serotypes, and two to E. coli O157. The number of culture-confirmed cases in each of these outbreaks ranged from eight to more than 500, and more than 1,200 cases have been reported overall (Tables 1 and 2). Some of the outbreaks involved multiple states and other countries, while others occurred in one or two states. Alfalfa sprouts, the most common form of green sprouts available in the marketplace, were implicated in all outbreaks, although in two outbreaks, other types of sprouts were also either culture-positive, or were mixed with the alfalfa sprouts. In all of the reported outbreaks, the likely source of contamination was seed. However, in the large 1996 Salmonella Montevideo/Meleagridis outbreak unsafe sprouting practices may also have contributed to the contamination of sprouts.
The problem is not restricted to the U.S.; sprout-associated outbreaks have also been reported from other countries, including the United Kingdom, Sweden, Finland, Japan, Denmark, and Canada. Alfalfa sprouts were often implicated in these non-U.S. outbreaks, but mung bean and cress sprouts also were associated with outbreaks. Radish sprouts were associated with a very large E. coli O157:H7 outbreak in Japan in 1996 and a smaller outbreak in 1997. The frequency of foodborne disease attributable to mung beans and soy bean sprouts is generally lower than that associated with "green" sprouts (e.g., alfalfa, clover, radish) since sprouts of beans are typically cooked before consumption.
Salmonella Stanley
In 1995, a S. Stanley outbreak raised concerns and provided important insights about sprout-associated salmonellosis (Mahon et al., 1997; Puohiniemi et al., 1997). Between March and June of that year, there was a marked increase in the number of cases of S. Stanley in Arizona, Michigan and Finland. Ultimately, 242 culture-confirmed cases in 17 states and Finland were attributed to this international outbreak. Initially, 22 S. Stanley cases in Arizona were identified and a case-control study implicated consumption of alfalfa sprouts as being significantly associated with illness. Alfalfa sprouts were also identified as the vehicle by independent case-control studies in Michigan and Finland (Mahon et al., 1997).
Laboratory analyses of S. Stanley patient isolates indicated that the U.S. and Finland cases were related. The S. Stanley isolates from the U.S. and Finland had a unique antimicrobial resistance pattern (resistance to tetracycline, bactrim, and kanamycin, and sensitivity to ampicillin). Molecular subtyping by pulsed-field gel electrophoresis (PFGE) also indicated the same unique pattern for the U.S. and Finnish isolates. Analysis of alfalfa seeds and sprouts from the small amount of the seed lot remaining did not yield S. Stanley.
Follow-up investigations of the outbreak indicated that contaminated alfalfa sprouts were grown by at least nine different sprout producers. These producers obtained alfalfa seed from a single U.S. seed supplier who purchased the seeds from a seed shipper in the Netherlands. This same Dutch shipper also supplied the seed for the sprouts eaten by the patients in Finland. The seed was not grown in the Netherlands, but, due to mixing of seed lots, it was not possible to determine the ultimate source of the seed. Evidence of rodents and birds was observed within the seed conditioning facility.
Salmonella Newport
In late 1995 and early 1996, an outbreak of 133 cases of S. Newport infection in Oregon and British Columbia was recognized. Case-control studies implicated alfalfa sprouts. S. Newport was isolated from both alfalfa sprouts and seeds from which they were grown and molecular subtyping by PFGE showed that a single strain caused the outbreaks. A traceback showed that the contaminated seed came from a single lot from a Dutch shipper. Subsequent investigation indicated S. Newport outbreaks due to this contaminated seed lot occurred in six states and Denmark (Van Beneden et al., 1999). Analysis of the implicated seed lot by the most probable number method (MPN) yielded 0.1-0.6 CFU of S. Newport/25 g of seed. Sprouting of the implicated seed lot also yielded S. Newport.
Salmonella Montevideo and Salmonella Meleagridis
In May through July 1996, approximately 500 culture-confirmed cases of Salmonella serotypes Montevideo and Meleagridis occurred in Nevada and California (Farrar and Mohle-Boetani, 1999). One elderly patient died from sepsis. A case-control study revealed that alfalfa sprouts were the only item associated with illness. The same strain of S. Meleagridis was isolated from patients as well as from sprouts obtained from retail stores and the sprouting facility. Seed samples, however, did not yield either serotype. All of the implicated sprouts were produced at one facility and were sprouted from alfalfa seed grown locally in California.
Investigation of the sprout facility revealed unsanitary sprouting practices such as the presence of flies and rodent droppings, and use of the same plastic buckets to collect both finished sprouts and sprouts that had fallen on the floor. Sub-optimal employee hygiene was also noted. At the farm where the alfalfa seed was grown, several potential risk factors were observed including: 1) use of chicken manure to fertilize the field, 2) use of canal water for watering crops, 3) transport of alfalfa in unclean vehicles, and 4) the presence of livestock next to the alfalfa field.
Salmonella Infantis and Salmonella Anatum
An outbreak of Salmonella serotypes Infantis and Anatum in Kansas and Missouri in 1997 resulted in 109 culture-confirmed cases. Alfalfa, rose, radish and snow pea sprouts yielded both serotypes, and the alfalfa seeds yielded S. Anatum. The implicated sprouts were produced at a single facility. The seed was locally grown and came from many surrounding farms (Pezzino et al., 1998; Slutsker, 1999).
Salmonella Senftenberg
Two clusters of Salmonella Senftenberg infection occurred in Nevada and California in late 1997 through July 1998. The 60 culture-confirmed cases in both clusters were shown to be due to the same strain of S. Senftenberg. The implicated sprouts were an alfalfa/clover sprout mixture from a single local sprout producer. The sprout producer associated with this outbreak inconsistently used a chlorine disinfection treatment on seed prior to sprouting (Farrar and Mohle-Boetani, 1999).
Cultures of immature sprouts from a growing drum in the facility associated with the outbreak yielded the pathogen but analysis of the clover and alfalfa seeds did not yield the pathogen. It was not possible to determine definitively which type of sprout in the alfalfa/clover mix caused the outbreak. However, clover may be more likely, since clover seeds from one production lot were used during the entire outbreak period, whereas the alfalfa seed source changed in March 1998. Further, 10,000 pounds of alfalfa seed from one of the implicated seed lots was also distributed to sprouting facilities throughout Washington without reports of Salmonella Senftenberg infections.
Salmonella Havana/Cubana
An outbreak of 18 cases of S. Havana infection in Arizona and California in May of 1998 was linked to consumption of alfalfa sprouts produced by one large California sprout producer and, possibly, one small producer. The large sprouting facility claimed to soak seeds in 2,000 ppm chlorine for 30 minutes, followed by a 300 ppm chlorine soak for several hours prior to sprouting. However, documentation at the facility prompted questions about the consistency of the application of chlorine treatments. The small sprouting facility utilized a pre-soak treatment, containing approximately 1,000 ppm chlorine, for seed disinfection (Farrar and Mohle-Boetani, 1999).
Subsequently, a cluster of 22 cases of S. Cubana occurred during May through August of 1998 in Arizona, California, Maryland, New Mexico, and Utah that was linked to consumption of alfalfa sprouts from the same large California sprout producer identified in the S. Havana outbreak. The same seed lot was used by the implicated producer to produce the sprouts associated with both outbreaks. Analysis of the implicated seed lot yielded S. Havana, S. Cubana, and S. Tennessee. The S. Havana and S. Cubana strains isolated from the implicated seed lot had PFGE patterns which were indistinguishable from patient isolates.
Salmonella Mbandaka
In January through March 1999, a Salmonella Mbandaka outbreak occurred in Oregon, Washington, Idaho, and California that was associated with alfalfa sprouts. A total of 75 cases were reported (Oregon, 31; Washington, 19; Idaho, 5; California, 20) at the time that this manuscript was prepared. Cases occurring in Oregon, Washington, and Idaho were associated with sprouts produced from a single Washington sprouting facility that was not consistently treating seed with a disinfectant (Keene, 1999). A single lot of seed, grown in Southern California and obtained from a seed conditioning facility in California was used by the Washington sprouting facility. S. Mbandaka was isolated from alfalfa seed from the implicated seed lot, alfalfa sprouts from the Washington manufacturing facility, and from alfalfa sprouts from aseptically sprouted seeds. Subsequent investigations of the distribution of the contaminated seed lot provided interesting information concerning the effectiveness of seed treatments to reduce pathogen levels. The implicated seed lot was distributed to and sprouted by 4 other sprout producers in California (3) and Florida (1) (Table 3). Two of the California sprout producers used a calcium hypochlorite seed treatment (2,000 - 20,000 ppm) before germination of the seeds. The third California sprouter was apparently not using a chlorine treatment for seeds. The Florida sprouter presoaked the implicated seed lot in 20,000 ppm calcium hypochlorite, 3 times for 20 minutes per soak prior to sprout germination. Only the facilities that did not consistently pretreat seeds were linked to S. Mbandaka infections.
Michigan/Virginia
In 1997, an outbreak of 108 cases of E. coli O157:H7 in Michigan and Virginia was epidemiologically associated with sprouts (CDC, 1997). The isolates were indistinguishable on molecular subtyping by PFGE, suggesting a common source. Traceback revealed that all implicated alfalfa sprouts were produced at a single sprouting facility in each state. Sprouts grown by the Michigan sprouter at the time of the outbreak came from two lots of seeds; one from Idaho and the other from Australia. The Virginia sprout manufacturer used the same lot of Idaho seeds as one of the lots used in Michigan. Cultures from this seed lot did not yield E. coli O157:H7.
Further investigations revealed that seed may have been contaminated at the farm where the alfalfa was grown. On the alfalfa farm in Idaho where the seeds were harvested, several possible sources of contamination from cow and deer manure were noted. Some fields were irrigated with water drained from neighboring fields where manure was applied and some alfalfa fields were directly adjacent to cattle feed lots. Some alfalfa was grown next to a deer refuge, and deer were observed in these fields daily.
California
In June of 1998, another sprout outbreak occurred in Nevada and California involving a non-motile strain of E. coli O157. Eight culture-confirmed cases were linked to consumption of an alfalfa/clover sprout mixture from the same sprout producer implicated in the Salmonella Senftenberg outbreak. This producer inconsistently used chlorine disinfection of seed prior to sprouting. Laboratory analysis of seeds, sprouted seeds, and environmental samples did not yield E. coli O157:NM (Farrar and Mohle-Boetani, 1999).
Japan
In contrast to the size of the outbreaks in the U.S., a very large outbreak of over 6,000 culture-confirmed cases of E. coli O157:H7 infections occurred in Japan in 1996. Consumption of radish sprouts was strongly linked to illness (Nat'l Inst. Inf. Dis., Ministry of Health and Welfare, Japan, 1997). In 1997, a smaller outbreak occurred with 126 cases, in which an identical strain of E. coli O157:H7 was isolated from patients and from radish sprouts obtained from a patient's refrigerator (Gutierrez, 1997).
Outbreak investigations identified several factors that affect the microbial safety of sprouted seeds. To date, contaminated seed have been the likely source for most, if not all, outbreaks. Seed contamination could have occurred at the farm, seed processor, or sprouting facility. The processes used for the production of sprouted seed offer ample opportunity for cross contamination from a few seeds or sprouts to the entire production lot. Most seed used for sprouting is not grown for human consumption. The seed is generally grown, milled, and stored under conditions where contamination can readily occur. Frequent failures to isolate pathogens from implicated seeds suggests that seed contamination may be intermittent, at very low levels, or unequally distributed within seed lots. However, even low levels of human pathogens are a concern. Conditions during sprouting (time, temperature, water activity, pH, and nutrients) are ideal for growth of pathogenic bacteria such as Salmonella and E. coli.
In recent outbreaks, investigations have attempted to determine the extent to which certain practices, such as seed disinfection treatments, are being used by sprout producers associated with an outbreak. In general, facilities associated with recent outbreaks often did not apply seed disinfection treatments, applied treatments inconsistently, or used disinfectants at relatively low levels. Conversely, facilities that traceforward investigations have identified as having used seed from the same lot as an implicated facility, but that have not been associated with any reported illnesses, appear to have been consistently using seed disinfection treatments, such as 20,000 ppm calcium hypochlorite, to disinfect seed prior to sprouting. While there may be other mitigating factors (such as product volume and amount of implicated seed used) these observations support the efficacy of seed disinfection treatments as a means to reduce the potential of sprout-associated foodborne illness outbreaks.
Prior to sprouting, seeds can harbor significant levels of microorganisms. Various surveys have revealed aerobic plate counts (APCs) of 3 - 6 x 104 CFU/g (Andrews et al., 1979), 9 x 105 CFU/g (Andrews et al., 1982), and 5 - 400 x 103 CFU/g (Prokopowich and Blank, 1991) on alfalfa seeds; 1 - 20 x 104 CFU/g on mung beans (Andrews et al., 1982); 1 x 105 CFU/g on onion seeds (Prokopowich and Blank, 1991); and 3 x 107 CFU/g on rice seeds (Piernas and Guiraud, 1997a).
