Guidance for Industry
Control of Listeria monocytogenes in Refrigerated or Frozen
Ready-To-Eat Foods
Draft Guidance
Contains Nonbinding Recommendations
This guidance document is being distributed
for comment purposes only.
Comments and suggestions regarding this draft document
should be submitted within 60 days of publication in the Federal Register
of the notice announcing the availability of the draft guidance. Submit
comments to the Division of Dockets Management (HFA-305), Food and Drug
Administration, 5630 Fishers Lane, rm. 1061, Rockville, MD 20852. All comments should be identified with the docket number listed in the notice of
availability that publishes in the Federal Register.
For questions regarding this draft document contact the
Center for Food Safety and Applied Nutrition (CFSAN) at 301-436-1400.
U.S. Department of Health and Human Services
Food and Drug Administration
Center for Food Safety and Applied Nutrition
February 2008
Table of Contents
- Introduction
- Background
- Listeria monocytogenes
- How the Recommendations in this Guidance can Help Prevent Foodborne Illness
- Who Should Follow the
Recommendations in this Guidance?
- How Does this Guidance Relate to
Our Current Good Manufacturing Practice Regulations in 21 CFR Part 110?
- How Does this Guidance Relate to
Other Regulations and Guidance that Apply to Refrigerated or Frozen
Ready-to-eat foods?
- What Definitions Apply to this
Guidance?
- How Should I Formulate a RF-RTE
Food to Prevent the Growth of L. monocytogenes?
- How Should I Use a Listericidal
Measure to Control L. monocytogenes?
- What Controls Should I Establish
and Implement on Ingredients and Other Raw Materials?
- Ingredients that are Potential Sources of L. monocytogenes
- Controlling L. monocytogenes in Ingredients that are Reasonably Likely to Be Contaminated with L. monocytogenes
- Applicable Sections of 21 CFR part 110
- What Storage Practices Should I
Establish and Implement?
- What Controls Should I Establish
and Implement Regarding Personnel?
- What Controls Should I Establish
and Implement on the Design, Construction and Operation of My Plant?
- Design and Construction of the Plant
- General Operation of the Plant
- Use of Compressed Gas, Water, and Ice in the Plant
- What Controls Should I
Establish and Implement for the Design, Construction and Maintenance of
Equipment?
- Design and Construction of Equipment
- Maintenance of Equipment
- What Controls Should I Establish
and Implement Regarding Sanitation?
- General Sanitation Program
- Cleaning Drains
- Sanitizers
- Sanitation Monitoring
- Sanitation Control Records
- What Transportation Controls
Should I Establish and Implement?
- What Should I Do to Monitor
Critical Surfaces and Areas to Detect Locations that Harbor Listeria
Species or L. monocytogenes?
- Written Plan
- Critical Food-Contact Surfaces
- Critical Non-Food-Contact Surfaces and Areas
- Procedures for Collecting Environmental Samples
- Applicable Sections of 21 CFR part 110
- What Sampling Should I Do of
Finished RF-RTE Foods?
- What Should I Do if I Detect
Contamination of a Critical Food-Contact Surface or Food With Listeria
Species?
- What Corrective Actions Should I
Take Regarding my Plant and my Processing if I Detect Listeria Species
or L. monocytogenes on a Critical Surface or Area or in Food?
- What Corrective Actions Should I
Take Regarding Food if I Detect Contamination of a Critical Food-Contact
Surface or Food With L. monocytogenes, or if I Assume that Listeria
Species on a Critical Food-Contact Surface or in Food is L. monocytogenes?
- Food that Supports the Growth of L. monocytogenes
- Food that Does Not Support the Growth of L. monocytogenes
- Records of Corrective Actions
- Applicable Sections of 21 CFR Part 110
- What Training is Recommended?
- What Analytical Methods Can I
Use to Detect Listeria Species, and to Detect, Confirm, or Enumerate L.
monocytogenes?
- What Records Should I Make and
Keep?
- References
- List of Appendices
Contains Nonbinding Recommendations
Guidance for Industry(1)
Control of Listeria monocytogenes In Refrigerated or Frozen
Ready-To-Eat Foods
Draft Guidance
This draft guidance, when
finalized, will represent the Food and Drug Administration's (FDA's) current
thinking on this topic. It does not create or confer any rights for or on any
person and does not operate to bind FDA or the public. You can use an
alternative approach if the approach satisfies the requirements of the
applicable statutes and regulations. If you want to discuss an alternative
approach, contact the FDA staff responsible for implementing this guidance. If
you cannot identify the appropriate FDA staff, call the appropriate telephone
number listed on the title page of this guidance.
I. Introduction
The purpose of this document is to provide guidance to
industry on how to control Listeria monocytogenes (L. monocytogenes)
in refrigerated or frozen ready-to-eat (RF-RTE) foods. Specifically, this
document provides guidance on:
- The formulation of RF-RTE foods to prevent the growth of L.
monocytogenes;
- Treatment of foods to destroy viable cells of L.
monocytogenes;
- Controls on ingredients;
- Controls on storing RF-RTE foods;
- Controls on:
- Employee hygiene;
- Design, construction, and operation of your physical
plant;
- Design, construction and maintenance of equipment; and
- Transportation.
- Sanitation controls and monitoring;
- Monitoring critical surfaces and areas to detect locations
that harbor Listeria species or L. monocytogenes;
- Corrective actions to consider if you detect Listeria
species or L. monocytogenes on critical surfaces or areas or in
food;
- Recommended training;
- Methods for collecting environmental samples;
- Methods for preparing environmental samples for analysis;
- Recommended methods to detect or enumerate Listeria
species or L. monocytogenes;
- Recommended records of the controls that you establish and
implement.
We recommend that you assess this guidance as it relates to
each of your operations and tailor your control strategies to the specific
circumstances of each operation.
FDA's guidance documents, including this guidance, do not
establish legally enforceable responsibilities. Instead, guidance describes
the Agency's current thinking on a topic and should be viewed only as
recommendations, unless specific regulatory or statutory requirements are
cited. The use of the word should in Agency guidance means that
something is suggested or recommended, but not required.
II. Background
A. Listeria monocytogenes
L. monocytogenes is a bacterium that can contaminate
foods and cause a mild non-invasive illness (called listerial gastroenteritis)
or a severe, sometimes life-threatening, illness (called invasive listeriosis).
Persons who have the greatest risk of experiencing listeriosis after consuming
foods contaminated with L. monocytogenes are fetuses and neonates who
are infected after the mother is exposed to L. monocytogenes during
pregnancy, the elderly, and persons with weakened immune systems. Invasive
listeriosis is characterized by a high case-fatality rate, ranging from 20
percent to 30 percent (Ref. 1).
L. monocytogenes is widespread in the environment. It
is found in soil, water, sewage, and decaying vegetation. It can be readily
isolated from humans, domestic animals, raw agricultural commodities, and food
processing environments (particularly cool damp areas) (Refs. 2 through 4).
Control of L. monocytogenes in the food processing environment has been
the subject of a number of scientific publications (Refs. 5 through 10). L.
monocytogenes can multiply slowly at refrigeration temperatures, thereby
challenging an important defense against food-borne pathogens, refrigeration
(Refs. 1, 11, and 12).
Most cases of human listeriosis occur sporadically - that is,
in an isolated manner without any apparent pattern. However, much of what is
known about the epidemiology of the disease has been derived from
outbreak-associated cases, in which there is an abrupt increase in reports of
the disease. With rare exceptions, foods that have been reported to be
associated with outbreaks or sporadic cases of listeriosis have been foods that
can support the growth of L. monocytogenes and that are ready-to-eat
(including coleslaw, fresh soft cheese made with unpasteurized milk,
frankfurters,(2)
deli meats, and butter) (Ref. 1). Outbreaks of listeriosis are often
associated with a processing or production failure (Ref. 13); this association has
been less evident among sporadic cases (Ref. 14).
In addition to this information obtained from reported cases
of listeriosis, contamination data (largely obtained from samples of foods
collected at retail or during storage before sale) are available in published
scientific literature, government documents and industry documents, or were made
available to us from unpublished government and industry documents (Ref. 1).
These contamination data show that L. monocytogenes has been detected to
varying degrees in unpasteurized and pasteurized milk, high fat dairy products,
soft unripened cheese (cottage cheese, cream cheese, ricotta), cooked
ready-to-eat crustaceans, smoked seafood, fresh soft cheese (queso fresco),
semi-soft cheese (blue, brick, monterey), soft-ripened cheese (brie, camembert,
feta), deli-type salads, sandwiches, fresh-cut fruits and vegetables, and raw
molluscan shellfish (Ref. 1). However, these data also show that most RTE
foods do not contain detectable numbers of L. monocytogenes. For many
RTE foods, contamination of foods with L. monocytogenes can be avoided -
e.g., through the application of current good manufacturing practices
that establish controls on ingredients, listericidal and listeristatic
processes, segregation of foods that have been cooked from those that have not,
and sanitation. Sanitation controls include effective environmental monitoring
programs designed to identify and eliminate L. monocytogenes in and on
surfaces and areas in the plant.
In 1996, we published a paper entitled "U.S. position on Listeria monocytogenes in foods" (Ref. 15). In that paper, we described
outbreaks of listeriosis and an active surveillance program for invasive
listeriosis initiated by the Center for Disease Control and Prevention (CDC).
