Dermatologists Release Psoriasis Care Guidelines
Biologic use is expected to enhance quality of treatment and patient safety.
|
E-mail this article
Subscribe to news
Printer friendly version
|
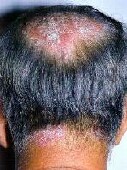
(SOURCE: American Academy of Dermatology, news releaes, May 21, 2008)
FRIDAY, May 30 (HealthDay News) -- The use of biologics is the focus of new guidelines for the management of mild to severe psoriasis and psoriatic arthritis released by the American Academy of Dermatology (ADA).
Biologics -- given by injection or infusion -- are systemic medications that pinpoint immune responses involved with psoriasis and psoriatic arthritis.
About 6 million to 7 million Americans have psoriasis, and between 300,000 and 750,000 have psoriatic arthritis. The new guidelines, based on an analysis of scientific studies of the conditions, were published in the May issue of the Journal of the American Academy of Dermatology.
"As a result of the impartial analysis of the most current research, physicians now have evidence-based guidelines which will help enhance the quality of care for patients and ultimately their safety," ADA President Dr. C. William Hanke said in a prepared statement.
"The guidelines for psoriasis and psoriatic arthritis, which focus on the use of biologics, are the first phase of the academy's efforts. The academy is currently working on the second phase of these guidelines, which will provide evidence-based advice for the management of psoriasis with topical therapies, ultraviolet light therapy, and systemic non-biologic therapies for psoriasis."
The new guidelines advise that topical treatments are appropriate for patients who are good candidates for localized therapy. But, in many cases, topical treatments should not be used exclusively to treat psoriasis if patients would benefit from a combination of systemic and/or phototherapy treatments.
Currently, five biologics are approved by the U.S. Food and Drug Administration for treatment of psoriasis, and three of those five are approved for psoriatic arthritis.
Because biologics target the immune system, it's important to use all approaches, including vaccination, to prevent infection, the guidelines state. However, once biologic therapy has started, patients should avoid vaccinations with live vaccines and consult their dermatologist before receiving vaccination of any kind.
Patients undergoing biologic therapy need to have periodic re-evaluations by their dermatologist to check for the development of new symptoms, including infections and potential cancers.
"Regardless of the therapy that is used to treat psoriasis or psoriatic arthritis, patients need to be in constant contact with their dermatologists to report any unusual side effects or fluctuations in their condition that may require an adjustment in their medications or indicate the onset of psoriatic arthritis or another secondary medical condition," Hanke said. "These guidelines should help further the understanding of the current psoriasis therapies and help enhance the overall health and quality of life of patients."
More information
The U.S. National Institute of Arthritis and Musculoskeletal and Skin Diseases has more about psoriasis.
Copyright © 2008 ScoutNews, LLC. All rights reserved. 
HealthDayNews articles are derived from various sources and do not reflect federal policy. healthfinder.gov does not endorse opinions, products, or services that may appear in news stories. For more information on health topics in the news, visit the healthfinder.gov health library.