The microflora of the seeds increases during sprouting, and high levels of microorganisms typically associated with commercially sprouted seeds have been recognized for many years. Alfalfa sprouts and bean sprouts from retail outlets had APCs of 108 - 109 CFU/g (Patterson and Woodburn, 1980), and retail onion sprouts had > 109 CFU/g (Prokopowich and Blank, 1991). Mung bean sprouts obtained from restaurants had APCs of > 106 CFU/g (Sly and Ross, 1982). It is important to note that high microbial levels per se are not necessarily of public health concern in sprouts. It is the presence and growth of pathogenic microbes that is the concern.
While fresh produce, including sprouts, can serve as a potential source of many types of foodborne pathogens (e.g., bacterial, viruses, protozoa, fungi, and helminths) (NACMCF, 1998), bacterial pathogens are of particular concern with sprouts. This reflects the fact that the environmental conditions and nutrients present during sprout production provide excellent conditions for the outgrowth of most pathogenic bacteria, if present. Pathogens can grow to elevated levels since there are no inherent steps in the production of sprouts that either prevent bacterial growth or eliminate them entirely.
Pathogenic bacteria could be introduced to sprouted seed by a number of pathways, including via the seeds, the water used during germination and sprouting, unsanitary production practices, or mishandling by the consumer (Patterson and Woodburn, 1980). However, epidemiological investigations suggest that seed are the likely source in most, if not all, sprout-associated illness outbreaks (Puohiniemi et al., 1991; CDC, 1997a; Mahon et al., 1997).
Little information is available on how seeds become contaminated with bacterial pathogens. Since seeds are raw agricultural products, they could be contaminated by a variety of potential sources of fecal contamination, including contaminated agricultural water, use of inadequately treated manure as a fertilizer, location of fields near animal rearing facilities, access by feral animals, and inadequate agricultural worker hygiene (NACMCF, 1998).
Once present on or in seed, pathogens are likely to survive for extended periods of time. Studies have shown that Salmonella can survive for months under dry conditions such as those used to store alfalfa seeds (Mistcherlich and Marth, 1984). The natural microflora on rice seed showed long-term viability, decreasing by less than 0.5 log after 260 days of refrigerated storage and approximately 2 logs after 277 days at ambient conditions (Piernas and Guiraud, 1997a). Erwinia herbicola, a common saprophyte of alfalfa seed, could be isolated for at least 3 years after treatment of seeds with 1% sodium hypochlorite (Moline and Kulik, 1997).
Many of the sources of contamination described above (e.g., untreated or improperly treated water, animal waste or manure, poor sanitation of equipment and poor personal hygiene) could also be potential sources of contamination for seeds or sprouts at the sprouting facility. Although seeds are suspected to be the most likely source of contamination, contamination of water used during sprouting could be a source of initial contamination or a vehicle for subsequent cross contamination. When only the roots of fully developed radish sprouts were immersed in water containing E. coli O157:H7, the pathogen was found throughout the edible portion (Hara-Kudo et al., 1997). Likewise, E. coli O157:H7 was found both on the outer surfaces and the inner tissue of radish sprouts grown from artificially inoculated seeds (Itoh et al., 1998).
The key aspect of sprouts that increases the risk of foodborne disease compared to other fresh produce, is the exponential growth of bacteria during sprouting. Microorganisms on seeds can grow quickly under the favorable conditions of the sprouting process (e.g., water activity, temperature, pH, time, and nutrients). An overnight soak of seeds in tap water caused a 10-fold increase in aerobic plate counts (Prokopowich and Blank, 1991). Within the first two days of sprouting, microbial populations on rice seeds increased approximately 2 logs (Piernas and Guiraud, 1997a) and approximately 3 logs on alfalfa seeds and mung beans (Andrews et al., 1982; Splittstoesser et al, 1983). Sprouts germinated in sterile containers in the laboratory had APCs as high as 9.2 x 108 CFU/g, an indication that microbial growth during sprout production can occur under sanitary conditions (Splittstoesser et al., 1983).
If pathogenic bacteria are present, they can grow to high levels. In artificial inoculations of 200 - 500 spores/g, B. cereus increased to 103 - 107 CFU/g during sprouting of mung bean, alfalfa and wheat seeds (Harmon et al., 1987). On naturally contaminated alfalfa, mung bean, and wheat seeds harboring <3 - >100 MPN B. cereus /g, pathogen levels increased 1,000- to 10,000-fold to <103 to >106 CFU/g during sprouting (Harmon et al., 1987). Rice seeds artificially inoculated with Listeria innocua (500 CFU/g) and B. cereus (3,100 CFU/g) supported growth of the microorganisms to 108 and 107 CFU/g, respectively, after 48 hours of sprouting (Piernas and Guiraud, 1997a). S. Stanley increased by 2.5 logs in 24 hours during the germination of contaminated alfalfa seed and by another 1 log during the first 18 hours of sprouting (Jaquette et al., 1996). These levels decreased only slightly during 10 days of refrigerated storage of the sprouts. Pooled S. Anatum and S. Montevideo inoculated onto mung bean seeds increased by 4 - 5 logs during sprouting, and pooled Salmonella Eimsbuettel and Salmonella Poona inoculated on alfalfa seeds increased by 3 - 4 logs during sprouting (Andrews et al., 1982). E. coli O157:H7 inoculated onto radish seeds increased by 4 - 5 logs during 24 hours of sprouting (Hara-Kudo et al., 1997).
As indicated above, sprouted seeds represent a unique microbial food safety concern due to the potential for certain pathogenic bacteria to grow rapidly during the germination and sprouting of the seeds. For sprouts contaminated by pathogens that do not grow during sprouting (protozoa, viruses), the risks of adverse public health consequences are similar to those already noted for fresh produce (NACMCF, 1998). In fact, the risks associated with those microorganisms might be reduced due to the extensive washing that sprouts receive during their production. Thus, pathogenic bacteria that either cannot grow under the conditions encountered during sprout production (e.g., Campylobacter jejuni) or that are not likely to be competitive enough to reach the levels needed to have an adverse public health impact (e.g., Staphylococcus aureus) are not considered to be an increased risk in sprouts compared to other fresh produce.
The following are pathogens that have either been implicated with sprouted seed-associated outbreaks or have been identified as being a potential source of increased risk due to their ability to proliferate during sprouting.
Salmonella
As noted in the preceding section, Salmonella has been responsible for several large sprout-associated outbreaks worldwide (O'Mahony et al., 1990; Oregon Health Division, 1995; Mahon et al., 1997; Puohiniemi et al., 1997; Farrar and Mohle-Boetani, 1999). Reported outbreaks of salmonellosis associated with sprouted seeds suggest an initial low level contamination of the seeds, followed by growth during sprouting (Splittstoesser et al., 1983; Mahon et al., 1997). Salmonellae have been shown to grow during the sprouting process (Splittstoesser et al., 1983; Andrews et al., 1983).
Enterohemorrhagic Escherichia coli
Enterohemorrhagic E. coli have been responsible for several large sprout-associated (radish and alfalfa) outbreaks (CDC, 1997; Wantabe and Okasa, 1997). Epidemiological and/or microbiological evidence suggest that seeds were the source of the pathogen (Itoh et al., 1998). E. coli O157 can grow rapidly to large populations during sprout production.
Listeria monocytogenes
L. monocytogenes is associated with soil, plant and animal products, and food processing environments. Because it is so ubiquitous, there are multiple opportunities for L. monocytogenes to contaminate either seeds or sprouts. L. monocytogenes can grow at refrigeration temperatures on a variety of produce, including sprouts (Buchanan, 1999; Lovett, 1989). This pathogen has been isolated from commercially produced sprouted seeds but no cases of human listeriosis have been linked to those sprouts. Whether the growth amplification kinetics seen with Salmonella and E. coli O157 during the sprouting process also occur with L. monocytogenes is unknown. At the present time, it is unclear whether the presence and growth of L. monocytogenes in sprouts is significantly different from the presence and growth of L. monocytogenes in other fresh produce.
Bacillus cereus
B. cereus is a ubiquitous spore-forming bacterium. It is commonly found in soil and on plants (Kramer and Gilbert, 1989). In 1973, an outbreak was associated with the consumption of sprouts (a mixture of soy, cress, and mustard seeds packaged in a seed sprouting kit) contaminated with B. cereus. As with other pathogens associated with outbreaks from contaminated sprouts, the likely source was seed. B. cereus is capable of growth under seed sprouting conditions (Harmon et al., 1987).
Yersinia enterocolitica
Yersinia enterocolitica can be found in diverse foods of animal origin including pork, beef, poultry, and dairy products, and is commonly isolated from different environments such as lakes, rivers, wells, and soil (Kapperud, 1991). An outbreak of yersiniosis was associated with eating non-commercially produced bean sprouts that were grown using pond water (Cover and Aber, 1989). As a psychrotroph, it must be assumed that Y. enterocolitica can grow on sprouted seeds during refrigerated storage (Chao et al., 1988) just as it can with other products. The bacterium is likely to be capable of growth during sprout production, but no specific data are available.
Shigella
Shigella, because of its low infectious dose (10-100 organisms), its dissemination in fecally-contaminated water, its ability to proliferate in vegetables, and the high degree of handling often associated with the production and packaging of sprouts, must be assumed to be a potential cause of foodborne disease in sprouted seeds (Rafii et al., 1995). However, no reports of this pathogen linked to foodborne disease outbreaks associated with sprouted seeds were identified.
Contamination of sprouted seed can occur at any point in the farm to fork continuum. However, because of the special nature of sprouts, this discussion will focus on two areas of concern, i.e., seed production in the field and seed conditioning facility, and sprouted seed production. As mentioned previously, seed appears to be the primary source of contamination in sprout-associated outbreaks. Further, the conditions during the sprouting process are nearly ideal for the exponential growth of pathogenic bacteria if they are present on or in the seed. After sprouting (e.g., during packaging, distribution, retail sale, and preparation for consumption) microbial hazards are similar to those previously identified for fresh produce (NACMCF, 1998).
Between December 1997 and March 1998, the California Department of Health Services, Food and Drug Branch (CDHS/FDB) and the United States Food and Drug Administration (FDA) conducted an inspection survey of 45 sprout producers in California. The purpose of the survey was to identify products produced and processes used, and to obtain baseline information on current industry practices in California as they may impact on the microbiological safety of sprouts.
Information collected was compared against the Voluntary California Sprout Growers Guidelines (California Sprout Working Group, 1997) which was distributed to all known sprout producers in 1997. Information was also collected on demographics of the firm, and on subjects such as seed source and handling, water source and quality, facility sanitation, and employee hygiene. A report of survey results and recommendations was released in May, 1998 (CDHS and FDA, 1998) and is further discussed in the sprouted seed production section below and in Appendix 2.
In July 1998, the FPWG, FDA, and CDHS toured seed production fields, seed distributors, and sprout manufacturers in California to further understand current industry practices and to observe practices used to reduce or prevent contamination of sprouts. The basic processes included in typical seed and sprout production operations, and a discussion of recent efforts to improve the safety of sprouted seeds follows.
Plants for seed production are grown in typical agricultural environments and seed are generally treated as a raw agricultural product. Potential sources of contamination in the field include agricultural water, improperly managed animal manure, contact with wild animals, and inadequate worker hygiene. In addition, domestic animals may be allowed to graze on alfalfa fields. While such contact is not likely to be a significant problem for the primary use of seed, i.e., seed for forage production, even low level, sporadic contamination of seed for food use may result in significant public health concerns because the sprouting process amplifies pathogen levels.
This overview focuses on alfalfa seed production, though many aspects of production (e.g., Good Agricultural Practices (GAPs) in the field and Good Manufacturing Practices (GMPs) during conditioning) would be equally applicable to other types of seed. Seed production consists broadly of the steps described in Figure 1. Some growers may modify some of these practices depending on many factors, such as the needs of the crop, resources of the operation, and requirements, if any, imposed by the buyer or distributor. Alfalfa seed production and conditioning are described in more detail in Appendix 1.
Harvesting procedures expose the seed to a substantial amount of dirt and debris and likely spread localized contamination throughout the harvested seed. The processes used to sort, clean, store, and package seeds at seed mills may reduce, but are unlikely to eliminate pathogenic microorganisms. If performed incorrectly, these steps could serve as a source of contamination or cross contamination. Further, the FPWG noted the possibility that damage to seeds, either inadvertently or purposefully to change the seeds' germination characteristics (e.g., scarification), could aggravate contamination by making removal of pathogenic microorganisms during subsequent steps more difficult.
For most crops, only a small proportion of harvested seed goes to sprout manufacturers. Further, the decision whether to direct seed to agricultural uses or to sprouting is often not made until after harvest. Thus, the seed grower does not necessarily know whether seed will be sold for food use and, therefore, may have little incentive for following GAPs. Finally, seed processing, shipping and selling practices often involve mixing multiple lots of seeds of different origins, complicating traceback and providing an opportunity for cross contamination.