In 2003, FDA and FSIS, in consultation with CDC, released a quantitative
assessment (the Risk Assessment) (Ref. 1) of relative risk associated with
consumption of certain categories of ready-to-eat foods that had a history of contamination
with L. monocytogenes, or that were implicated epidemiologically with an
outbreak or a sporadic case of listeriosis. The Risk Assessment estimated that
the risk of listeriosis would vary widely among those food categories (Ref. 1).
The foods that the Risk Assessment estimated to pose the
highest risk support the growth of L. monocytogenes. In contrast, the
foods that the Risk Assessment estimated to pose
the lowest risk of being associated with listeriosis are foods that have
intrinsic or extrinsic factors to prevent the growth of L. monocytogenes,
or that are processed to alter the normal characteristics of the food.
(3) For example, it is well established
(Refs. 12 and 16 through 18) that L. monocytogenes does not grow when:
- The pH of the food is less than or equal to 4.4;
- The water activity of the food is less than or equal to 0.92; or
- The food is frozen.
Foods may naturally have a pH or water activity that
prevents growth of L. monocytogenes or may be deliberately processed to
achieve those characteristics (e.g., by adding acid to deli-type salads
to bring the pH to less than or equal to 4.4). Listeristatic control measures,
such as antimicrobial substances, can prevent L. monocytogenes from
growing in food (Ref. 12). Antimicrobial substances such as sorbic acid are
commonly used to prevent the growth of L. monocytogenes in foods such as
cheeses. Antimicrobial substances such as sorbic acid and benzoic acid are
commonly used to prevent the growth of L. monocytogenes in foods such as
deli-type salads. Naturally occurring or added antimicrobial substances can
have an interactive or synergistic effect with other parameters of the
formulation, such as pH, water activity, the presence of other preservatives,
and processing temperature. A concept known as the "hurdle concept" states that
several inhibitory factors (hurdles), while individually unable to inhibit
microorganisms, will, nevertheless, be effective in combination (Refs 12 and
19). For reasons such as these, whether the addition of a particular
anti-microbial substance to a particular food is effective in preventing the
growth of L. monocytogenes is a case-by-case determination that can be
demonstrated through scientific studies. A listeristatic control measure is generally
considered to be effective if growth studies show less than a one log increase
in the number of L. monocytogenes during replicate trials with the food
of interest. For an example of how such studies are conducted, see Reference
20.
L. monocytogenes can survive longer under adverse
environmental conditions than can many other vegetative bacteria that present a
food safety concern. In addition to being able to survive and grow at
refrigeration temperatures, L. monocytogenes tolerates high salt
concentrations (such as in non-chlorinated brine chiller solutions) and survives
frozen storage for extended periods. It is more resistant to nitrite and
acidity than many other food-borne pathogens. It also is more resistant to
heat than many other non-spore forming food-borne pathogens, although it can be
killed by heating procedures such as those used to pasteurize milk
(4) (Ref. 21).
Several scientific publications provide detailed
recommendations for the control of L. monocytogenes in the food
processing environment (see, e.g., Refs. 2 and 5 through 10). Many of the
recommendations in this guidance are adapted from these published recommendations.
In the following tables, we list potential sources of L.
monocytogenes and examples of scenarios that could lead to contamination of
RF-RTE foods with L. monocytogenes.
Table 1. Potential Sources of L.
monocytogenes (Adapted from Ref. 5)
|
Category
|
Potential Sources of L. monocytogenes
|
|
A. Ingredients
|
- Raw foods, such as:
- Raw meat, poultry, and seafood
- Raw milk
- Raw produce
|
|
B. Processing aids
|
- Compressed air
- Ice
- Brine solutions used in chilling refrigerated RTE foods
|
|
C. Contact surfaces for RF-RTE foods
|
- Fibrous and porous-type conveyor belts
- Filling and packaging equipment
- Belts, peelers, and collators
- Containers, bins, tubs and baskets
- Slicers, dicers, shredders and blenders
- Utensils
- Gloves
|
|
D. Surfaces that do not contact RF-RTE foods
|
- In-floor weighing equipment
- Cracked hoses
- Hollow rollers for conveyances
- Equipment framework
- Wet, rusting, or hollow framework
- Open bearings within equipment
- Poorly maintained compressed air filters
- Condensate drip pans
- Motor housings
- Maintenance tools (e.g., wrenches and screw
drivers)
- Forklifts, hand trucks, trolleys, and racks
- On/off switches
- Vacuum cleaners and floor scrubbers
- Trash cans and other such ancillary items
- Tools for cleaning equipment (e.g., brushes and
scouring pads)
- Spiral freezers/blast freezers
- Ice makers
- Aprons
|
|
E. Plant environment
|
- Floors, walls and drains
- Ceilings, overhead structures, and catwalks
- Wash areas (e.g., sinks), condensate, and
standing water
- Wet insulation in walls or around pipes and cooling
units
- Rubber seals around doors, especially in coolers
- Contents of vacuum cleaners
|
Table 2 provides examples of scenarios that could lead to
contamination of a RF-RTE food with L. monocytogenes (Refs. 5 and 9).
Table 2
Scenarios That Could
Lead to Contamination with L. monocytogenes
- A packaging line is moved or modified significantly.
- Used equipment is brought from storage or another plant
and installed into the process flow.
- An equipment breakdown occurs.
- Construction or major modifications are made to an area
where RTE foods are processed or exposed (e.g., replacing refrigeration
units or floors, replacing or building walls, modifications to sewer
lines).
- A new employee, unfamiliar with the operation and L.
monocytogenes controls, has been hired to work in, or to clean
equipment in, the area where RTE foods are processed or exposed.
- Personnel who handle RTE foods touch surfaces or
equipment likely to be contaminated (e.g., floor, trash cans) and
do not change gloves or follow other required procedures before handling
the food.
- Periods of heavy production make it difficult to clean
the floors of holding coolers as scheduled.
- A drain backs up.
- Product is caught or hung-up on equipment. (Stagnant
product in a system can be a major site of microbial growth during
production.)
- Raw or under-processed foods are placed in an area
designated for cooked foods.
- Frequent product changes on a packaging line cause you
to change packaging film, labels, forming pockets or molds, line speeds,
etc.
- Personnel are used interchangeably for packaging raw and
cooked foods.
- Increased production causes you to perform wet cleaning
of lines that have been taken down from production in the same room as
lines that are running product.
- Heat exchangers have become compromised (e.g.,
with pinholes).
- Equipment parts, tubs, screens, etc. are cleaned on the
floor.
- Waste bins in the RTE area are not properly maintained,
cleaned and sanitized.
- Personnel handling RTE foods may come into contact with
these items and then contaminate the foods and/or food contact surfaces.
- Re-circulating pumps and lines are not cleaned and
sanitized.
- Indiscriminate use of high-pressure hoses in cleaning.
- Inappropriate use of footbaths in dry processing areas.
- Water is sprayed on wheels on transport cars when
in-process product is stored near the wheels.
B. How the Recommendations in this Guidance can
Help Prevent Foodborne Illness
The Risk Assessment estimated that the risk of foodborne
illness from consumption of RTE foods that do not support the growth of L.
monocytogenes would be much lower than the risk from consumption of RTE
foods that do support the growth of L. monocytogenes. Therefore, we
provide recommendations on formulating RF-RTE foods so that they will not
support the growth of L. monocytogenes.
Treatment of ingredients and in-process materials with a
listericidal process can destroy viable cells of L. monocytogenes that
enter a facility with new shipments of ingredients (such as raw seafood) or
that are present in an unknown location on a food-contact surface or in an area
that is in close proximity to a food-contact surface. Therefore, we provide
recommendations on listericidal control measures that you could incorporate
into your manufacturing process.
Raw foods (such as raw seafood, raw produce, and
unpasteurized milk) are potential sources of contamination with L.
monocytogenes. In addition, L. monocytogenes has been detected in a
number of finished RF-RTE foods that could be purchased and used as an
ingredient of other finished RF-RTE foods. Therefore, we provide
recommendations on the control of ingredients used in RF-RTE foods.
The Risk Assessment discussed the effect of storage times
and temperatures on the potential for viable cells of L. monocytogenes
to increase in number. We provide recommendations on storage practices that
are consistent with the discussion in the Risk Assessment.
The processing environment may be a primary source of L.
monocytogenes (Refs. 2 and 5 through 10). Therefore, we provide
recommendations on controls to reduce the potential for contamination of a
RF-RTE food from:
- Personnel;
- The design, construction and operation of your plant;
- The design, construction and maintenance of equipment; and
- Sanitation controls
Because of its pervasiveness in the environment, L.
monocytogenes may be introduced into your plant. Therefore, we provide
recommendations on monitoring critical surfaces and areas in your plant for L.
monocytogenes.
Likewise, contamination of a RF-RTE food with L. monocytogenes can occur during
transportation, and viable cells of L. monocytogenes can grow if the
temperature is not controlled during transportation. Therefore, we provide
recommendations for controls on transportation.
Periodic sampling and testing of finished RF-RTE foods that
you process can be an important reference for you to use in evaluating your
control of L. monocytogenes over time. Therefore, we provide
recommendations for such periodic sampling and testing. Although testing a
single lot of finished product is not a practical means of assuring that the
lot is free from contamination by L. monocytogenes, results from such
testing, performed on many lots over a long period of time (many months to
several years), can serve to document a baseline of performance and to validate
the adequacy of other control measures.
Because we provide recommendations for monitoring your plant
for the presence of L. monocytogenes, and for periodic sampling and
testing of finished RF-RTE foods, we also provide recommendations for
corrective actions to take if you detect L. monocytogenes in your plant
or in food.
Because L. monocytogenes may be introduced into a
food processing facility, we provide recommendations for training all employees
as well as for training supervisors and other persons who are responsible for certain
key operations.
We recommend that you make and keep certain records as an
aid to controlling L. monocytogenes in your plant.
This document contains Appendices that describe procedures
you can use to collect environmental samples and prepare those samples for
analysis for L. monocytogenes. We refer you to the procedures that we
use for detection and enumeration of L. monocytogenes in food and in the
plant environment.
III. Who Should Follow
the Recommendations in this Guidance?
We recommend that any processor of a RF-RTE food, including
processors of fresh-cut fruits and vegetables, and processors of frozen fruits
and vegetables, follow the recommendations in this guidance. Our
recommendations apply regardless of whether or not the food that is processed
supports the growth of L. monocytogenes. The fact that a food does not
support the growth of L. monocytogenes does not affect a manufacturer's
responsibility under section 402(a)(4) of the Federal Food, Drug, and Cosmetic Act
(the Act) to ensure that food is not prepared, packed, or held under insanitary
conditions whereby it may become contaminated with filth, or whereby it may be
rendered injurious to health.
We recommend that persons who transport RF-RTE foods follow
the recommendations in section XV of this document.
In developing this guidance, we did not include specific
recommendations that would apply to a person who harvests, picks, transports,
or processes raw intact fruits and vegetables. Although persons who are engaged
in these activities could find this guidance useful, we recommend that such
persons refer to our guidance document entitled "Guide to Minimize Microbial Food Safety Hazards for Fresh
Fruits and Vegetables" as the primary guidance on preventing food-borne illness.
The "FDA Food Code" is a model code developed by
FDA to assist and promote consistent implementation of national food safety
regulatory policy among the local, State, and tribal governmental agencies that
have primary responsibility for the regulation of oversight of retail level
food operations. The FDA Food Code provides a scientifically sound technical
and legal basis for regulating the retail and food service segment of the
industry. The FDA Food Code also gives other users of the Food Code, such as
educators, trainers and the retail and food service industries (restaurants and
grocery stores and institutions such as nursing homes) up-to-date information
on mitigating risk factors that can contribute to food-borne illnesses.
Although the retail and food service industries may find the guidance in this
document useful, we recommend that the retail and food service industries refer
to the Food Code and its Annexes as the primary source of information on
preventing food-borne illness. The FDA Food Code and its Annexes are available
online.
IV. How Does this Guidance Relate to
Our Current Good Manufacturing Practice Regulations in 21 CFR Part 110?
In Title 21 of
the Code of Federal Regulations (21 CFR part 110), we have established
regulations for current good manufacturing practice (CGMP) in preparing,
packing or holding food. These regulations include both binding requirements
and non-binding recommendations relating to personnel, buildings and
facilities, equipment, and production and process controls. In general, 21 CFR
part 110 uses the word "shall" to identify binding requirements and the word
"should" to identify non-binding recommendations. For example, under 21 CFR
110.80(a)(1), raw materials and other ingredients shall be
inspected and segregated or otherwise handled as necessary to ascertain that
they are clean and suitable for processing into food and shall be
stored under conditions that will protect against contamination and minimize
deterioration. Raw materials shall be washed or cleaned as
necessary to remove soil or other contamination. Containers and carriers of raw
materials should be inspected on receipt to ensure that their
condition has not contributed to the contamination or deterioration of food.
The controls and practices provided in this guidance are
recommendations and guidance to the refrigerated and frozen ready-to-eat foods
industry. The recommendations in this guidance on control of L.
monocytogenes in RF-RTE foods are recommendations that complement, but do
not supersede, the requirements and recommendations in 21 CFR part 110. The
recommendations in this guidance are intended to assist processors of RF-RTE
foods in meeting the requirements and recommendations in 21 CFR part 110 with
respect to the control of L. monocytogenes. They can also serve as a
tool to be used by Federal and State regulatory officials in their evaluation
of a firm's implementation of the requirements in 21 CFR part 110. This
guidance provides information that would likely result in controls that are
acceptable to FDA. However, it is not a binding set of requirements.
Processors may choose to use other control measures to comply with the
requirements in 21 CFR part 110, as long as they provide an equivalent level of
assurance of safety for the product. However, processors who choose to use
other control measures are responsible for establishing their adequacy.
For your convenience, in this document we identify those
sections of 21 CFR part 110 that are most relevant to specific recommendations
in this guidance. For example, under 21 CFR 110.40(g), compressed air or other
gases mechanically introduced into food or used to clean food-contact surfaces
or equipment shall be treated in such a way that food is not contaminated with
unlawful indirect food additives. Within this guidance, we are making a
specific recommendation for how to treat compressed gases used directly in or
on RF-RTE foods, or on surfaces that contact RF-RTE foods. Because this
specific recommendation is related to 21 CFR 110.40(g), we refer you to 21 CFR
110.40(g) when we state this recommendation. Importantly, it is your
responsibility to comply with all applicable requirements in 21 CFR part 110,
regardless of whether or not we refer to them in this guidance.
You can access 21 CFR part 110 on the Internet from the home
page for FDA's Center for Food Safety and Applied Nutrition (CFSAN). For your
convenience, when we refer you to a single section of 21 CFR part 110, we
repeat the text of the section in this guidance. However, when we refer you to
more than one section, we do not repeat the text of the section in this document,
because it is simply impractical to do so.
V. How Does this Guidance Relate to Other Regulations and Guidance that Apply to Refrigerated or Frozen Ready-to-eat foods?
The recommendations in this guidance do not change the applicability of other
federal or state regulations or your responsibility to comply with those
regulations. For example, if you process seafood for use as sushi, nothing in
this guidance alters your responsibility to comply with the requirements of 21
CFR part 123 for Fish and Fishery Products. As another example, this guidance
does not affect the responsibility of the Grade "A" dairy industry and the
molluscan shellfish industries to operate in accordance with state regulations
based on the Grade "A" Pasteurized Milk Ordinance
and the National Shellfish Sanitation Program Model Ordinance,
respectively.
In addition, in general the recommendations in this guidance
complement, but do not supersede, recommendations that we have issued in other
guidance documents. For example, if you process seafood for use as sushi, we
continue to recommend that you follow the guidance in our document entitled "Fish and Fisheries Products. Hazards and Controls Guide".
VI. What Definitions Apply to this Guidance?
We recommend that you refer to 21 CFR 110.3 for definitions
of terms such as "adequate," "food-contact surface," "lot," "plant," and
"rework."
In addition, in this section we define the following terms
for the purpose of this guidance.
Clean in place (CIP) means a system used to
clean process piping, bins, tanks, mixing equipment, or larger pieces of
equipment without disassembly, where interior product zones are fully exposed
and soil can be readily washed away by the flow of the cleaning solution.
Clean out of place (COP) means a system (e.g.
cleaning tanks) used to clean equipment parts, piping, etc. after disassembly.
Critical food-contact surface means a surface
that contacts food, or a surface from which drainage onto the food or onto
surfaces that contact food ordinarily occurs during the normal course of
operations after the food is subjected to a listericidal control process, or
when the food is not subjected to any listericidal control process at the
processor's facility.
Critical non-food-contact surface or area
means a surface (other than a food-contact surface) or area that could, through
the action of man or equipment, contaminate a food that will not be subjected
to a listericidal control measure after the exposure of food or a food-contact
surface to the surface or area. Critical non-food-contact surfaces and areas
include equipment, vents, fixtures, drains, walls, floors, employee clothing,
shoes, and accessories, and other surfaces in the plant that do not (or are not
intended to) contact food.
Critical surfaces and areas mean critical
food-contact surfaces and critical non-food-contact surfaces and areas.
Environmental sample means a sample that is
collected from a surface or area of the plant for the purpose of testing the
surface or area for the presence of Listeria species or L.
monocytogenes.
Finished RF-RTE food means a refrigerated or frozen
ready-to-eat food that is packaged.
High efficiency particulate air (HEPA) filter
means an air filter that has an efficiency of 99.97 percent to 99.99 percent
when tested using the dioctylphthalate (DOP) test with a particle size of 0.3
microns. Such filters can remove all yeast, mold, bacteria and other particles
that are larger than 0.3 microns.
Listeria species means microorganisms within
the genus Listeria, including L. monocytogenes.
Listericidal control measure means a control
measure that will consistently destroy viable cells of L. monocytogenes
and consistently lead to a
finished food that contains less than 0.04 cfu of L. monocytogenes per
gram (g) of food.
Listeristatic control measure means a control
measure that has been scientifically demonstrated to prevent the growth of L.
monocytogenes in a food.
Monitor means to conduct a planned sequence of
observations or measurements to assess whether a process or procedure is under
control.
Process or processing means any activity that
is directly related to the production of a food, including any packaging
activity.
Processor means any person engaged in
commercial, custom, or institutional processing of a food.