The CDHS/FDA survey found sprouts being produced in buildings, sheds, greenhouses, modified buses, agricultural fields, or a combination of these. The 45 firms covered by the inspection survey produced 24 different types of sprouts, the most frequently observed products were mung bean, alfalfa, clover, and radish sprouts. Only 25 firms reported producing over 5,000 pounds of sprouts per day (CDHS/FDA, 1998).
Sprout production consisted broadly of the steps depicted in Figure 2. Some firms may add to or omit some of these practices depending on a number of factors, including the type of seed being sprouted and the size and resources of the firm. Sprout production is described in more detail in Appendix 2.
Although seed appears to be the primary source of contamination in sprout-associated foodborne illness outbreaks, practices at the sprouting establishment may increase or decrease the extent of the microbial hazards. Poor sanitation and inadequate hygiene at the sprout production facility can exacerbate the problem of sprout-associated foodborne illness. The CDHS/FDA survey indicated there were significant gaps in sprout manufacturers' understanding and knowledge of food safety, GMPs, and regulatory requirements. In addition, most sprout producers were not registered as food processing establishments as required by California regulations, and thus had not been previously inspected for compliance with GMPs.
Examples of gaps in knowledge of GMPs observed during the inspection survey include, but are not limited to:
In August 1998, FDA issued a national field assignment directing district offices 1) to inspect 100 sprouting facilities, 2) to collect samples of alfalfa, radish, broccoli, and mung beans at different stages of the sprouting process for microbial analysis, and 3) to conduct a survey to develop a profile of the sanitary operations currently in use in the U.S. Districts were also instructed to identify firms operating under insanitary conditions, document the conditions, and take appropriate regulatory actions. Based on preliminary evaluation of data from this national survey, FDA expects that the current practices and needs of U.S. sprout producers are similar to those identified by the CDHS/FDA survey of California producers (Smith, 1999).
In August 1997, copies of the Voluntary California Sprout Growers Guidelines (the Guidelines) were mailed to 85 of the 109 firms initially identified as sprouting operations (California Sprout Working Group, 1997). Of the 45 firms that were found to be sprouters and therefore covered by the CDHS/FDA inspection survey, 11 stated that they did not receive the Guidelines and had no opportunity to make improvements. Twenty four firms did not comment on whether they had received the Guidelines or made improvements. Eight firms reported making changes and improvements; 7 as a result of the Guidelines and 1 based on sanitation guidelines received from the International Sprout Growers' Association (ISGA). Changes made by these firms indicate the types of improvements that might be made by other firms that are effectively reached by education and/or inspection. Changes included:
CDHS/FDB developed and implemented a sprout specific training course to educate sprouted seed producers on the potential risk of their products, as well as federal, state, and local requirements, GMPs and standard operating procedures (SOPs), appropriate sanitation and disinfection procedures, and additional technical assistance resources available to assist sprout producers.
In August 1998, CDHS and FDA's Center for Food Safety and Applied Nutrition (CFSAN) initiated activities that led to California obtaining clearance for the use of 20,000 ppm calcium hypochlorite for disinfecting seeds used for sprouting within California. USDA's Agricultural Research Service (ARS) assisted in developing an emergency permit application, and the Environmental Protection Agency (EPA) quickly reviewed the application for the use of 20,000 ppm calcium hypochlorite for disinfecting seeds for sprouting throughout the U.S. The exemption was granted by EPA in November 1998. The exemption is only valid for one year. One of the stipulations on extending an emergency permit, if necessary, is that the registrants be actively pursuing a full registration.
The International Sprout Growers Association is instituting a voluntary quality assurance program
in which members agree to follow ISGA sanitary guidelines based on GMPs. Sprout producers
that pass a third party inspection may choose to label their products as ISGA-certified. ISGA
certification requires that sprout producers apply a seed disinfection treatment of 20,000 ppm
calcium hypochlorite according to ISGA or EPA directions. An alternative treatment may be used
if it reduces CFUs by ![]() 3.5 log as shown by
data in a refereed scientific journal and complies with
EPA and FDA requirements. ISGA recommends that, at a minimum, third party inspectors look
for general good sanitary conditions; current state and federal food processors registration; proof
that seed disinfection is being done; written SOPs and standard sanitary operating procedures
(SSOPs); written pest control management procedures and evidence that procedures are being
followed (e.g., a pest control log); evidence of employee or manager GMP training within the last
year; and verification of product cold chain and shipping at
3.5 log as shown by
data in a refereed scientific journal and complies with
EPA and FDA requirements. ISGA recommends that, at a minimum, third party inspectors look
for general good sanitary conditions; current state and federal food processors registration; proof
that seed disinfection is being done; written SOPs and standard sanitary operating procedures
(SSOPs); written pest control management procedures and evidence that procedures are being
followed (e.g., a pest control log); evidence of employee or manager GMP training within the last
year; and verification of product cold chain and shipping at
![]() 45°F.
45°F.
In August 1998, a Sprouts Task Force initiated research at the National Center for Food Safety and Technology (NCFST) in Illinois to study the effects of commercial sprouting conditions on pathogens and of potential seed treatment interventions (including chemical, heat, and irradiation). Several treatments show potential for significant reduction, but not elimination, of pathogens. The task force is considering the feasibility of different treatment combinations and criteria for microbial testing of seeds, sprouts, and irrigation water as an additional control in sprout production.
Sprouts research projects have also been included in FDA's and USDA's research plans for produce. These projects include identifying and characterizing natural microflora, the incidence of pathogens on seeds and sprouts, and competitive interactions between natural microflora and Listeria monocytogenes on sprouts.
Prevention or intervention steps might be taken at one or more points during sprouted seed production. Broadly, opportunities for prevention or intervention include strategies that 1) target seed (e.g., in the field, during conditioning, prior to distribution, or at the sprouting facility prior to sprouting), 2) may be applied during sprouting (e.g., to processing water or to immature sprouts), or 3) relate to the finished product. Such strategies may include prevention of contamination, chemical and physical treatments to reduce or eliminate contamination, microbial testing, or combinations of these.
There is relatively little scientific literature available pertaining to techniques for the reduction or elimination of pathogenic bacteria on seeds and sprouts. However, there has been a flurry of research activity in the past two years which has been communicated directly to the FPWG. Since the seed used for sprouting appears to be a primary source of pathogenic bacteria causing sprout-associated illnesses, much of the research on prevention and intervention technologies has focused on eliminating pathogens from the seed prior to sprouting.
Seed production
Sources of seed contamination in the agricultural environment are similar to those described for fresh produce (NACMCF, 1998). The likelihood of contamination in the field may be reduced by systematic implementation of GAPs.
The FPWG found little scientific information available on the impact that seed conditioning and handling practices have on the microbiological safety of sprouted seeds. However, as noted earlier, opportunities for contamination or cross contamination exist. Furthermore, damage to seed may make subsequent removal of pathogens more difficult. Risk of contamination of seeds with foodborne pathogens at this stage might be reduced by development of systematic procedures to minimize contamination or cross contamination.
Seed storage
As noted earlier, once pathogens are present in seeds, they are likely to survive for extended periods of time under normal seed storage conditions. Several researchers have investigated pathogen survivability on seed stored under different conditions (Jaquette et al., 1996, Taormina and Beuchat, 1999). In one study, S. Stanley was inoculated onto alfalfa seeds at 102-3 CFU/g. Salmonella populations decreased 1 log after 8 - 9 weeks storage at 8°C. Increasing storage temperature to 21°C resulted in a 2 log reduction in S. Stanley after 9 weeks (Jaquette et al., 1996). In another study, populations of E. coli O157:H7 inoculated onto alfalfa seeds (initial level of 103 log CFU/g) remained relatively unchanged after 38 weeks storage at 5°. However, within one week of storage at 25 and 37C, E. coli O157:H7 populations decreased significantly. When seeds were stored at 5, 25, or 37°C for 54, 13, or 8 weeks respectively, pathogens were not detectable by direct plating; however, culture enrichment revealed the presence of pathogens in seeds after 38 weeks of storage at 25 and 37°C but not after 54 weeks (Taormina and Beuchat, 1999).
Seed testing
Although epidemiological investigations have frequently identified seeds as the most likely source of contamination of sprouts, laboratory analyses have often been unable to isolate pathogens from implicated seed. This suggests that contamination may be sporadic and at low levels. In the 1995-1996 S. Newport outbreak, analysis of the implicated seed lot by MPN yielded 0.1-0.6 CFU of S. Newport/25 g of seed (4 - 24 cells/1000 g seed) (Van Benden et al., 1999). Analysis of the seed implicated in the 1998 S. Havana outbreak revealed S. Tennessee, S. Cubana, and S. Havana at levels of approximately 4 CFU/1,000 g (Farrar and Mohle-Boetani, 1999). Nonetheless, sprout producers may find advantages to testing seeds for pathogens. While a negative result does not guarantee the absence of pathogens, a positive result would allow a producer to avoid using seed lots that have been shown to contain pathogens.
Seed decontamination
In general, sanitizing is more effective for reducing contamination on seeds than on sprouted seeds (Caetano-Anolles et al., 1990). This may be due to a combination of lower levels of both microorganisms and organic material present on seeds than on sprouts, and the internalization of bacteria into sprout tissues during sprouting making them physically inaccessible to sanitizers (Hara-Kudo et al., 1997; Itoh et al., 1998).
A successful seed decontamination treatment must inactivate microbial pathogens while preserving seed viability, germination, and vigor. Seeds vary in sensitivity to antimicrobial agents and other treatments, which determines how well they germinate and grow after treatment. In addition, a treatment that is effective for one type of seed may not be applicable to all types of seeds. Seeds vary in surface features, which may influence how well an antimicrobial agent can access and inactivate pathogens on or in the seed. Washing seed with water alone decreases levels of B. cereus, E. coli, or Salmonella by 1 log and hence is only marginally effective in reducing pathogens (Harmon et al., 1987; Potter and Ehrenfeld, 1998). Several investigators indicated to the FPWG that alfalfa seeds are generally the most difficult type of seed to sanitize effectively.
A number of researchers have investigated the ability of chlorine compounds to inactivate pathogenic bacteria on seeds, particularly alfalfa seeds. Pretreatment of the seeds prior to germination with 150 ppm of hypochlorite did not eliminate S. Newport (Aabo and Baggesen, 1997). Jaquette et al. (1996) examined the effects of chlorine (sodium hypochlorite treatments ranging from 100 - 2040 ppm active chlorine) on alfalfa seeds inoculated with 102-3 CFU of S. Stanley/g. Treating seed with 100 ppm active chlorine solution for 5 or 10 minutes significantly reduced S. Stanley. A further reduction, but not elimination, occurred after treatment with 290 ppm active chlorine. Treatment with 1,010 ppm active chlorine did not result in additional reduction compared to 290 ppm. Treatment with 2,040 ppm active chlorine reduced S. Stanley to undetectable levels (<1 CFU/g). A study of disinfection procedures for rice seeds showed that ethanol, hydrogen peroxide, or 1,000 ppm sodium hypochlorite reduced APCs by 2-3 logs, but did not eliminate microflora (Piernas and Guiraud, 1997).
In another study, alfalfa seeds were inoculated with a mixture of 5 Salmonella serovars and subjected to a variety of treatments at different concentrations using a variety of antimicrobial chemicals (i.e., calcium and sodium hypochlorite, hydrogen peroxide, and ethanol) (Beuchat, 1997). Significant reductions in Salmonella populations were observed with most increases in concentration of the test chemical. No adverse effects on percent germination were observed for any treatment at any concentration. Treatment solutions containing calcium hypochlorite and sodium hypochlorite at concentrations of 1,800 and 2,000 ppm active chlorine, respectively, 6% hydrogen peroxide, or 80% ethanol were effective in reducing populations by 1000-fold after a 10 minute treatment (Beuchat, 1997). Consistent with Jaquette et al. (1996), Salmonella were not detected by direct plating after treatment with 2,000 ppm active chlorine. However, in this study, where seed disinfection treatments resulted in < 1 CFU Salmonella/g, enrichment procedures were employed, and, in all cases, viable Salmonella were recovered. The author speculated that Salmonella trapped in cracks and crevices on the seed were inaccessible to lethal concentrations of chemicals (Beuchat, 1997).
In a subsequent study, alfalfa seeds inoculated with E. coli O157:H7 at 1-2 X 106 were treated for 10 minutes with 2.0%, 2.5%, or 3.0% (w/v) calcium hypochlorite solutions containing 13,800, 17,000, or 20,670 ppm active chlorine, respectively (Fett, 1998). The lowest concentration (2.0%) resulted in a 2 log reduction while higher concentrations (2.5 and 3.0%) reduced E. coli O157:H7 by approximately 4 logs. No viable bacterial cells were detected on seed treated with 2.5 or 3.0% calcium hypochlorite when plating was done on selective E. coli/coliform Petrifilm count plates. However, survivors were recovered by both direct plating onto Tryptic Soy Agar (TSA) as well as by enrichment. Plating on both selective and nonselective medium indicated that some of the viable cells of E. coli present after treatment with calcium hypochlorite were injured. The author suggested that additional reductions may occur when chemical treatment is used in combination with physical treatments such as low dose irradiation or heat (Fett, 1998).