Ready-to-eat (RTE) food
means a food that is customarily consumed
without cooking by the consumer, or that reasonably appears to be suitable for
consumption without cooking by the consumer.
Screening test for Listeria species
means a test that detects L. monocytogenes and other Listeria
species.
We, us, and our refer to the U.S. Food and
Drug Administration.
You, I, and my refer to the processor.
VII. How Should I Formulate a RF-RTE Food to Prevent the Growth of L. monocytogenes?
As discussed above (see Section II), the Risk Assessment
estimated that some foods present a lower risk of transmission of foodborne
illness than others. The foods that the Risk Assessment estimated to pose the
lowest risk had physical or chemical characteristics that do not support the
growth of L. monocytogenes. Therefore, we recommend that, whenever practical,
you formulate a finished RF-RTE food using one or more of the following
listeristatic control measures:
- pH less than or equal to 4.4;
- Water activity less than or equal to 0.92;
- Formulated to contain one or more inhibitory substances that,
alone or in combination, prevent the growth of L. monocytogenes.
If you use a listeristatic control measure that is generally
recognized to prevent the growth of L. monocytogenes (i.e.,
through control of pH or water activity), we recommend that you establish and
implement controls to ensure that the control measure is consistently applied
to your manufacturing process and is consistently achieved. For example, you could test each batch of material for pH
or water activity in-process.
If you use a formulation that contains one or more
inhibitory substances, we recommend that you demonstrate, through scientific
studies, that the formulation consistently prevents the growth of L.
monocytogenes. A listeristatic control measure is generally considered to
be effective if growth studies show less than one log increase in the number of
L. monocytogenes during replicate trials with the food of interest. For
an example of how such studies are conducted, see Reference 20.
We also recommend that you establish and implement controls
to ensure that any formulation designed to prevent the growth of L.
monocytogenes is consistently applied to your manufacturing process and is
consistently achieved. For example, you could establish a standard operating
procedure in which one or more individuals (other than those responsible for
batch operations) are responsible for verifying the addition of particular
ingredients in specific amounts.
Freezing a food is generally recognized as effective in
preventing the growth of any L. monocytogenes that may be present in the
food. However, whether freezing can effectively prevent the transmission of
foodborne illness when L. monocytogenes is present in low numbers
depends on whether the frozen food is consumed in a frozen state (e.g., ice
cream), or whether it is thawed and held in a refrigerator before consumption (e.g.,
frozen crabmeat). Thus, freezing could be an effective control for ice cream,
but may not be an effective control for crabmeat.
Sections of 21 CFR part 110 that are applicable to the use
of listeristatic control measures include 21 CFR 110.80(b)(4), which requires
that measures such as sterilizing, irradiating, pasteurizing, freezing,
refrigerating, controlling pH or controlling water activity, that are taken to
destroy or prevent the growth of undesirable microorganisms, particularly those
of public health significance, be adequate under the conditions of manufacture,
handling, and distribution to prevent food from being adulterated within the
meaning of the Act.
VIII. How Should I Use a Listericidal Measure to Control L. monocytogenes?
We recommend that you demonstrate, through scientific
studies, that a listericidal control measure that you establish and use
consistently destroys viable cells of L. monocytogenes and consistently
leads to a finished food that contains less than 0.04 cfu/g of L.
monocytogenes. Determining the extent of the process necessary to achieve
that goal depends, in part, upon determining the likely levels of L.
monocytogenes prior to application of the listericidal control measure. In
the absence of scientific studies, a listericidal control measure that provides
a reduction of the number of viable cells of L. monocytogenes of six
orders of magnitude (six logarithms) may be acceptable to achieve that goal.
This is called a "6D" process.
There are several approaches available for demonstrating
that your process consistently destroys viable cells of L. monocytogenes,
including:
- Reference to scientific literature or previous validation
studies;
- Scientifically valid experimental trials; and
- Statistically designed surveys and/or mathematical
modeling.
In looking for listericidal control measures, we recommend
that you consider measures such as:
- Thermal processing;
- Irradiation, provided that irradiation has been approved
and listed in 21 CFR part 179;
- Ultraviolet light, high pressure, or pulsed electric
field; and
- Decontamination through the use of listericidal agents.
If you establish and use a listericidal control measure, we
recommend that you establish and implement controls to ensure that the control
measure is consistently applied to your manufacturing process and is
consistently achieved.
When demonstrating that a listericidal control measure that
you establish and implement is effective and when monitoring a listericidal
control measure to ensure that it is consistently achieved, we recommend that
you monitor parameters such as time, temperature, and concentration of a
listericidal agent, on a continuous basis. If you determine that a listericidal
control measure was not achieved or is otherwise ineffective, we recommend that
you identify and implement appropriate corrective actions.
Sections of 21 CFR part 110 that are applicable to the use
of listericidal control measures include 21 CFR 110.80(b)(4), which requires
that measures such as sterilizing, irradiating, pasteurizing, freezing, refrigerating, controlling pH or controlling water
activity, that are taken to destroy or prevent the growth of undesirable
microorganisms, particularly those of public health significance, be adequate
under the conditions of manufacture, handling, and distribution to prevent food
from being adulterated within the meaning of the Act.
IX. What Controls Should I Establish and Implement on Ingredients and Other Raw Materials?
In this section, we recommend controls to reduce the
potential that an ingredient or other raw material will contaminate a finished
RF-RTE food with L. monocytogenes.
A. Ingredients that are Potential Sources of L.
monocytogenes
As discussed in the background section of this document (see
section II), raw foods (such as raw seafood, raw produce, and unpasteurized
milk) are potential sources of contamination with L. monocytogenes. In
addition, because L. monocytogenes has been detected in a number of
finished RF-RTE foods, a RF-RTE food that you purchase and use as an ingredient
of a food that you manufacture is also a potential source of contamination.
We recommend
that you establish a list of ingredients (such as raw foods) that are
reasonably likely to be contaminated with L. monocytogenes and then
establish controls that are appropriate for each of these ingredients.
Depending on how an ingredient that you purchase is processed, it may be more
or less likely to be contaminated with L. monocytogenes. For example, ingredients
processed in the following manner are not reasonably likely to contain L.
monocytogenes
- Aseptically processed and packaged;
- Retorted (e.g., canned);
- Ethylene oxide treated or irradiated in the package;
- Pasteurized (or equivalent treatment) in the package;
- Other approved lethal technologies (in the package)
We recommend that you handle raw foods, and any other food
ingredients that could be contaminated with L. monocytogenes, as if they
are contaminated with L. monocytogenes.
B. Controlling L. monocytogenes in
Ingredients that are Reasonably Likely to Be Contaminated with L.
monocytogenes
We recommend that you treat ingredients or other raw
materials that are reasonably likely to be contaminated with L.
monocytogenes with a listericidal control measure during the manufacturing
process. For example, you could treat an ingredient before it is used in
manufacture, or you could treat a mixture of ingredients at an in-process stage
of manufacture.
If you do not use a listericidal control measure, then we
recommend that you establish and implement controls designed to reduce the
potential that the ingredient or other raw material received from a supplier is
contaminated with L. monocytogenes. For example, you could:
- Obtain the ingredient under a supplier's Certificate of
Conformance (COC) (guarantee). We recommend that any COC that you rely on
in following this guidance include the period of guarantee and include a
statement that the supplier's ingredients are produced under conditions
that are consistent with this guidance.
- Obtain the ingredient under a supplier's Certificate of
Analysis (COA). We recommend that any COA that you rely on in following
this guidance include the sampling plan and the analytical results of
testing to detect or enumerate Listeria species or L.
monocytogenes, including the analytical method used and limits of the
analytical method.
- Test the ingredient or other raw material for the presence
of Listeria species or L. monocytogenes.
Although our recommended measures to control ingredients include obtaining an ingredient
under a COA, or testing the ingredient yourself, testing a single lot of a food
product for L. monocytogenes is of limited value in establishing the
acceptability of that lot. It is preferable that your supplier produces food
products under conditions that are consistent with this guidance. Product
testing adds value primarily as part of a history of test results that is used
to verify the adequacy of control measures over time.
If your supplier provides a COC, we recommend that you
obtain that COC and conduct an on-site audit of the supplier on an annual
basis. We also recommend that you periodically (e.g., weekly, monthly,
or quarterly) test ingredients that you receive under a COC to verify the
absence of L. monocytogenes.
If your supplier provides a COA, we recommend that you
obtain the COA for each lot of ingredients, either prior to delivery or on
receipt. We also recommend that you verify the results of the supplier's COA
on each shipment of each lot of ingredients that you receive until you have
enough experience with that supplier to be confident in the results provided on
the COA. After that, we recommend that you continue to test ingredients that
you receive under a COA on a periodic basis (e.g., weekly, monthly, or
quarterly) to verify the absence of L. monocytogenes.
If you test an incoming ingredient for the presence of Listeria
spp. rather than receive the ingredient under a COC or COA, we recommend that
you:
- Test every lot of the ingredient if your final product
supports the growth of L. monocytogenes; or
- Test incoming ingredients on a periodic basis (e.g.,
weekly, monthly, or quarterly) if your final product does not support the
growth of L. monocytogenes.
For your convenience, in Table 3 we present these
recommendations in the form of a decision tree.
Table 3. Decision
Tree for Control of Ingredients
- Is the ingredient reasonably likely to contain L. monocytogenes?
- Yes
↓
- Does your process include a listericidal step after the ingredient is used?
- Yes →
- We recommend that you control the listericidal step
- No
↓
- Can you obtain the ingredient under a Certificate of Conformance (COC) from your supplier?
- Yes →
-
We recommend that you:
- Obtain COC at least annually;
- Conduct annual on-site audit of supplier; and
- Conduct periodic verification testing for Listeria species in the ingredient (e.g., weekly, monthly, or quarterly)
- No
↓
- Can you obtain the ingredient under a Certificate of Analysis (COA) from your supplier?
- Yes →
- We recommend that you:
- Obtain COA for each lot, prior to or on receipt;
- Conduct periodic verification testing for Listeria species in the ingredient (e.g., weekly, monthly, or quarterly); and
- Be aware that testing a single lot of a food product for L. monocytogenes is of limited value in establishing the acceptability of that lot.
- No
↓
- Does your final product support the growth of L. monocytogenes?
- Yes →
- We recommend that you:
- Test every lot of ingredient for Listeria species; and
- Be aware that testing a single lot of a food product for L. monocytogenes is of limited value in establishing the acceptability of that lot.
- No
↓
- We recommend that you conduct periodic testing of ingredient lots for Listeria species (e.g., weekly, monthly, or quarterly)
- No
→
C. Applicable Sections of 21 CFR part 110
Sections of 21 CFR part 110 that are applicable to control
of ingredients include 21 CFR 110.80, 110.80(a)(2) and 110.80(b)(4).
X. What Storage Practices Should I Establish and Implement?
We recommend that you establish and implement procedures to
minimize the amount of time that ingredients and other raw materials,
in-process materials, and finished foods are stored. In particular, we
recommend that you establish and follow procedures to use such materials on a
first-in, first-out basis.
We also recommend that you transport and store ingredients
and other raw materials, in-process materials, and finished foods at an
internal temperature of less than or equal to 4 degrees C
(less than or equal to 40 degrees F) and that you establish and use
control measures to consistently achieve this internal temperature.
We also recommend that, during processing, you establish and
use controls on the amount of time that ingredients and other raw materials,
in-process materials, and finished foods are held above 4 degrees C (40 degrees
F) and on the temperature during this time period.
Sections of 21 CFR part 110 that are applicable to storage
practices to reduce the potential of foodborne illness from the consumption of
a RF-RTE food include 21 CFR 110.40(e), 110.80(a)(5), 110.80(b)(2),
110.80(b)(3) and 110.80(b)(4).
XI. What Controls Should I Establish and Implement Regarding Personnel?
We recommend that you provide training in employee health
and hygienic practices for all employees and contractors who enter production
and storage areas (e.g., employees who conduct production, maintenance,
quality assurance, quality control, or warehousing operations), including
employees and contractors who are temporary or seasonal. We also recommend
that the training be conducted before the employee performs job activities with
refresher training on an annual basis.
We recommend excluding employees who exhibit symptoms of
gastroenteritis from working in food production areas.
We recommend that all persons who will enter an area where
RF-RTE foods are processed or exposed thoroughly wash their hands before doing
so. We also recommend that employees use suitable utensils (such as spatulas
or tongs), or wear gloves, when touching exposed RF-RTE foods, food-contact
surfaces, and packaging materials, and not touch exposed RF-RTE foods,
food-contact surfaces, and packaging with bare hands.
We recommend that gloves and footwear worn by employees who
handle RF-RTE foods or who work in areas where RF-RTE are processed or exposed
be made of impermeable material, in good repair, easily cleanable or
disposable, and used only in RF-RTE areas. Employees should not use cleated
footwear unless it is necessary for their safety, because cleated footwear can
collect large particles of dirt or other waste from the plant.
When gloves are used, we recommend that:
- Employees wash their hands before putting the gloves on;
- Multi-use gloves be washed and sanitized after the employee
touches any non-food-contact surface;
- Single use gloves be discarded and replaced after the employee
touches any non-food-contact surface; and
- Gloves worn outside areas where RF-RTE foods are processed or
exposed (e.g., restrooms) be discarded before returning to the RF-RTE
area.
We recommend that you consider the use of footbaths
containing sanitizer when entering areas where RF-RTE foods are processed or
exposed. We do not recommend the use of footbaths in dry processing
environments, because the absence of water in the environment prevents the
growth of L. monocytogenes. In dry environments it may be more
appropriate to use a dry powdered sanitizer.
An example of a footbath is an automatic spray of foam
disinfectant on the floor where people and equipment (such as carts and
forklifts) enter the RF-RTE area. A footbath may also be a low flat container
or a water tight recess in the floor with a non-slip surface and filled with a
suitable sanitizer. If non-automatic footbaths are used, we recommend checking
them at regular intervals, such as hourly, to ensure that they are filled with
sanitizer and that the sanitizer is diluted to the proper concentration.
With respect to clothing, we recommend that:
- Employees not wear street clothes in areas where RF-RTE
foods are processed or exposed unless the street clothes are adequately
covered above the knees (e.g., with a clean smock);
- Smocks for employees in areas where RF-RTE foods are
processed or exposed be worn only in the RF-RTE area and an adjacent
vestibule (i.e., the area where the smock would be put on);
- Smocks be laundered or disposed of daily;
- Employees change into a clean uniform or smock before
entering areas where RF-RTE foods are processed or exposed;
- Smocks or uniforms that will be used in areas where RF-RTE
foods are processed or exposed be distinguished from smocks or uniforms
that will be used in other areas (particularly areas where raw foods are
processed or exposed) using a mechanism such as color coding; and
- Smocks or uniforms be distinguished according to the task
that the employees perform (e.g., production or maintenance). For
example, if you restrict the access of maintenance employees to areas of
the plant where finished product is exposed, such color coding helps to
identify the employees with such restricted access.
We recommend that you provide separate locker areas, break
areas, and cafeteria areas for employees who handle RF-RTE foods and employees
who handle raw foods. We also recommend that you ensure that employees who
handle trash, offal, floor sweepings, drains, production waste, or scrap
product do not handle RF-RTE food, and do not touch RF-RTE food-contact
surfaces or food packaging material, unless they change their smocks or
uniforms, wash and sanitize hands, wear clean gloves, and don and sanitize
footwear.
We recommend that maintenance personnel in areas where
RF-RTE foods are processed or exposed comply with the same hygiene requirements
as production employees in those areas.
Sections of 21 CFR part 110 that are applicable to employee
hygiene include 21 CFR 110.10(a), 110.10(b)(1), 110.10(b)(3), 110.10(b)(5),
110.10(b)(9), 110.10(c), and 110.37(f).
XII. What Controls Should I Establish and Implement on the Design, Construction and Operation of My Plant?
A. Design and
Construction of the Plant
To reduce the potential for contamination of RF-RTE foods
via air, aerosols, or traffic of employees or equipment, we recommend that you
design and construct the plant to separate areas where RF-RTE foods are
processed, exposed or stored from areas where raw foods are processed, exposed
or stored, and from equipment washing areas, microbiological laboratories,
maintenance areas, waste areas, offices, and toilet facilities. When possible,
microbiological laboratories should be located as far away as possible from the
processing area, preferably in another building. In designing the air flow in
your plant, we recommend that you maintain positive air pressure on the RF-RTE
side of the operation relative to the "raw" side (i.e., maintain higher
air pressures in RF-RTE areas and lower air pressures in raw areas). We
recommend that you consult individuals with appropriate engineering skills to
determine how to achieve proper air balance, including determining the number,
size, and location of intake and exhaust fans. Appendix 1 of this document
provides some schematic diagrams relevant to these recommendations.
Figure 1 of Appendix 1 relates to air flow, and simply shows
that the recommended air flow should have negative pressure in the raw
processing area rather than in the RTE processing area. In Figures 2 and 3 of
Appendix 1, we provide examples of plant design, including schematic
recommendations related to air flow, product flow and the use of partitions in
the design of the plant. In Figure 2 of Appendix 1, the example of a plant
design has an additional partition between the raw processing area and the RTE
processing area. In Figure 3, the example of a plant design does not have this
additional partition between the raw processing area and the RTE processing
area; instead, the plant design in Figure 3 relies on additional positive air
pressure in the RTE processing area.
To reduce the potential for contamination of RF-RTE foods
with microorganisms (including L. monocytogenes), we recommend that you
design and construct the plant so that the air in rooms where RF-RTE foods are
processed or exposed will be filtered. To remove bacteria (including L. monocytogenes)
as well as yeasts and molds, we recommend that you filter the air in areas
where RF-RTE foods are processed or exposed through High Efficiency Particle
Air (HEPA) filters that have an efficiency of 99.97-99.99 percent at 0.3
micron. If you are not able to use such filters, we recommend that the final
filter have an efficiency of at least 90-95 percent at 1 micron as rated in
ASHRAE standard 52.2-1999.(5)
To avoid contamination of intake air, we recommend that the location of the air
intake not be adjacent to the location of the air exhaust or other sources of
airborne contamination such as waste disposal areas. Our recommendations to filter
air are particularly important when major construction or remodeling occurs in
an existing plant, because using air-tight barriers and limiting access between
construction and food production areas can prevent introduction of
contamination into the plant environment.