Another researcher treated seed with sodium- or calcium hypochlorite, ozonated water and hydrogen peroxide. Of these treatments, 2% calcium hypochlorite showed the greatest reduction but did not completely eliminate the natural microflora. Sodium hypochlorite at concentrations greater than 1% inhibited seed germination. Treatment of over a dozen types of seeds in 2% calcium hypochlorite or 6% hydrogen peroxide resulted in variable germination rates. For example, 2% calcium hypochlorite allowed good germination of alfalfa seeds but drastically reduced germination of onion seeds (Moline, 1999).
Alfalfa seeds were artificially contaminated with E. coli O157:H7 and the effectiveness of sodium hypochlorite, calcium hypochlorite, hydrogen peroxide, ethanol, chlorine dioxide, acidified sodium chlorite, and a consumer product (Vegi-CleanTM, Microcide, Inc., Detroit, Mich.) were tested for produce sanitation. In no instance was E. coli O157:H7 eliminated (L. Beuchat, personal communication). Calcium and sodium forms of hypochlorite at a concentration of 2% appear to be equally effective for E. coli O157:H7 inactivation. However, calcium hypochlorite may be preferable to sodium hypochlorite because it is not as volatile. In addition, calcium hypochlorite solutions up to 20,000 ppm did not affect percent germination of alfalfa seeds. However, at high concentrations, alfalfa seed vigor (rate of germination) was slowed (Beuchat, 1999). Percent germination of alfalfa seed treated for 10 minutes in 3 % calcium hypochlorite solution was not significantly different from untreated seed after 2 days of sprouting, however, the rate of germination was slower (Fett, 1998).
The effectiveness of few other sanitizing agents for reducing the pathogen levels on seeds has been reported in the literature. A 1 - 2 log reduction in microbial counts on rice seed was achieved with a treatment with 1% hydrogen peroxide (Piernas and Guiraud, 1997b). Treatment with 70% ethanol was effective for reducing pathogen levels but significantly inhibited germination. Reducing the ethanol concentration to 10% improved germination but decreased its microbiocidal effect (Piernas and Guiraud, 1997b). Percent germination of alfalfa seed was also significantly decreased by treatment with 30 or 70% ethanol, precluding the use of ethanol as a sanitizer (Taormina and Beuchat, 1999).
Preliminary trials with commercially available sanitizers, such as GlycorineTM (a mixture of lauric acid and sodium lauryl sulfate), have been found to be effective in reducing E. coli O157:H7 by > 2 logs per gram of alfalfa seeds. Trials with these sanitizers have been at least as effective as limited trials with hydrogen peroxide or other chemical seed treatments. Research is continuing to optimize exposure time and concentrations (Slade, 1999). TsunamiTM (active oxygen solution, Ecolab, Mendota Heights, Minn.) and VortexTM (active oxygen solution, Ecolab) at 80 ppm and Vegi-CleanTM at 20,000 ppm for 10 minutes reduced E. coli O157:H7 to < 0.5 CFU/g on alfalfa seeds. However, E. coli O157:H7 was detected from treated seeds after culture enrichment (Beuchat, 1999).
Ozone and ozonated water decreased microbial levels without adversely affecting the sprouting of black matpe and alfalfa seeds; and decreased the natural microflora on beans, peas, grain and spices by 1 - 3 logs (Naito and Shiga, 1989; Naito et al., 1988). Hydronium ion treatment of alfalfa seed has been initiated (Slade, 1999).
Potassium sorbate, calcium propionate, gallic acid, benzoic acid, salicylic acid, dihydroxybenzoic acids, hydroxybenzoic acids, riboflavin, phloxine b, eosin b, nisin, and calcium-EDTA were also tested. Of these treatment options, only calcium-EDTA and 4-hydroxybenzoic acid appear to warrant further study (Moline, 1999).
Surfactants
It has been suggested that the barrier to disinfecting seeds is not in the lethality of the treatment solutions but in the ability of treatments to reach pathogens in the seeds. Microbial cells in seed crevices may be protected from exposure to lethal concentrations of sanitizers and related surface treatments (Caetano-Anolles, 1990; Beuchat, 1997). The use of surfactants alone reduced E. coli O157:H7 populations by approximately 1 log (Beuchat, 1999). Pre-treatments or co-treatment with surfactants have been proposed as a possible means of improving the effectiveness of sanitizing agents. Early research has not been promising. The use of surfactants Tween 80 and benzalkonium chloride had little effect on improving decontamination of rice seed (Piernas and Guiraud, 1997b) or alfalfa seed (Beuchat, 1999) compared to disinfectant treatments alone. However, research is continuing in this area..
Heat
Heat treatments have also been explored as a possible means for reducing pathogen levels on seeds. S. Stanley levels were unchanged after a 10 minute soak in water at 21°C. Soaking seed in 54°C water for 5 or 10 minutes significantly reduced but did not eliminate the pathogen. Soaking seed at 57 or 60° 5 minutes reduced S. Stanley to < 1 CFU/g, without substantial loss of germination. Higher temperatures and longer times (> 5 minutes) caused significant declines in germination. Treatments up to 66°C for 10 minutes caused germination to decline to 6% (Jaquette et al., 1996). The narrow temperature range between treatment efficacy and seed injury may make relying on heat alone to eliminate pathogens difficult on a commercial scale.
Heat and disinfectants
It has been suggested that pathogens that survive chemical disinfection treatments may be injured (Fett, 1998). Microbial populations (APCs) on rice seed decreased at least 5 log cycles when a 5-minute sodium hypochlorite soak was combined with heating at 60°C for 5 minutes (Piernas and Guiraud, 1997b). Alfalfa seed were inoculated with E. coli O157:H7 and treated with various solutions at 55°C for 3 minutes. Treatment with calcium hypochlorite (20,000 ppm active chlorine) eliminated pathogens from three of three samples as determined by direct plating. When seeds were subjected to two 3-minute rinses at 55°C and at the same concentration of calcium hypochlorite, E. coli O157:H7 was detected in one of three samples. The pathogen was detected in all samples after culture enrichment (Taormina and Beuchat, 1999).
In most patents associated with sprouting systems, the decontamination step, if listed, is recommended primarily to inhibit spoilage rather than for pathogen reduction. One U.S. patent, issued for sterilizing and cultivating seeds for sprouting, describes a procedure in which the seeds are exposed to heat for a short period, quickly cooled, and then soaked in chlorine solution (Suzuki and Takizawa, 1997). This treatment reduced but did not eliminate pathogens. Precise treatment conditions were not provided because of variability in the effects of heat on viability of different types of seeds, but 70°C for 20-30 seconds or 90°C for 10 seconds was provided as a guideline.
Radiation
Gamma irradiation has been shown in preliminary studies to be an effective antimicrobial treatment for both seeds and sprouts. Using Cs137 and temperature control (5°C), E. coli O157:H7 and Salmonella were inactivated while seed viability was maintained. However, the dose required to inactivate these pathogens (up to 5 kGy) exceeds current allowable limits (1 kGy) for treatment of produce. Use of gamma irradiation in conjunction with chemical treatments (e.g., 20,000 ppm calcium hypochlorite) is being investigated (Thayer, 1999).
Preliminary trials with electron beam irradiation of alfalfa seeds at 2.5 - 10 kGy at various accelerated voltages appear promising. Researchers observed a linear reduction in the levels of Salmonella and natural microflora at increasing dose levels. Pathogens were not detected in seeds treated at 10 kGy. However, enrichment was not done prior to analysis. Percent germination was not reduced at the highest radiation level tested. However, researchers noted physiological changes (i.e., shortening, thickening, and curling) in the roots of sprouts grown from treated seeds. Additional research on depth of penetration and combining electron beam irradiation at different levels with chemical treatments is planned (Slade, 1999).
Other
The use of a number of physical, non-thermal processing technologies, alone or in combination with antimicrobial chemicals, is virtually unexplored for seed decontamination. Several relatively inexpensive technologies have not been investigated but may have potential use in sprout production. Ultraviolet light might be useful for surface disinfection of seeds, and its activity may be enhanced by antimicrobial chemicals such as hydrogen peroxide. At certain ultrasound frequencies, light is produced (soniluminescence), which might be useful in peroxide activation for microbial control. Flashes of bright white light (PureBright® technology) have been effective for killing microorganisms on food surfaces. The feasibility of using high pressure processing for reducing microbial levels on vegetables has been shown (Arroyo et al., 1997). Pulsed electrical or magnetic field technologies are additional processes that have not been tested and might be applicable to seeds. Inactivation of microbial cells lodged in seed crevices might be enhanced by treatment of the seeds with gaseous antimicrobial compounds (e.g., gas phase peroxide). Incorporation of these compounds into packaging materials might also be useful for controlling microbial growth after sprouting. In addition, vacuum infiltration of calcium or sodium hypochlorite may allow sanitation of surfaces of seeds that have been resistant to current treatments.
Seed disinfection treatments may be done by the seed supplier or by the sprout producer. Some types of treatments may be more easily done at one location than another. In addition, because of the potential for survivors or for recontamination of treated seed, sprout producers may need to implement additional treatments just prior to sprouting.
A reduction in pathogens of 1 - 2 logs has little practical significance because pathogens surviving on seeds would be expected to grow during the spouting process. Some disinfection processes (e.g., 20,000 ppm calcium hypochlorite) have been identified that achieve higher reductions. However, to date, no single treatment has been identified that can eliminate pathogens, if present, or one that can be applied to all types of seed. It is possible that combining treatments may provide sufficient cumulative reductions to meet food safety goals.
Treatments/interventions during the sprouting process
It may be possible to implement treatments during the sprouting process to prevent or inhibit the growth of pathogens that survive seed treatments. However, there is little published literature in this area. Use of rinse water, chlorinated at 100 ppm, during sprouting of mung beans decreased counts of the natural microflora by < 1 log (Splittstoesser et al., 1983). Preliminary trials have begun for a number of other disinfectant treatments that might be applied to sprouts or irrigation water during sprouting. Treatments under investigation include the use of sodium and calcium hypochlorite, hydrogen peroxide, TsunamiTM, sodium EDTA, and sodium chlorite (Fett,1999). As with seed, successful treatments will need to be effective in killing pathogens without being phytotoxic to growing sprouts.
Biofilms tend to protect entrapped foodborne pathogens from the antimicrobial activity of sanitizers and may also interfere with the activity of disinfectants applied to sprouted seed during or after sprouting. Studies on biofilm formation on sprouts have been initiated. Scanning electron microscopy has shown extensive formation of biofilms on commercially obtained sprouts and on 4-day old sprouts grown in the laboratory (Fett, 1999).
Competitive exclusion techniques, where non-pathogenic microorganisms are used to repress the growth of pathogenic bacteria during sprouting have been suggested; however, there are few reports in the literature. When co-inoculated with L. monocytogenes, nisin-producing lactococci isolated from bean sprouts reduced the levels of the pathogen by 1 log (Cai et al., 1997). Work has been initiated on using competitive exclusion during sprouting (Fett, 1999). Naturally occurring microbes from commercial alfalfa sprouts were isolated and tested for their ability to repress the growth of a mixture of Salmonella strains. Preliminary trials have shown 1 - 3 log reductions in Salmonella counts during a 7-day grow-out after challenge with the various microflora isolates.
While contamination of seeds may be at low levels and difficult to detect, microbial populations increase significantly during the sprouting process (Jaquette et al., 1996). Significant levels of microorganisms are recovered in the irrigation water during sprouting. Testing of sprout irrigation water for total counts and coliforms indicate that the water contains at least 90 % of the counts found in the sprouts themselves. It is possible that testing spent irrigation water may be useful for monitoring microbial levels and detecting pathogens that may be present during sprouting. Research is needed to determine an optimum testing protocol, including when and how to test, to maximize accuracy and achieve economic feasibility. The effect of disinfectant treatments applied during sprouting on the level of pathogens and microflora in spent irrigation water is unknown. If disinfectants kill pathogens in irrigation water but allow pathogens to survive in sprouts, testing of irrigation water would be meaningless (Tortorello and Fu, 1999).
Interventions for sprouted seed
There have been few studies on reducing pathogen levels in finished product. Washing mature sprouts with water decreased levels of B. cereus, E. coli or Salmonella by no more than 1 log (Harmon et al., 1987; Potter and Ehrenfeld, 1998). Soaking of fully developed mung bean sprouts for 30 minutes in 0.5% sodium hypochlorite resulted in a 2 log decrease in counts (Splittstoesser et al., 1983). Treating mung bean sprouts with 500 ppm sulfur dioxide reduced microbial counts by 1 - 2 log (Splittstoesser et al., 1983).
As noted earlier, gamma irradiation has been shown in preliminary studies to be an effective antimicrobial treatment for both seeds and sprouts. However, the dose required to inactivate these pathogens (up to 5 kGy) exceeds current allowable limits (1 kGy) for treatment of produce (Thayer, 1999).