We recommend that water systems be adequately designed,
installed, and maintained to prevent backflow and cross-connections between
potable and non potable water lines or systems. We recommend that the areas
for washing equipment that contacts RF-RTE foods be located in a room that is
separate both from areas where RF-RTE foods are processed or exposed and from
areas where equipment that contacts raw foods are washed.
To prevent the accumulation of standing water in or around
drains, we recommend that:
- You design and construct the plant in a way that will make drains
adequately accessible for cleaning and function;
- You eliminate trench drains in areas where RF-RTE foods are
processed or exposed and replace them with enclosed plumbing to a floor drain;
when this is not possible, we recommend that you equip trench drains for
automatic flushing;
- You design and construct drains so that the drains conform to
applicable plumbing codes and do not flow from areas where raw foods are
processed or exposed to areas where RF-RTE foods are processed or exposed;
- You design and construct drains so that restroom drains are downstream
of drains serving areas where RF-RTE foods are processed or exposed;
- The slope of floors to drains be at least 0.25 inch per foot
(0.64 cm per 30.5 cm); and
- Sewer lines not be located above areas where RF-RTE foods, food-contact
surfaces, or food packaging materials are processed or exposed. If this is not
possible, we recommend that you take steps to protect RF-RTE foods from
potential leakage, e.g., by shielding the sewer lines to prevent or
convey leakage.
We recommend that you design and construct the plant in a
manner that will prevent condensate from contacting exposed RF-RTE foods,
food-contact surfaces, and food packaging material. Measures that can help
prevent the formation of condensate include exhausting vapors from cooking
operations, using dehumidifiers, and providing adequate ventilation.
We recommend that you design and construct the plant so that
walls, ceilings, windows, doors, floors, drains, and overhead fixtures (e.g.
pipes, air vents, and lights) in areas where RF-RTE foods are processed or
exposed are accessible for cleaning, resist deterioration by product or
cleaning chemicals, and prevent harborage of microorganisms. We also recommend
that you design and construct the roof so that it drains freely and does not
leak. We recommend that you not place windows that can be opened in areas where
RF-RTE foods are processed or exposed. To prevent harborage of microorganisms,
we also recommend not using construction materials made of wood in areas where RF-RTE
areas are processed or exposed and in other wet processing areas in the
facility.
Sections of 21 CFR part 110 that are applicable to the
design and construction of the plant include 21 CFR 110.20(b)(2), 110.20(b)(4),
110.20(b)(6), 110.20(b)(7), 110.20(d)(4), 110.35(a), 110.35(c), 110.37(b), 110.40(a),
and 110.80(b)(13).
B. General operation of
the plant
To minimize the potential for transfer of L.
monocytogenes between areas where raw foods are processed or exposed and
areas where RF-RTE foods are processed or exposed, we recommend that you control
traffic flow patterns for employees, food products, and equipment.
In areas where RF-RTE foods are processed or exposed, we
recommend that you use pallets that can easily be cleaned and keep them in good
condition, and that you not use wood pallets in areas where RF-RTE foods are
processed or exposed or in other areas for wet processing and storage. We also
recommend that you clean and sanitize pallets and wheels of transport equipment
(e.g., carts, forklifts, and mobile racks) before they enter an area
where RF-RTE foods are processed or exposed. We recommend that you designate
one set of equipment such as carts, forklifts, mobile racks, and pallets to
areas where raw foods are processed or exposed and designate a separate set of
such equipment to areas where RF-RTE foods are processed or exposed.
With respect to containers, we recommend that you:
- Distinguish between containers used for product, rework, or
waste;
- Clearly label containers;
- Select containers that can easily be cleaned;
- Dedicate containers by function (e.g., in-process
products, rework and waste);
- Dedicate containers by area (e.g., use one set of
containers in areas where RF-RTE foods are processed or exposed and a different
set of containers in areas where raw foods are processed or exposed); and
- Use a mechanism such as color-coding to identify containers by
function and area.
We recommend that you use a mechanism such as color-coding
to distinguish between portable equipment (such as utensils, racks, and totes)
that is used with RF-RTE foods and portable equipment that is used with raw
foods.
We recommend that you discard or decontaminate continuous
use brines and recycled process water used in direct contact with RF-RTE with
sufficient frequency to control L. monocytogenes (see our
recommendations in Appendix 2). To decontaminate such brines and water, we
recommend that you use measures such as chlorination, heat treatment, or other
effective treatment. In determining the frequency of decontamination
treatment, you should consider the results of environmental monitoring for L.
monocytogenes (see section XVI of this document).
To prevent aerosols from contacting RF-RTE food, food-contact
surfaces and food packaging materials, employees should not use high-pressure
water hoses in areas where RF-RTE foods are processed or exposed during
production or after equipment has been cleaned and sanitized.
Sections of 21 CFR part 110 that are applicable to the
operation of the plant include 21 CFR 110.35(a), 110.40(a), 110.80(a)(2),
110.80(a)(5), 110.80(b)(1), and 110.80(b)(6).
C. Use of compressed
gas, water, and ice in the plant
We recommend that you dry and filter compressed gases or air
used directly in or on RF-RTE food, or on RTE food-contact surfaces. We
recommend that dehydration be done at the source of gas or air supply and that
filtration be done at the point of use, using a filter that can retain
particles larger than 0.3 micron. You should take appropriate steps to maintain
the filters.
We recommend that you maintain and inspect water treatment
systems to ensure that they do not become a source of microbial contamination.
We also recommend that you handle and store ice and ice utensils in a manner
that protects ice from microbial contamination.
We recommend that you make mixed hot and cold water
available at hand washing stations.
Sections of 21 CFR part 110 that are applicable to the use
of compressed gas, water and ice include 21 CFR 110.37(a), 110.37(e), 110.40(g),
110.80(a)(1), and 110.80(b)(16).
XIII. What Controls Should I Establish and Implement for the Design, Construction and Maintenance of Equipment?
A. Design and
Construction of Equipment
We recommend that the equipment that you purchase and use to
process RF-RTE foods be designed and constructed to facilitate cleaning and to
minimize sites where microbial harborage and multiplication can occur. We also
recommend that you or the manufacturer of the equipment review the design of
equipment regardless of whether the equipment is new or existing, including
existing equipment that is being modified.
We recommend that you design and construct equipment such as
catwalk framework, table legs, conveyor rollers, and racks so that they cannot
collect water that could harbor L. monocytogenes (e.g. not hollow
or foam filled). Ladders and stairs in areas where RF-RTE foods are processed
or exposed should have plating underneath that prevents any debris from shoes or
other items from personnel who are using them from falling onto the processing
line. We recommend that these plates have up-turned edges so that debris that
falls onto the plates cannot roll off and onto the lines.
To prevent contamination of RF-RTE foods, we recommend that
you not position catwalks and stairs with open grating over exposed RF-RTE food
or food-contact surfaces. To prevent contamination of stationary equipment
used to process RF-RTE foods, you should not install such equipment over floor
drains. We recommend that to prevent contamination of RF-RTE foods and
food-contact surfaces from floor splash, you should sufficiently elevate food-contact
surfaces (including conveyors) above the floor. When possible, you should
avoid overhead conveyors. If overhead conveyors cannot be avoided, we recommend
that they be designed to be easily accessible for cleaning. To prevent
contamination of RF-RTE food and food-contact surfaces from wheel spray, we
recommend that you place cleanable cover guards over the wheels of racks used
for transporting exposed RF-RTE foods.
We recommend that you add listericidal substances (e.g.,
sodium benzoate) to lubricants (e.g., chain, valve, and seal lubricants)
so that the lubricants do not become contaminated with product residue and,
thus, become a niche for L. monocytogenes.
We recommend that condensate from refrigeration evaporation
coils be directed to a drain through a hose or, alternatively, collected in a
pan that drains through a hose or suitable pipe to a drain. An air gap or other
back flow mechanism should be in the drain line to prevent back flow from the
sewer system to the drip pan. We recommend that you regularly inspect the pan
and drain to ensure that the hose or pipe does not become clogged.
To prevent hose nozzles and employee hands from becoming contaminated,
we recommend that you keep hose nozzles off the floor or other unclean surfaces
unless they are not intended to make contact with RF-RTE food, food-contact
surfaces, or packaging material. For example, you could install and use automatically
retractable hoses (including spring tension or spring loaded retractable hoses)
or fixed length hoses that do not touch the floor.
If you use raw product to cool RF-RTE product, we recommend
that heat exchangers have higher pressure on the RF-RTE side than on the raw
side.
We recommend that food-contact surfaces be smooth,
non-absorbent, sealed, easily cleanable, sloped to drain freely, and made of
durable, non-corrosive and non-toxic materials. We also recommend that
food-contact surfaces be smoothly bonded (e.g., free of pits, folds,
cracks, crevices, open seams, cotter pins, exposed threads and piano hinges)
and that junctures be covered. Piping used to convey RF-RTE foods should not
have dead ends or cross-connections between raw and RF-RTE foods.
Sections of 21 CFR part 110 that are applicable to the
design and construction of equipment include 21 CFR 110.20(b)(4), 110.37(b)(3),
110.40(a), 110.40(b), 110.40(c), and 110.40(d).
B. Maintenance of Equipment
We recommend that you establish and use a preventive
maintenance program that is designed to minimize breakdowns and prevent
contamination that could occur during repair of equipment. We recommend that such
a preventive maintenance program include a written program containing defined schedules
for examination and maintenance of equipment such as valves, gaskets, o-rings,
pumps, screens, filters, and heat exchanger plates.
We recommend that you examine and change filters used on
intake air either at a frequency based on the manufacturer's specification or
more frequently based on pressure differential or the results of environmental
monitoring.
We recommend that tools intended for maintenance of
equipment used in areas where RF-RTE foods are processed or exposed be
dedicated to those areas and be washed and sanitized prior to use.
We recommend that equipment and food-contact surfaces used
in areas where RF-RTE foods are processed or exposed be cleaned and sanitized
after maintenance and prior to use in production. Equipment that could become contaminated
as a result of maintenance on facility utilities (e.g., air or water systems)
or remodeling should be cleaned and sanitized prior to use.
Sections of 21 CFR part 110 that are applicable to maintenance
of equipment include 21 CFR 110.35(d)(1), 110.40(a), 110.40(g), 110.80(b)(1),
and 110.80(b)(2).
XIV. What Controls Should I Establish and Implement Regarding Sanitation?
A. General Sanitation Program
To minimize the potential for contamination of RF-RTE food
and food-contact surfaces with L. monocytogenes, we recommend that you establish
and use a sanitation program that includes a written sanitation standard
operating procedure (SSOP) and a sanitation maintenance schedule for areas
where RF-RTE foods or food-contact surfaces are processed or exposed. In
general, we recommend that a written SSOP address:
- The condition and cleanliness of food-contact surfaces,
including equipment, utensils, gloves, and outer garments; and
- The prevention of cross-contamination:
- From insanitary objects to food;
- From insanitary objects to food packaging material and
other food-contact surfaces (including equipment, utensils, gloves, and
outer garments);
- From raw food to cooked food;
- After any listericidal step; and
- As a result of traffic flow patterns for employees and
for equipment.
We recommend that if you establish a written SSOP that you make
the SSOP readily available to personnel who are responsible for cleaning and that
you monitor adherence to the SSOP. We provide recommendations for schedules
for routine cleaning and sanitation in Appendix 2.
We recommend that SSOPs for cleaning equipment and floors identify:
- Equipment or area to be cleaned;
- Disassembly of equipment, if applicable;
- Frequency of cleaning;
- Type and concentration of cleaning compounds and sanitizers;
- Time/temperature of cleaning solutions; and
- Flow rate (velocity) or pressure of cleaning solution, if
applicable.
We recommend that SSOPs for cleaning and sanitizing equipment
include the following steps:
- Removal of heavy soil or debris from equipment, if needed;
- Pre-rinsing the equipment;
- Foaming and scrubbing the equipment with an effective
cleaner;
- Rinsing the equipment after treating it with the cleaner;
- Sanitizing equipment; and
- Visual inspection of food-contact surfaces for residues of
cleaning agents or sanitizing agents.
We recommend that SSOPs for cleaning and sanitizing floors
include the following steps:
- Removal of heavy soil or debris from floors (e.g.,
with brooms or shovels), if needed;
- Cleaning other debris from floors;
- Rinsing floors with water using a low pressure/low volume
hose;
- Using a dedicated brush or floor scrubber to scrub floors
with an effective cleaner, applying water as needed;
- Thoroughly rinsing floors using a low pressure/low volume
hose;
- Sanitizing floors; and
- Removing excess water from floors if needed.
For clean-in-place (CIP) systems, we recommend that you monitor
the concentration of cleaning solutions and sanitizers and verify flow rate,
duration of the cleaning cycle and temperature. For clean-out-of-place (COP)
systems, we recommend that you monitor the concentration of cleaning solutions
and sanitizers.
With respect to wet cleaning, we recommend that you:
- Not perform wet cleaning of equipment (e.g., down lines,
storage and spiral coolers, and spiral freezers) in a room where RF-RTE food is
exposed, even if you cover the RF-RTE food (e.g., with plastic or
paper);
- If wet cleaning does occur, remove all exposed RF-RTE food from a
room before beginning any wet cleaning of equipment, floors, etc; and
- Remove all exposed RF-RTE food from a cooler prior to cleaning the
cooler, refrigeration condenser units or condensate drip pans and hoses.
When assembling cleaned and sanitized equipment (e.g.,
pump impellers, pipes), you should not place the equipment directly on the
floor. We recommend that you take steps to prevent water from the floor or
unclean equipment from splashing onto clean equipment.
When using CIP systems, we recommend that you dedicate separate
CIP systems for cleaning equipment used to process RF-RTE
food and for cleaning equipment used to process raw food. If you are
unable to designate separate CIP systems and, thus, use a common CIP system, we
recommend that you maintain the temperature of an alkaline cleaning solution at
or above 71 degrees C (160 degrees F).
When using COP systems (e.g., wash tanks), we
recommend that you dedicate separate COP systems for cleaning equipment used to
process RF-RTE food and for cleaning equipment used to process raw food.
We recommend that all wipes be disposable and discarded
after each use on RTE food-contact surfaces and that scouring pads be discarded
daily. When scouring pads are not in use during the day, we recommend they be
kept dry or placed in a sanitizer solution.
We recommend that you maintain and clean equipment used for
cleaning (e.g., brushes, mops, floor scrubbers, and vacuum cleaners) so that
it does not become a source of contamination. We recommend that you dedicate cleaning
equipment either to areas where RF-RTE foods are processed or exposed, or to
areas where raw foods are processed or exposed, using a mechanism (such as
color coding) that easily distinguishes the equipment dedicated to the two areas.
Sections of 21 CFR part 110 that are applicable to a general
sanitation program include 21 CFR 110.35(a), 110.35(d)(1), 110.80(b)(1), and
110.80(b)(7).
B. Cleaning Drains
We recommend that you clean and
sanitize floor drains in a manner that prevents contamination of other surfaces
in the room. To do so, we recommend that:
- You not clean floor drains during times when RF-RTE foods are
processed or exposed;
- You not use high-pressure hoses to clear or clean a drain,
because use of such hoses could create aerosols that may spread contamination
throughout the room;
- Brushes used to clean floor drains be at least ¼ inch (0.64 cm) smaller
than the diameter of the drain opening;
- You use a splashguard to prevent splashing during cleaning;
- Utensils that you use for cleaning drains be dedicated to that
purpose and be easily distinguishable from utensils used for other purposes (e.g.,
by color-coding).
If a drain backs up and water flows into an area where RF-RTE
foods are being processed or exposed, we recommend that you take steps to avoid
splashing any equipment and follow the sequence of steps described below to
clear the drain and clean the area around it:
- Stop any production;
- Remove any uncovered RF-RTE foods from the affected area;
- Clear the drain;
- Clean the affected area with an effective cleaner, rinse
it, and sanitize it; and
- Remove excess water from the floor.
After cleaning a drain, we recommend that employees change
clothes and wash and sanitize hands before touching a RF-RTE food-contact
surface.
If you use bactericidal drain rings, we recommend that you monitor
them and replace them when appropriate.
Sections of 21 CFR part 110 that are applicable to cleaning
drains include 21 CFR 110.10(b)(1), 110.10(b)(3), 110.35(a), and 110.37(b)(3).
C. Sanitizers
Sanitizers containing quaternary ammonium compounds (QACs), peroxyacetic
acid, iodine, or chlorine have been used to control L. monocytogenes in
various situations (Ref. 5). We recommend the use of QACs for many
applications, because QACs have been found to be effective against L.
monocytogenes and leave a residual germicidal effect on surfaces (Ref. 5).
We note that peroxyacetic acid sanitizers have been shown to be effective
against biofilms containing L. monocytogenes (Ref. 5). We recommend
that you rotate sanitizers, because this has been reported to provide for
greater effectiveness (Ref. 5).
Sanitizers are subject to registration under the Federal
Insecticide, Fungicide, and Rodenticide Act (FIFRA), which is administered by
the U.S. Environmental Protection Agency. You should use sanitizing solutions
in accordance with the conditions of use authorized under FIFRA, which should
be on the product label. You should direct questions regarding the use of the sanitizer
to control L. monocytogenes to the supplier of the sanitizer, who may
have specific recommendations regarding factors (such as temperature, pH, and
water hardness) that could influence the effectiveness of the sanitizer.
Hot water or steam has been used to sanitize racks and
equipment that is difficult to clean (Ref. 5).
Sections of 21 CFR part 110 that are applicable to sanitizing
procedures and solutions include 21 CFR 110.35(a), 110.35(b), 110.35(d),
110.35(d)(1), 110.35(d)(2), 110.35(d)(5), and 110.80(b)(1).
D. Sanitation Monitoring
We recommend that you monitor sanitation conditions and
practices during processing with sufficient frequency to ensure the cleanliness
of food-contact surfaces and prevent cross-contamination. We also recommend
that you correct, in a timely manner, any monitored sanitation conditions,
cleanliness and practices that are not implemented in accordance with your
SSOP.
Sections of 21 CFR part 110 that are applicable to
sanitation monitoring include 21 CFR 110.35(a), which requires, in relevant
part, that cleaning and sanitizing of utensils and equipment be conducted in a
manner that protects against contamination of food, food-contact surfaces, or
food-packaging materials.