Finding 1. Food Safety Knowledge
At the time of the charge from FDA, there was a lack of fundamental food safety knowledge along the continuum from seed production through sprout consumption. This included a general perception that the sprouted seed industry was producing an agricultural commodity and not a "ready-to-eat" food. Since then, most within the sprouted seed industry have become aware of the potential for this food to be a vehicle for foodborne disease and the need for appropriate controls. This includes seed producers, processors and distributors, sprout producers, sprout retailers, and consumers. However, such awareness is not universal.
Recommendation
Industry and government should enhance the knowledge of all interested parties pertaining to the microbiological safety of sprouted seeds by:
a. Ensuring that all stakeholders recognize that seeds and sprouted seeds could be a source of foodborne pathogens.
b. Developing education programs for sprout growers and seed producers on basic principles of food safety microbiology, GMPs, GAPs and HACCP.
c. Educating sprout retailers and consumers about the public health risk associated with the consumption of sprouted seeds.
Finding 2. Seed Production
Contaminated seed is the likely source for most, if not all, reported sprout-associated outbreaks. Seeds for sprouting are not currently produced in a manner different from that for seeds used for planting. There are multiple opportunities during seed production and harvest by which contamination with foodborne pathogenic microorganisms can occur. Once present on seeds, these pathogens are likely to remain viable for extended periods. The potential for seed contamination is likely to be reduced by systematic implementation of good agricultural practices (FDA, 1998). However, prevention of all contamination is unlikely.
Recommendation
Seeds for sprout production should be grown under conditions that minimize exposure to foodborne pathogenic bacteria.
a. Growers should consider the presence of animal production facilities and related factors (e.g., slope of land, runoff controls) that could lead to the crop being inadvertently exposed to untreated manure and take steps to avoid contamination.
b. The use of manure or domestic animal grazing on fields destined for seed production should be managed in accordance with good agricultural practices (FDA, 1998).
c. Sources of agricultural water should be evaluated and managed in accordance with good agricultural practices (FDA, 1998).
Finding 3. Seed Handling
In addition to contamination during their production, seeds for sprouted seed production can be contaminated during post-harvest operations such as transport, conditioning, grading, storage, and packaging. The risk that contamination with foodborne pathogenic microorganisms occurs during this phase can be reduced by developing procedures and barriers that prevent introduction of the pathogen.
Recommendation
The potential for seed to become contaminated after harvest can be reduced by developing and implementing seed cleaning, storage and handling practices that minimize potential sources of contamination that could lead to the introduction of pathogenic microorganisms.
Finding 4. Seed Treatment
A number of treatments have been shown to reduce levels of pathogenic bacteria present on seeds, but none have totally eliminated pathogenic microorganisms. Their routine use is likely to reduce the level of contamination if present and, in turn, decrease the risk for foodborne disease with sprouted seeds. Some of these technologies can be applied at the sprouting facility while others can be applied at earlier steps in the seed production continuum.
Recommendation
Seeds should be treated with one or preferably more than one treatment that has been shown to reduce the level of pathogenic bacteria on seeds.
a. Just prior to sprouting, seeds should be subjected to one or more treatments that can effectively reduce or eliminate pathogenic bacteria (e.g. 20,000 ppm calcium hypochlorite).
b. The Food and Drug Administration and/or other appropriate regulatory agencies should expedite the review of irradiation and other promising new technologies for the treatment of seeds to reduce or eliminate pathogens prior to or at the sprout production facility.
c. Based on currently available data on quantitation of pathogens in seeds and achievable reductions, seeds should undergo a combination of treatment strategies that will achieve a 5-log reduction in the levels of Salmonella spp. and enterohemorrhagic Escherichia coli O157. (See Appendix 3.) This recommendation should be reevaluated as additional data become available. Intervention strategies that deliver less than a 5 log reduction should be coupled with microbiological testing of sprouts or spent irrigation water.
Finding 5. Sprout Production
While seeds have been identified as the primary source of foodborne pathogens on sprouted seeds, the procedures and practices used by sprout producers have a substantial impact on the likelihood that pathogenic bacteria will survive and proliferate in sprouts. This was aggravated by what appears to have been an incomplete understanding by a segment of the sprouted seed industry of the requirements for manufacturing and marketing of ready-to-eat foods. There is a clearly identified need to establish good manufacturing practices and food safety systems that systematically look for means for preventing sprouted seeds from serving as the vehicle for foodborne disease.
Recommendation
The microbiological safety of sprout production could be enhanced by:
a. Classifying sprout production facilities as food processing operations.
b. Ensuring that the sprout industry, in consultation with the Food and Drug Administration and other appropriate regulatory agencies, establish and implement good manufacturing practices for sprout production.
c. Requiring the use of validated microbiological assays to test sprouts or their irrigation water prior to harvest for pathogenic bacteria (see recommendation 4c).
d. The adoption of HACCP programs by sprout producers.
e. Development, implementation, and testing of systems that can be used by sprout producers and seed mill operators to conduct traceback and recalls.
f. The Food and Drug Administration expediting the review of irradiation and other promising new technologies that can be used to treat sprouts to reduce or eliminate pathogens.
Finding 6. Research
There were substantial data gaps in the scientific information related to the microbiological safety of sprouted seeds, particularly in relation to intervention technologies that can be used to prevent contamination or growth of pathogenic bacteria during seed germination and sprouting.
Recommendation
It is recommended that the government and industry fund research related to the microbiological safety of sprouted seeds, particularly in the areas of:
a. The development of additional technologies that can reduce or eliminate pathogenic bacteria on seeds or finished sprouts. The research should focus on technologies that are appropriate for the small business character of the sprout industry.
b. Identification of the sources of contamination of seeds and means for prevention.
c. The development of means for preventing or retarding the growth of pathogenic bacteria (e.g., competitive exclusion) during seed germination and sprout outgrowth.
d. Evaluation of seed characteristics and handling practices to determine their contribution to seed contamination, ease of decontamination, or internalization of pathogenic bacteria.
e. Design of sprouting equipment that is easily cleaned and sanitized.
f. Evaluate the additional risk reduction that could be achieved through the use of various labeling strategies.
g. Development of better methods for evaluating sprouts or spent irrigation water for the presence of pathogenic microorganisms.
TABLE 1. REPORTED UNITED STATES SPROUT OUTBREAKS, 1995-1998 | |||||||
| Year | Pathogen | No. of Cases | Location of Outbreak | Type of Sprout | Likely Source of Contamination | Reference | |
| 1995 | S. Stanley | 242 | 17 states and Finland | Alfalfa | Seed | Mahon et al., 1997 | |
| 1995-96 | S. Newport | >133 | 7 states and Canada | Alfalfa | Seed | Van Beneden et al., 1999 | |
| 1996 | S. Montevideo/ Meleagridis | >500 | California | Alfalfa | Sprouter/Seed | Farrar and Mohle-Boetani, 1999 | |
| 1997 | S. Infantis/ Anatum | 90 | Kansas, Missouri | Alfalfa | Seed | Slutsker, 1999 | |
| 1997 | E. coli O157:H7 | 108 | Michigan, Virginia | Alfalfa | Seed | CDC, 1997 | |
| 1997/98 | S. Senftenberg | 60 | California, Nevada | Clover/Alfalfa | Sprouter/Seed | Farrar and Mohle-Boetani, 1999 | |
| 1998 | E. coli O157:NM | 8 | California | Clover/Alfalfa | Seed | Farrar and Mohle-Boetani, 1999 | |
| 1998 | S. Havana/Cubana | 18 | California | Alfalfa | Seed | Farrar and Mohle-Boetani, 1999 | |
| 1999 | S. Mbandaka | 75 | Oregon, Washington | Alfalfa | Seed | Keene, 1999 | |
TABLE 2. REPORTED INTERNATIONAL SPROUT OUTBREAKS, 1988-1997 | |||||||
| Year | Pathogen | No. of Cases | Location of Outbreak | Type of Sprout | Likely Source of Contamination | Reference | |
| 1988 | S. Saint-Paul | 143 | United Kingdom | Mung Bean | Seed | O'Mahony et al., 1990 | |
| 1989 | S. Gold-Coast | 31 | United Kingdom | Cress | Unkown | Joce et al., 1990 | |
| 1994 | S. Bovismorbificans | 492 | Sweden, Finland | Alfalfa | Seed | Ponka et al., 1995 Puohiniemi et al., 1997 | |
| 1995 | S. Stanley | 114 | Finland | Alfalfa | Seed | Kontiainen et al., 1996 Mahon et al., 1997 | |
| 1995 | S. Newport | ???? | Denmark, Canada | Alfalfa | Seed | Oregon Health Division, 1995 Aabo and Baggesen, 1997 | |
| 1996 | E. coli O157:H7 | >6,000 | Japan | Radish | Unknown | Nat'l Inst. Infect. Dis. and Infect. Dis. Ctrl Div., Ministry of Health and Welfare of Japan, 1997 | |
| 1997 | S. Meleagridis | 78 | Canada | Alfalfa | Seed | Buck et al., 1998 | |
| 1997 | E. coli O157:H7 | 126 | Japan | Radish | Unkown | Gutierrez, 1997 | |
TABLE 3. Distribution of the seed lot implicated in the 1998/99 Salmonella Mbandaka outbreak, associated seed treatment strategy, and association with foodborne illness outbreak*. | |||||
| Sprouter | Pounds of implicated seed lot received | Pounds of implicated seed lot sprouted | Pounds of implicated seed lot embargoed | Seed treatment strategy | Facility implicated in outbreak |
| Sprouter #1 - WA | 2,000 | 1,800 | 200 | no consistent treatment | YES |
| Sprouter #2 - CA | 2,000 | 0 | 2,000 | N/A | N/A |
| Sprouter #3 - CA | 2,000 | 100 | 1,900 | 2,000-20,000 ppm calcium hypochlorite | NO |
| Sprouter #4 - CA | 1,000 | 675 | 325 | 2,000-20,000 ppm calcium hypochlorite | NO |
| Sprouter #5 - CA | 4,000 | 3,850 | 150 | no consistent treatment | YES |
| Sprouter #6 - FL | 2,000 | 2,000 | 0 | 20,000 ppm bleach, 3X for 20 min. each | NO |
| *A total of 40,000 pounds of the implicated seed lot was obtained from farmer A and processed at seed mill B. A total of 15,000 pounds of the original 40,000 pounds of the implicated seed lot remained under the possession of farmer A and was embargoed by the state of California. A total of 12,000 pounds of the original 40,000 pounds of the implicated seed lot was under the ownership of seed distributor C and physically located at seed mill B and was embargoed by the state of California. A total of 13,000 pounds of the implicated seed lot was distributed by seed distributor C to six sprouters as described above. | |||||
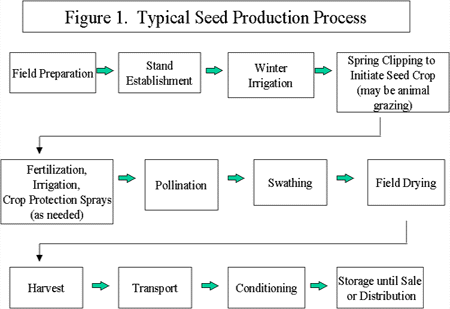
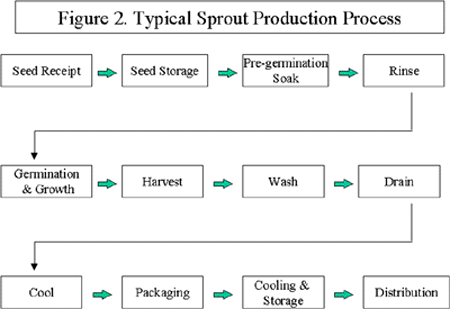
Specific agricultural practices may vary with the type of crop. Some crops are grown exclusively for seed while other crops may be grown for both foliage and seed. For example, beans, including mung beans, are annuals with a single harvest per crop. Other crops, such as alfalfa, are biennials or perennials and may be subject to repeated harvests of foliage (hay or silage) or seed or both.
Alfalfa Seed Production
Approximately 80 million pounds of alfalfa seed are produced each year in the United States (Mueller, 1999). California, Idaho, Oregon, Washington, and Nevada provide 85% of this production. Non-dormant varieties are planted for forage in areas where winters are mild. Over 90% of the seed produced in California are non-dormant seed (i.e., seed from varieties that continue to grow, albeit slowly, during the winter months) and approximately 9% are seed of semi-dormant varieties (i.e., varieties that stop growth for a shorter time (e.g., 2 months vs. 5), compared to dormant varieties). Seed of the more dormant varieties (i.e., varieties that do not grow from about November through March) is typically produced in the Pacific Northwest.
Current seed certification programs are a marketing and quality assurance tool for the forage market. Use of certified seed is a guarantee to customers that the seed is of the stated variety and will perform to expectations in terms of yield and forage quality. Many of the quality attributes of certified seed have significantly less relevance for sprout producers compared to forage producers. Thus, uncertified, or common seed, is seed that most often is used for sprouting. However, there may be aspects of the current seed certification system, such as the ability to trace seed back to its source, that might be considered for incorporation into programs to improve the safety of seed for sprouting.
Seed for certification must pass a series of inspections and be grown under specified conditions. Each state has a seed certifying agency. In California, seed is certified by the California Crop Improvement Association (CCIA). Growers wishing to certify seed submit an application to CCIA with information regarding previous crop history, seed lot planted, etc. CCIA agents inspect the fields for weed control, genetic purity, isolation from other fields, and other factors. CCIA agents certify that the seed is of the stated variety, purity, and quality. Certified seed retains its identity through sale to the end user. Certified seed can be traced back to the field in which it was grown. More than 70% of California's total seed production is certified.
Agricultural Production Practices
Properly timed cultural practices are the keys to any successful agricultural production system. Among the most important production factors that have an impact on seed yield and quality are pollination, irrigation, insects, and weed infestations. For alfalfa seed production in California, this includes:
Irrigation
An essential component of alfalfa seed production is timely irrigation scheduling. Highest seed yields are obtained when irrigation practices prevent severe plant stress and promote slow, continuous growth through the entire production period without excessive stimulation of vegetative growth. Deciding when to terminate irrigation for the season is also critical. Enough water is required to mature the seed, but soil moisture must be depleted prior to desiccation or the plant will not dry down adequately to prepare for harvest.
Insect Control
The most important pests in alfalfa seed fields are lygus bugs that feed on the buds, blooms, and developing seeds of alfalfa throughout the season though other pests may also cause significant yield reductions in some years. Cultural and biological control options are limited in availability and effectiveness for controlling the most significant insect pests. Currently, growers must rely on chemical control of pests in order to maintain economic yields.
Insecticides must be carefully selected and applied to kill the target insects without harming bees required for pollination. There are few chemicals registered for alfalfa seed that effectively control insect pests. Furthermore, in order to use them, growers must comply with label restrictions to insure none of the treated seed or crop residue enters the food chain. This means that seed may not be used for sprouting if the grower relies on currently registered chemicals to control insect pests. Chemical companies typically cannot afford to establish tolerances for their materials to allow them to be registered on alfalfa seed as a food crop.
Weed Control
Control of weeds in alfalfa seed production is important to prevent competition and encourage the growth of the alfalfa. In addition, seed for certification must meet stringent requirements for purity. Weed control in alfalfa seed fields is continuous from stand establishment to the final cleaning process. Mechanical cultivation and use of herbicides registered for alfalfa effectively control most winter and summer annual and perennial weeds. Herbicides can be applied by ground or by air. Rainfall or irrigation is often required to activate them. In some areas, sheep graze seedling fields as a means of weed control.
Since chemical control is not always 100% effective, large weeding crews are often employed to remove remaining weeds from seed fields late in the season. Modern seed cleaning equipment is available to separate and remove weed seeds from alfalfa seed, although a significant quantity of alfalfa seed can be lost in the process. Therefore, control of weeds in the field is a more efficient and less expensive practice.
Pollination
Alfalfa must be cross-pollinated to maintain high forage and seed yields. Pollinator activity significantly affects seed yield. Honeybees, leafcutter bees, alkali bees, or a combination are used to pollinate alfalfa for seed production in the United States.
Honeybees are used almost exclusively to pollinate alfalfa for seed production throughout California. They are relatively inexpensive and readily available, but they are not the most enthusiastic pollinators of alfalfa. Because of their inefficiency, it typically requires a long pollination season to set seed with honeybees.
Desiccation and Harvest(1)
Harvesting alfalfa seed involves either 1) cutting and windrow curing followed by threshing with a pickup combine, or 2) chemically desiccating followed by direct combining the standing crop. Desiccants currently registered restrict using the seed from treated plants for sprouting. Therefore, this discussion will focus on harvesting seed from plants that have been air-dried.
Alfalfa seed should be swathed during periods of high humidity or dew after 2/3 or 3/4 of the pods have changed from green to a dark brown color. The swather cuts the alfalfa just above the crown, and lays it in long strips, or windrows, on the stubble in the field. Physiologically mature seed will ripen in the windrow. Windrows are ready for threshing when the moisture content of the foliage is from 12-18%. Harvesting and combining equipment may occasionally "clip" the ground, taking in dirt and any contamination that is present.
Mechanical damage caused by excessive cylinder speed and lack of proper clearance between cylinder bars and concave has been related directly to seed damage and subsequent poor seed germination. Damaged seed may be more difficult to disinfect as pathogens in cracks and crevices may be more difficult to reach with liquid treatments. Mechanical damage can be identified and monitored with a hand lens or microscope. Damage can be reduced and percentage germination maintained above 90% by the following methods:
Seed is transported from the field to the conditioning facility in steel bins loaded on a flatbed truck or in bottom unloading bulk trucks. Storage bins may be uncovered or covered with metal, paper, or plastic to prevent birds or rodents from entering the bin. Bins are often stacked, and in some cases only the top bin is covered. Bins hold 2,700 pounds of field run seed or 3,200-3,300 pounds of clean seed.
Seed Conditioning
Conditioning removes soil, weed seeds, and other debris from the alfalfa seed. This is accomplished by equipment that utilizes differences in physical characteristics of alfalfa seed and the non-seed fraction, such as particle size, shape, density, and surface texture. Separating machines include 1) air-screen cleaner, 2) specific gravity separator, 3) velvet roll seed separator, 4) magnetic separator, 5) indent disc and cylinder separators, and 6) spiral separators.
The air-screen cleaner makes separations on the basis of particle size, shape, and density. The field-run seed is fed into an air stream that removes lightweight seeds and impurities. The heavier seed and impurities are directed to and flow down or fall through a series of inclined reciprocating screens with different diameter circular and/or rectangular slotted holes. The top screen removes impurities larger than the seed; the last screen separates the seed from fine particles of soil, broken seed, and other impurities that fall through. Cleaned seed is then passed through an air stream that removes any remaining light material.
The specific gravity separator separates on the basis of specific gravity and particle size. The separating unit of the gravity table is a quadrangle-shaped, perforated, tilted, and wire mesh or cloth-covered surface. Air is forced up through this surface as the deck mechanism oscillates on both horizontal and vertical planes. Seed is introduced at the lower corner of the deck and moves up the inclined plane to the opposite sides. The path of the seed and foreign material is dependent upon the specific gravity of each particle and the output is divided into a series of distinct fractions.
The velvet roll seed separator is used for more precise cleaning and separation based upon surface texture and shapes. A series of paired velvet covered rolls are mounted with a few degrees tilt from the horizontal with spacing between the rolls so that at the upper end the velvet makes substantial contact and at the lower end the rolls are completely separate. This separator removes dirt, sand, rough-coated weed seeds, and other foreign materials that have rough edges that cling to the velvet. In most plants, the velvet roll seed separator is used as a finishing machine; however, in a modern installation it is an in-line operation following the gravity separator.
The magnetic separator also makes separations based upon surface texture. Seeds are fed into a screw conveyor with a regulated amount of water and finely ground iron powder. The iron powder clings to rough seed coats, cracks in the seeds, chaff, soil particles, and seeds whose surface becomes sticky when moistened. The mixture is passed over a high-intensity, magnetically charged, rotating drum. Sound seed does not attach to the drum, whereas those particles to which the iron powder adheres are attracted to the drum and later removed. The magnetic separator is used extensively to remove rough-coated dodder seed.
The indent disc and indent cylinder separators are used to remove seeds with the same width or thickness on the basis of length differences. The short seeds are lifted into pockets or indentations in a rotating disc or cylinder. The sizes of the indentations are shallower than alfalfa seed; thus alfalfa seed is not picked up.
The spiral separator is a nonmoving spiral, and its function is based on differences in movement characteristics of particles. Spherical seeds or particles roll down the spiral, whereas the characteristically flat, irregular shaped alfalfa seeds tend to slide. Round particles travel faster and discharge at different distances from the center of the spiral.
Scarification
Some legume seed has a hard seed coat that is more impermeable to water compared to other ("non-hard") seed. Consequently, this hard seed does not readily absorb the moisture needed for germination. Mechanical scarification, or scratching the seed coat, improves percent and uniformity of germination of hard seed.
Seed is generally scarified only if it has a high hard seed percentage. The proportion of hard seed in a seed lot varies with the type of seed (dormant > semi-dormant > non-dormant seed) and where the seed is grown. Seed grown in California from non-dormant varieties is generally between 2 and 12 percent hard seed. When the same non-dormant varieties are grown outside areas of their adaptation (e.g., in the Pacific Northwest), the percentage hard seed increases greatly (to 20 - 40%).
Equipment
All equipment should be thoroughly dry cleaned between seed lots. When seed is intended for sprouting, some conditioners run additional raw seed between lots to clean out lines and minimize risk of contamination from agricultural seed treated with chemicals that are not approved for food use.
Seed Quality
All seed lots are tested for purity, germination rate, and noxious weed content prior to marketing. Seed is stored in metal bins in the warehouse until it is bagged.
1. Information for this section was taken from Rincker, C.M. et al., 1988. Seed Production Practices. In: Alfalfa and Alfalfa Improvement. Hanson, A.A., D.K. Barnes, and R.R. Hill, eds. Agronomy Series 29. American Society of Agronomy, Crop Science Society of America, Soil Science Society of America. Madison, WI.
The following discussion is based on results of the CDHS/FDA survey and inspections and on observations by the FPWG during site visits.
Seed were usually received by the firm in bags or sacks. The most common size noted was 50 pounds (74% of firms), and the most common type bag was cloth. Seed bags usually bore a tag listing the following information: supplier name, supplier address, lot number, seed type name, and country of origin.
Approximately 83% of the manufacturers surveyed stocked seed sacks that were traceable to the source. Ninety-seven % of suppliers were identifiable from labeling and 71% of seed sacks showed imprinted lot codes. Fifty-one % of the bags originated from the United States. There were two firms that did not have supplier identification. One of these firm received seed from China. The other bought its seed as needed from a health food store.
Seven producers said they regularly perform microbiological testing. Amount of seed tested varied from under one ounce to three pounds. One manufacturer followed testing procedures in the FDA Bacteriological Analytical Manual. The majority of manufacturers (51 %) replied that they conducted no testing; though their suppliers provided them purity test analysis for such items as % Germination, % Dormant, and % Hard Seed. These purity tests do not include assays for the presence of pathogenic organisms.
The majority of firms surveyed (41/45) reported that they did not blacklight seed sacks or visually inspect incoming seeds.
It is important that seed are stored off the floor and away from walls to reduce rodent contamination and facilitate inspection for signs of rodent contamination. Open bags should be stored in a receptacle with a tight fitting lid or otherwise protected from contamination. Eight firms were cited for seed storage concerns. Citations included failure to exclude pests, seed storage in a garage among personal items, and standing water and debris in the seed storage area.
Types of pest control used were closely divided (51% in-house and 47% contractor). Firms that reported using contract pest control still showed evidence of rodent, insect, and bird activity. One of five firms showed evidence of domestic pets, three of nine firms showed evidence of rodents, four of nine firms showed evidence of insects, and five of ten firms showed evidence of bird activity.
Soaking causes seeds to swell and softens hulls so a sprout will grow out of the seed. This step may incorporate chlorination/disinfection. Eighteen of the 45 firms surveyed reported that they applied a disinfection treatment during soaking and/or rinsing.
Seeds were soaked in tubs, bins, and drums in either chlorinated or non-chlorinated water. Seeds were soaked for up to 12 hours in non-chlorinated water. When chlorine was used, soak time was generally shorter. However, there was no set relationship between chlorine concentration and length of soak time. Chlorine concentrations ranged from 25 to 5,000 ppm and soak time ranged from 5 to 120 minutes. Several firms reported using chlorine but did not report knowing chlorine concentration.
Rinsing removes residues from soaking. Chlorinated or non-chlorinated water may be used. Buckets and hand held screens were typically used for rinsing. Firms used different combinations of soak and rinse water type. Reported practices included 1) chlorinated water soak and non-chlorinated water rinse, 2) non-chlorinated water soak and chlorinated water rinse, 3) chlorinated water for both soak and rinse, and 4) non-chlorinated water for both steps.
Methods used for germination and growth, and subsequent harvest and washing, varied depending on many factors, including the size of the operation and the type of sprout grown. Few of the firms surveyed utilized nutrients or other additives in their growing procedures.
Surveyed firms reported the following germination processes by sprout type:
| Rotating Drum | Alfalfa, Broccoli, Clover, and Radish |
| Growing Room | Mung Bean, Onion, Adzuki, Lentils, Garbanzo, Peas*, and Wheat berry |
| Soil Tray | Wheat Grass, Sunflower, Peas*, Buckwheat, Daikon (White radish), Broccoli and Sungreens |
| *Peas were grown both hydroponically and in soil. | |
Germination and growing times varied with type of sprout, time of year, and germination process used. Minimum and maximum ranges reported by product were:
| Alfalfa | 3-7 days | Onion | 6-10 days |
| Adzuki | 2-9 days | Peanuts | 5-6 days |
| Broccoli | 4-7 days | Radish | 3-14 days |
| Clover | 3-7 days | Soybean | 4-6 days |
| Garbanzo | 2-9 days | Sunflower | 6-14 days |
| Lentils | 2-9 days | Wheat | 6-7 days |
| Mung beans | 3-8 days | Wheat grass | 3-14 days |
5a. Hydroponic Processes
Hydroponic processes fell into two general categories; rotating drums, generally in a lighted room, and stationary containers (e.g., bins, beds, or buckets) in environmentally controlled rooms or free standing growth chambers which were often kept dark.
Rotating Drums
Rotating drums were usually hard plastic cylinders measuring approximately 5 feet in diameter by 5 feet deep. Drums were positioned sideways and off the ground with a chain attached to the back closed end for rotation to prevent clumping of sprouts. The inside of the drum was typically partitioned into quarters with the same plastic.
Typically, alfalfa, broccoli, clover, and radish seed were sprouted in rotating drums. Each drum can hold eight to 100 pounds of seeds. One hundred pounds of seed can yield approximately 500 pounds of finished product. Once the seed is placed in the drum, the lid is closed and the drum begins to rotate. Water is sprayed intermittently on the seed. For instance, seed may receive a water spray every 10 to 15 minutes for 10 seconds. Temperature of the water may be either ambient or heated to approximately 75°F. The purpose of the water is to 1) keep the sprouts from overheating, 2) remove by-product residues of growth, such as ethylene gas, and 3) irrigate the product.
Air may be blown into the drum to keep the product ventilated. Some manufacturers have drums automatically set for rotation speed, water temperature, and air ventilation.
After sprouts are removed from the rotating drum, they are washed with cool water to remove excess hulls. Sprouts may be washed in a bucket of water and sieved through a hand screen, or they may be placed in a bubbling tub of water to loosen and float off hulls. Sprouts are raked out and spin dried using a centrifuge for one minute, or, in some instances, a clothes dryer for two minutes. Sprouts may also be placed in a cold room or otherwise treated to remove heat generated during the sprouting process. When cool and dry, fully germinated sprouts are ready for packaging.
Some manufacturers germinate sprouts in drums, allow up to 60% of growth to occur, then rinse the sprouts, and transfer them to trays, cups, or final packages (such as clamshell type packages) for further growth. These containers are placed on a tiered growing table or in a growing rack for an average of three to five days. With this process, sprouts will grow in a more vertical, uniform manner and turn greener. Some processors opt to not expose these sprouts to light until the last day of growth.
Growing Rooms and Chambers
Mung beans and soybeans were generally grown in bins or beds in separate rooms. Rooms were kept dark, humid, and with temperatures between 70 to 79°F. Some bins were on wheels so that when the product finished growing, it could be wheeled out of the room to another area for rinsing and packaging.
Seeds were grown in bins or beds for approximately four to seven days. Stationary beds were usually separated with plastic dividers to separate sprouts at different stages of growth. An intermittent spray was applied either manually with a hose or by an automated watering system. The average water spray lasted less than a minute every four hours. Non-chlorinated municipal or well water was generally used.
Mung and onion seed were germinated and grown in large (32-gallon) barrels. Barrels had air ventilation through a pipe, typically had holes at the bottom to drain excess water, and were on pallets. Water (80 to 85°F) was delivered to each line for one minute every four hours. Germination and growth averaged 5 to 6 days. Eight pounds of mung beans yielded 75 pounds of sprouts; 12 pounds of onion seed yielded 25 pounds of sprouts.
Adzuki, lentils, garbanzos, peas, and wheat berry seeds were generally sprouted in 5-gallon buckets. Bucket germination and growth times averaged 2 days. Each 5-gallon bucket held 12 pounds of beans or seeds. Racks were used to hold the buckets of germinating beans or seeds. Temperature of the rooms containing buckets and barrels ranged from 68 to 75°F, with an average room size of 16' by 16'.
Seed may also be sprouted in a free standing growth chamber. A Sprouter Automatic Sprinkler System for germinating mung beans was seen at 1 firm. It was an 8' by 2' by 4' device to hold the seed in a dark, controlled environment in a lighted room. Growth chambers may be used for low poundage production, e.g., about 200 pounds of sprouts per production cycle.
5b. Soil
Ten of the firms surveyed were growing sprouts in soil. These firms were also producing hydroponically grown sprouts. Typically, soil-grown and hydroponic products were grown in separate rooms.
Sprouts were planted and grown in plastic trays containing a composted soil that was mixed to the producer's preferences. In one case, the soil mix included composted horse manure, bone meal, and oyster shell flour.
Seed used for soil trays was soaked and rinsed as described above for hydroponic operations. After rinsing, wet seed was held for 12 to 24 hours in buckets to allow for initial germination. Meanwhile, plastic trays (approximately 2' by 3') were filled with soil. When the seeds were ready, they were spread on top of the soil and leveled out. Growing usually took place in a green house. Water was sprayed on trays two times daily, usually by an automated pipe system overhead. Sprouts can take 5 to 10 days for optimal growth in wintertime, or 3 to 10 days in summertime.
Most soil-grown sprouts were harvested at the facility, washed, packaged, and delivered to the customer. Typically, soil-grown products were brought into the cutting and packaging room in soil trays. They were often there at the same time as the hydroponic items. At one firm the following was observed. Dirt trays were placed on one of three tables and the sprouts were cut from the stems and placed in large totes. Dirt trays were piled beside the table until cutting was completed. Soil-grown sprouts were then mixed with other types of sprouts and called "sprout salad" (sunflower, onion, radish, and clover) and "mixed bean snack" (whole peas, garbanzos, adzuki, and lentils.)
Alternatively, some processors delivered the product as is, in the tray, directly to the retailer. At the retailer the tray was inserted in a plastic bag and placed in a cooler. Typical retailers of this product were juice blending shops. These shops cut sprouts, primarily wheat grass, directly from the tray and drop them into a blender with other fruits, vegetables, and various ingredients. Apparently, some sprout producers and retailers view product that is allowed to continue growing in soil and is not harvested until just before use as more healthy and organic compared to product that is harvested and packaged by the producer.
When the retailer is finished cutting/snipping sprouts from the tray, the tray of soil with remaining product (e.g., roots) is returned to the sprout producer. The producer may pick up these trays while delivering new trays of freshly grown, uncut product. Soil and remaining spout parts were then deposited into a compost mound. The soil may be eventually reused for subsequent sprout production.
Firms reported various packaging processes, including 1) harvesting and washing sprouts at the facility and placing sprouts into either bulk or individual retail containers, 2) 60% growth in a drum followed by further growth in individual trays, cups, or clam shell type packaging, then closing and distributing as is, and 3) soil tray growth without harvest. Packaging was usually done manually, in a separate packaging room.
Types of packaging used to transport product during distribution included 1) plastic bags (with and without holes for respiration) with heat-sealed or wired-twist closures, resealable ziploc or tamper evident plastic bags; 2) heat sealed cellophane bags with partially-sealed film covers to allow respiration; 3) rigid plastic cup or box-type containers with completely sealed film cover; 4) clam-shell type packages; 5) uncovered and plastic-covered soil trays; and 5) used produce boxes.
Packaging usually occurred at the growing site. However, occasionally, product was transported in bulk to another location to be packaged. Some firms selling at farmers' markets transported the product in bulk and packaged the product into retail containers as it was sold.
Finished product may be sent directly to the retailer with no refrigeration, or may be placed into a cooler to await distribution. Of firms surveyed, time in the cooler ranged from one hour to five days. One firm, that was cited with many other objectionable conditions, kept product in their cooler for up to two weeks.
Temperatures in most coolers ranged from 34°F to 45°F. Five coolers were over 45°F. Some cooler thermometers were not operating or had inaccurate readings. Five firms had no refrigeration at the sprouting facility.
The 45 firms surveyed used a total of 116 trucks, vans, and cars for product delivery. Of these 116 vehicles, 51 percent were refrigerated. Some vehicles had refrigeration and thermometer gauges. The general condition of 64 percent of the delivery vehicles was rated by inspectors as "adequate".
Reported delivery times ranged from 20 minutes up to 9 hours. Ninety-seven percent of firms surveyed distributed to firms within California while 11 percent distributed outside California. One firm reported up to 100 miles for delivery distance. Maximum delivery times depended not only on delivery distance, but also on the number of delivery stops made.
Delivery distance, time, and refrigeration status of vehicles must take into account dock time and conditions. Dock time is the time between removal from refrigeration at the firm and the time product is placed in the delivery vehicle. Thirty-seven of the 45 firms surveyed answered the question on dock time. Fifty percent of these firms stated they had no dock time; 32 percent stated they had less than 30 minutes; and 21 percent stated they had dock time from one hour to 10 hours. Often, temperature was not controlled on loading docks. Where temperature was controlled, the set point was usually above 45°F.
Water Quality
Twenty two percent of firms used non-municipal well water. Half of these firms tested their water at least for coliforms. Seventy three percent used municipal water, and 4 % used both well and city water.
Sanitation
Facilities were evaluated by state or Federal investigators and subjectively assigned a score of 1 (poor) to 10 (good). Nineteen of 45 firms scored no more than 5. Many firms (23/45) firms did not have hot water for cleaning. Of those reporting to have hot water, only 10 had hot water between 110 and 160°F.
At 9 firms, equipment and/or utensils were broken or cracked potentially allowing microorganisms or mold to grow in crevices. Seventeen firms did not store equipment in a manner that would prevent contamination. Six firms had equipment that were made of wood, pitted plastic, or were not easily accessible for cleaning. Equipment surfaces had buildup of brown residue. At several firms, totes were noted in direct contact with the floor in foot traffic areas after washing and sanitizing. Some firms also reused produce boxes to distribute product. Used produce boxes may not have been adequately cleaned or protected during storage.
Restrooms
Restrooms were evaluated by state or Federal investigators and subjectively assigned a score of 1 (poor) to 10 (good). Fifteen of the 45 firms scored no more than 5.
Food residue, oil, and bacteria on hands can best be removed with hot water and soap. However, 15 firms had no hot water. There was no soap at 9 firms; no hand washing signs at 23; and no paper towels at 16. One firm had no hot water, soap, or paper towels. One firm had only hot water.
Restrooms need to be clean and easily accessible. They need to have hot water, soap, and sanitary hand drying. At a number of firms, restroom facilities were far away from the growing and processing facilities, in owners' homes, or were port-a-pots across fields. One had a toilet that opened into the processing area and had no door or vent to the outside of the building. At this firm, used toilet paper was held in an open 5-gallon container. A second firm also had used toilet paper stored in an open bucket in the bathroom. One firm had a portable toilet, no hand washing station, and eight employees.
Hand dip stations are important to sanitize clean hands and gloves. However, 30 firms did not have hand dip stations.
Training
Twenty-three firms reported that employees had received hygiene or sanitation training in the past year. Length and type of training ranged from one day for new employees at 5 firms to continuous on the job training at 7 firms. Twenty firms reported no current hygiene/sanitation supervision. There was a close correlation between the "no" answer on the question for whether training was given and the "no" answer to the question for whether there was current supervision.
Lack of awareness about contamination was a major issue on the FDA 483s (Inspectional Observations) and CDHS/FDB R.O.s (Report of Observations). Many individuals were observed handling unsanitized items and then returning to packaging sprouts without washing and sanitizing their hands and/or gloves. Food containers were placed on the floor to fill and move product from one operation to the next.
Airborne Hazards
The sprout processing facility was not fully enclosed at 11 firms. Some firms operated with open unscreened doors or windows and traffic pollutants, dirt, and dust could enter into the growing and packaging areas. At one firm, a wall humidifier was improperly maintained in a way that could trap and propagate molds, bacteria, and spores which could then blow across the growing trays.
Testing of Finished Product
A testing program is most effective when based on a written plan that specifies types of sprouts to be tested, sample size, frequency of testing, tests to be performed, and limits. The plan should include actions to be taken when limits are exceeded.
Ten firms reported that they had tested their finished product in the past, primarily for generic E. coli and Salmonella. Five of these firms stated they tested with a regular frequency of once each week or once each month. Though some firms grew many types of sprouts, alfalfa sprouts were the predominant sprout tested.
Record Keeping/Traceback Mechanisms
As mentioned previously, seed suppliers were identifiable from tags on seed bags at most firms (43/45). Further, 36 firms had seed receiving documents available for the past six months, and 32 firms could identify lot numbers from tags on seed sacks. Only 5 firms used a production lot number or color coding system on finished product.
Thorough record keeping allows traceback from finished product on the market optimally back to the seed grower. None of the firms surveyed kept adequate records for a complete traceback. This means that in the event of a foodborne illness outbreak, none of the firms surveyed have the capability to isolate when, where, and how the problem may have occurred.
Limited quantitative data is available on the level of foodborne pathogens present in seeds used for sprouting. Quantitative analyses performed on seeds associated with illness attributed to sprout consumption found pathogen levels ranging from <1 - 6/100 g of seed (Inami and Bryant, 1999). Therefore the worse case scenario for seed contamination was assumed to be 1 pathogen/10g of seed. It was also assumed that fifty kg of seeds is the amount of starting material for each batch of sprouts. This yields an initial level of 5,000 pathogens per batch of sprouts. Thus, a 1- log treatment reduction will yield 500 pathogens/batch; a 2-log treatment 50 pathogens/batch; a 3- log treatment 5 pathogens/batch; a 4-log treatment 0.5 pathogens/batch (one batch out of every two will contain a pathogen); and a 5-log treatment 0.05 pathogens/batch (one batch out of every twenty will contain a pathogen). It is realized that the actual extent of risk reduction achieved will likely be greater than this because the extent of initial contamination and the amount of seed used per batch of sprouts will typically be less than the values assumed in the current worst case calculation.
Aabo, S. and D.L. Baggesen. 1997. Growth of Salmonella Newport in naturally contaminated alfalfa sprouts and estimation of infectious dose in Danish Salmonella Newport outbreak due to alfalfa sprouts. In: Program and abstracts of Salmonella and Salmonellosis '97. Nice, France. pp 425-426.
Andrews, W.H., C.R. Wilson, P.L. Poelma, A. Romero, and P.B. Mislivec. 1979. Bacteriological survey of sixty health foods. Appl. Environ. Microbiol. 37:559-566.
Andrews, W.H., P.B. Mislivec, C.R. Wilson, V.R. Bruce, P.L. Polema, R. Gibson, M. W. Trucksess, and K. Young. 1982. Microbial hazards associated with bean sprouting. J. AOAC. 65:241-248.
Arroyo, G., P. D. Sanz, and G. Prestamo. 1997. Effect of high pressure on the reduction of microbial populations in vegetables. J. Appl. Microbiol. 82: 735 - 742.
Beuchat, L. R. 1996. Pathogenic microorganisms associated with fresh produce. J. Food Prot. 59: 204 - 216.
Beuchat, L. R. 1997. Comparison of chemical treatments to kill Salmonella on alfalfa seeds destined for sprout production. Int. J. Food Microbiol. 34: 329 - 333.
Beuchat, L.R. 1999. University of Georgia, Center for Food Safety and Quality Enhancement. Personal communication.
Buchanan, R. 1999. U.S. Food and Drug Administration, Center for Food Safety and Applied Nutrition, Washington, DC. Personal Communication.
Caetano-Anolles, G., G. Favelukes, and W. D. Bauer. 1990. Optimization of surface sterilization
for legume seed. Crop Sci. 30: 708 - 712.
Cai, Y., L.-K. Ng, and J. M. Farber. 1997. Isolation and characterization of nisin-producing
Lactococcus lactis subsp. lactis from bean sprouts. J. Appl. Microbiol. 83: 499 - 507.
California Department of Health Services and U.S. Food and Drug Administration. 1998.
California Sprout Industry Survey.
California Sprout Working Group. 1997. Voluntary California Sprout Growers Guidelines.
California Department of Health Services.
http://www.dhs.ca.gov/ps/dfdrs/fdb/food/sprouts.htm
Centers for Disease Control and Prevention. 1997. Outbreaks of Escherichia coli O157:H7
associated with eating alfalfa sprouts - Michigan and Virginia, June-July 1997. Morbid. Mortal.
Weekly Rep. 46: 741-744.
Chao, W.L., R.J. Ding, and R.S. Chen. 1988. Survival of Yersinia enterocolitica in the
environment. Can. J. Microbiol. 34:753-756.
Cover, T.L. and R.C. Aber. 1989. Yersinia enterocolitica. N. Engl. J. Med. 321:16-24.
Farrar, J. and J. Mohle-Boetani. 1999. California Department of Health Services. Personal
communication.
Fett, W. 1999. U.S. Department of Agriculture, Agriculture Research Service, Eastern Regional
Research Center. Personal communication.
Food and Drug Administration. 1998. Guidance for Industry - Guide to Minimize Microbial
Food Safety Hazards for Fresh Fruits and Vegetables.
http://www.foodsafety.gov/~dms/prodguid.html.
Gutierrez, E. 1997. Japan prepares as O157 strikes again. Lancet. 349:1156.
Hara-Kudo, Y., H. Konuma, M. Iwaki, F. Kasuga, Y. Sugita-Konishi, Y. Ito, and S. Kumagai.
1997. Potential hazard of radish sprouts as a vehicle of Escherichia coli O157:H7. J. Food Prot.
60: 1125-1127.
Harmon, S.M., D.A. Kautter, and H.M. Solomon. 1987. Bacillus cereus contamination of seeds
and vegetable sprouts grown in a home sprouting kit. J. Food Prot. 50:62-65.
Inami, G and R. Bryant. 1999. California Department of Health Services. Personal
communication.
Itoh, Y., Y. Sugita-Konishi, F. Kasuga, M. Iwaki, Y. Hara-Kudo, N. Saito, Y. Noguchi, H.
Konuma, and S. Kumagai. 1998. Enterohemorrhagic Escherichia coli O157:H7 present in radish
sprouts. Appl. Environ. Microbiol. 64:1532-1535.
Jackson, L.A. 1998. Overview of outbreaks of foodborne illness associated with fresh fruits and
vegetables. Symposium on Fresh Fruits and Vegetables: Food Safety Challenges, Institute of
Food Technologists and the National Center for Food Safety and Technology, Rosemont, IL, May
12 - 14.
Jaquette, C. B., L. R. Beuchat, and B. E. Mahon. 1996. Efficacy of chlorine and heat treatment in
killing Salmonella Stanley inoculated onto alfalfa seeds and growth and survival of the pathogen
during sprouting and storage. Appl. Environ. Microbiol. 62: 2212 - 2215.
Joce, R., D.G. Sullivan, C. Strong, B. Rowe, M.L.M. Hall, and E.J. Threlfall. 1990. A national
outbreak of Salmonella Gold-Coast. Comm. Dis. Rep. 4:3-4.
Keene, W.E. 1999. Oregon Health Division. Personal Communication.
Kapperud, G. 1991. Yersinia enterocolitica in food hygiene. Int. J. Food Microbiol. 12:53-65.
Kontiainen, S., K. Sammalkorpi, and A. Siitonen. 1996. High frequency of septicemia in an
outbreak of Salmonella Stanley infections. Scan. J. Infect. Dis. 28:207.
Kramer, J.M.. and R.J. Gilbert. 1989. Bacillus cereus and other Bacillus species, p. 21-70. In M.P.
Doyle (ed.), Foodborne Bacterial Pathogens. Marcel Dekker, New York.
Lovett, J. 1989. Listeria monocytogenes. P. 283-310. In Foodborne Pathogens. (ed.) M.P. Doyle.
Marcel Dekker, New York.
Mahon, B.E., A. Ponka, W. Hall, K. Komatsu, L. Beuchat, S. Shiflett, A. Siitonen, G. Cage, M.
Lambert, P. Hayes, N. Bean, P. Griffin, and L. Slutsker. 1997. An international outbreak of
Salmonella infections caused by alfalfa sprouts grown from contaminated seed. J. Infect. Dis.
175:876-882.
Mistcherlich, E. and E.H. Marth. 1984. Microbial survival in the environment. Berlin: Springer-Verlag.
Moline, H.E. 1999. U.S. Department of Agriculture, Agriculture Research Service, Beltsville
Agricultural Research Center (retired). Personal communication.
Moline, H.E. and M.M. Kulik. 1997. Contamination and deterioration of alfalfa sprouts caused
by a seedborne isolate of Erwinia herbicola. J. Food Quality 20: 53 - 60.
Mueller, S.C. 1999. University of California, Davis. U.S. Cooperative Extension Service.
Personal communication.
National Advisory Committee on Microbiological Criteria for Foods. 1998. Microbiological
Safety Evaluations and Recommendations on Fresh Produce. Food Control. 10:321-347.
Naito, S. and I. Shiga. 1989. Studies on utilization of ozone in food preservation. IX. Effect of
ozone treatment on elongation of hypocotyl and microbial counts of bean sprouts. J. Jap. Soc.
Food Sci. Technol. 36: 181 - 188. (Abstract in English).
Naito, S., Y. Okada, and T. Sakai. 1988. Studies on utilization of ozone in food preservation. V.
Changes in microflora of ozone-treated cereals, grain, peas, beans and spices during storage. J.
Jap. Soc. Food Sci. Technol. 35: 69 - 77. (Abstract in English).
National Institute of Infectious Diseases and Infectious Diseases Control Division, Ministry of
Health and Welfare of Japan. 1997. Verocytotoxin-producing Escherichia coli
(enterohemorrhagic E. coli) infection, Japan, 1996-June 1997. Infect. Agents Surveil. Rep.
18:153-154.
O'Mahony, M.J. Cowden, B. Smyth, D. Lynch, M. Hall, B. Rowe, E.L. Tearle, R.E. Tettmar, A.S.
Rampling, M. Coles, R.J. Gilbert, E. Kingcott, and C.L.R. Bartlett. 1990. An outbreak of
Salmonella saint-paul infection associated with bean sprouts. Epidemiol. Infect. 104:229-235.
Oregon Health Division. 1995. Salmonellosis outbreak traced to alfalfa sprouts-Oregon and BC.
Communicable Disease Summary. OR:OHD, 45.
Patterson, J.E. and M.J. Woodburn. 1980. Klebsiella and other bacteria on alfalfa and bean
sprouts at the retail level. J. Food Sci. 45:492-495.
Pezzino, G., C. Miller, R. Flahart, and S.R. Potsic. 1998. A multi-state outbreak of Salmonella
serotypes Infantitis and Anatum - Kansas and Missouri, 1997. Kansas Medicine. 98(3): 10-12.
Piernas, V. and J.P. Guiraud. 1997a. Microbial hazards related to rice sprouting. Int. J. Food Sci.
Technol. 32: 33 - 39.
Piernas V., and J. P. Guiraud. 1997b. Disinfection of rice seeds prior to sprouting. J. Food Sci.
62: 611 - 615.
Ponka, A., Y. Anderson, A. Sitonen, B. deJong, M. Jahkola, O. Haikala, A. Kuhmonen, and P.
Pakkala. 1995. Salmonella in alfalfa sprouts. Lancet. 345:462-463.
Potter, A.P. and E.E. Ehrenfeld. 1998. Microbial loads of bean sprouts: coliform, E. coli and
Salmonella. Poster Q-243, 98th General Meeting of the American Society for Microbiology,
Atlanta, GA, May 17 - 21.
Puohiniemi, R., T. Heiskanen, and A. Siitonen. 1997. Molecular epidemiology of two
international sprout-borne Salmonella outbreaks. J. Clin. Microbiol. 35:2487-2491.
Prokopowich, D. and G. Blank. 1991. Microbiological evaluation of vegetable sprouts and seeds.
J. Food Prot. 54: 560 - 562.
Rafii, F., M.A. Holland, W.E. Hill, and C.E. Cerniglia. 1995. Survival of Shigella flexneri on
vegetables and detection by polymerase chain reaction. J. Food Prot. 58:727-737.
Slade, P. 1999. National Center for Food Safety and Technology. Summit-Argo, IL. Personal
Communication.
Slutsker, L. 1998. Centers for Disease Control and Prevention. Personal communication.
Sly, T. and E. Ross. 1982. Chinese foods: relationship between hygiene and bacterial flora. J.
Food Prot. 45: 115 - 118.
Smith, M.A. 1999. Food and Drug Administration, Center for Food Safety and Applied
Nutrition, Washington, DC. Personal communication.
Splittstoesser, D.F., D.T. Queale, and B.W. Andaloro. 1983. The microbiology of vegetable
sprouts during commercial production. J. Food Safety. 5:79-86.
Suzuki, T. and T. Takizawa.. 1997. Sprouted vegetables seeds steril
Thayer, D. 1999. U.S. Department of Agriculture, Agriculture Research Service, Eastern
Regional Research Center. Personal communication.
Taormina, P.J. and L.R. Beuchat. 1999. Comparison of chemical treatments to eliminate
enterohemorrhagic Escherichia coli O157:H7 on alfalfa seeds. J. Food Prot. 62:318-324.
Tortorello, M.L. and T.J. Fu. 1999. U.S. Food and Drug Administration. Center for Food Safety
and Applied Nutrition. Personal communication.
Van Beneden, C.A., W.E. Keene, R.A. Strang, D.H. Werker, A.S. King, B. Mahon, K. Hedberg,
A. Bell, M.T. Kelly, V.K. Balan, W.R. MacKenzie, and D. Fleming. 1999. Multinational
outbreak of Salmonella enterica serotype Newport infections due to contaminated alfalfa sprouts.
JAMA. 281:158-162.
Wantabe, Y. and K. Ozasa.. 1997. An epidemiological study on an outbreak of Escherichia coli
O157:H7 infection. Rinsho Byori. 45:869-874.
Hypertext updated by ear 2001-AUG-15
Foodborne Illness
Foods Home
|
FDA Home
|
Search/Subject Index
|
Disclaimers & Privacy Policy
|
Accessibility/Help