E. Sanitation Control Records
We recommend that you establish and maintain sanitation
control records that document:
- Your sanitation monitoring; and
- Corrections of monitored sanitation conditions and practices that
are not implemented in accordance with your SSOP.
XV. What Transportation Controls Should I Establish and Implement?
We recommend that you inspect transportation vehicles for
structural integrity, cleanliness, and overall suitability when unloading
ingredients and prior to loading finished products. We also recommend that you
use transportation vehicles that are adequately equipped to control and monitor
the temperature of incoming ingredients and outgoing finished food products on
a continuous basis.
We recommend that any tankers used to transport ingredients
or finished food products be dedicated to those specific uses and that you
verify that the tanker has been adequately cleaned and sanitized prior to use.
Sections of 21 CFR part 110 that are applicable to transportation
include 21 CFR 110.93, which requires that storage and transportation of
finished food be under conditions that will protect food against physical,
chemical, and microbial contamination as well as against deterioration of the
food and the container.
XVI. What Should I Do to Monitor Critical Surfaces and Areas to Detect Locations that Harbor Listeria Species or L. monocytogenes?
See Section VI for the definitions that we are using in this
document for the terms:
- Critical food-contact surface;
- Critical non-food-contact surface or area; and
- Critical surfaces and areas.
A. Written Plan
We recommend that you establish and implement a written plan
for the collection of environmental samples from critical surfaces and areas,
and for testing those samples for the presence of Listeria species or L.
monocytogenes. We recommend that you evaluate each plant, product, and
process to determine the appropriate monitoring points.
We recommend that your program use screening tests for the
presence of Listeria species because these tests
usually are more rapid than tests for L. monocytogenes. We
recommend that you either test the individual samples that you collect, or test
a composite that you make from multiple samples taken from a given area. We
describe recommended procedures for compositing samples in Appendix 4.
B. Critical Food-Contact Surfaces
For critical food-contact surfaces, we recommend that your
written plan include collection and testing environmental samples from all, or
representative sets of, such surfaces at least once every week. If you will
test a representative set of samples (rather than all samples) each week, we
recommend that your plan for sample collection and testing be designed so that
all critical food-contact surfaces are tested at least once each month. The
most important time to collect these environmental samples is at a time that is
both prior to cleanup and no sooner than the middle of production.
The number of samples that you should collect depends on the
size of your production lines (Refs. 5, 6, 9 and 10). We recommend that even
the smallest processors collect samples from a minimum of 5 sites of
food-contact surfaces in each production line for RF-RTE foods. We recommend
that larger processors determine the appropriate number of sampling sites based
on the size of the facility. If you consistently see negative test results, we
recommend that you add other surfaces to your plan for sample collection and
testing. For examples of food-contact surfaces to sample, see the sites that we
identify as potential sources of contamination with L. monocytogenes in
section C of Table 1 (see section II, above).
C. Critical Non-Food-Contact Surfaces and Areas
For critical non-food-contact surfaces and areas, we
recommend that your written plan include collection and testing of
environmental samples from all, or representative sets of, such surfaces and
areas at least once every two weeks. If you will test a representative set of
samples (rather than all samples) each time, we recommend that your plan for
sample collection and testing be designed so that all critical non-food-contact
surfaces are tested at least once each quarter. Such samples could be taken at
any time (e.g., pre-production but post-sanitation; during production; or
after production).
The number of samples that you should collect depends on the
size of your production lines (Refs. 5, 6, 9, and 10). We recommend that even
the smallest processors collect samples from a minimum of 5 sites in each area
where RF-RTE foods are processed, exposed, or stored. We recommend that larger
processors determine the appropriate number of sampling sites based on the size
of the facility. In identifying the sites within each area, we recommend that
you focus on areas of high traffic and/or high moisture. We recommend that you
vary the sites of sample collection on a rotating basis, particularly if you
consistently see negative test results. For examples of sites to sample, see
the sites that we identify as potential sources of contamination with L.
monocytogenes in sections D and E of Table 1 (see section II, above).
D. Procedures for Collecting Environmental Samples
We describe recommended procedures to collect environmental samples
in Appendix 3.
E. Applicable Sections of 21 CFR part 110
Sections of 21 CFR part 110 that are applicable to monitoring
critical surfaces and areas include 21 CFR 110.80, which requires, in relevant
part, that chemical, microbial, or extraneous-material testing procedures be
used where necessary to identify sanitation failures or possible food
contamination.
XVII. What Sampling Should I Do of Finished RF-RTE Foods?
We recommend that you establish and implement a written plan
for the periodic collection of samples of finished RF-RTE food, and for testing
those samples for the presence of Listeria species or L.
monocytogenes. The purpose of the recommended periodic sample collection
and testing is to provide a historical reference for your production facility and
to validate the adequacy of your controls rather than to determine whether to
release individual lots of RF-RTE food that you process. We recommend that
your written plan include the frequency of sampling and the sampling plan to
accomplish those goals. For recommendations on corrective actions to take if
you find Listeria species or L. monocytogenes in samples of
finished RF-RTE food, see Sections XIX and XX.
Sections of 21 CFR part 110 that are applicable to sampling
of finished foods include 21 CFR 110.80, which requires, in relevant part, that
chemical, microbial, or extraneous-material testing procedures be used where
necessary to identify sanitation failures or possible food contamination.
XVIII. What Should I Do if I Detect Contamination of a Critical Food-Contact Surface or Food With Listeria Species?
If you detect contamination of a critical food-contact
surface or food with Listeria species, we recommend that you either conduct
a test to determine whether the Listeria species is L. monocytogenes,
or assume that the Listeria species is L. monocytogenes. Sections
of 21 CFR part 110 that are applicable to this recommendation include 21 CFR 110.80,
which requires, in relevant part, that chemical, microbial, or
extraneous-material testing procedures be used where necessary to identify
sanitation failures or possible food contamination.
If you determine that the Listeria species is NOT
L. monocytogenes, we recommend that you take corrective actions regarding
your plant and your processing, because the presence of any Listeria
species suggests that conditions also are suitable for survival and/or growth
of L. monocytogenes. In section XIX of this document, we
describe our recommended corrective actions for your plant and your processing
and provide citations to applicable sections of 21 CFR part 110.
If you either determine or assume that the Listeria
species IS L. monocytogenes, we recommend that you take the
corrective actions that we describe in sections XIX and XX of this document. In
Section XIX, we describe our recommendations regarding your plant and your
processing and provide the citations to applicable sections of 21 CFR part
110. In Section XX, we describe our recommendations regarding food and provide
citations to applicable sections of 21 CFR part 110.
XIX. What Corrective Actions Should I Take Regarding my Plant and my Processing if I Detect Listeria Species or L. monocytogenes on a Critical Surface or Area or in Food?
If you detect Listeria species or L. monocytogenes
on a critical surface or area or in food, we recommend that you follow a
corrective action plan that describes the steps to be taken, and assigns
responsibility for taking those steps, to ensure that the cause of the
contamination is corrected. We recommend that a corrective action plan include
the following corrective actions:
- Conduct additional sampling and testing as appropriate to
determine the specific surface or area that is contaminated with Listeria
species or L. monocytogenes. For example, if you initially tested a
composite sample, we recommend that you determine the source of the
contamination, either by testing the individual samples that had been
combined to form the composite or by collecting additional individual
samples from the affected area and testing the new samples; and
- Clean and sanitize the contaminated surface or area; and
- Conduct additional sampling and testing to determine
whether the contamination has been eliminated. If test results from the
additional testing continue to be positive for Listeria species or L.
monocytogenes, we recommend that you continue to clean, sanitize,
sample and test until the test results demonstrate that the contamination
with Listeria species or L. monocytogenes has been
eliminated; and
- Review production, maintenance and sanitation procedures
to determine whether to modify the procedures to prevent contamination and
make those modifications; and
- Review the scenarios that we provide in Table 2 (see
Section II of this document) as an aid to identifying causes of
contamination; and
- Check maintenance records for modifications or repairs to
major equipment; and
- Interview and observe sanitation, maintenance, and
production employees to determine whether procedures were followed; and
- Correct any identified problems that could lead to
contamination.
We also recommend that you make and keep a record of all
corrective actions taken.
Sections of 21 CFR part 110 that are applicable to
corrective actions in your plant if you detect Listeria species or L.
monocytogenes on a critical surface or area or in food include 21 CFR 110.35(d),
which requires that all food-contact surfaces, including utensils and
food-contact surfaces of equipment, be cleaned as frequently as necessary to
protect against contamination of food.
Sections of 21 CFR part 110 that are applicable to
corrective actions in your processing if you detect Listeria species or L.
monocytogenes on a critical surface or area or in food include 21 CFR 110.80
and 110.80(b)(2).
XX. What Corrective Actions Should I Take Regarding Food if I Detect Contamination of a Critical Food-Contact Surface or Food With
L. monocytogenes, or if I Assume that Listeria Species on a Critical Food-Contact Surface or in Food is L. monocytogenes?
In this section, we provide two sets of recommendations for corrective actions that you
should take regarding food if you detect L. monocytogenes on a critical
food-contact surface or in food (or if you assume that Listeria species
on a critical food-contact surface or in food is L. monocytogenes). Our
recommendations depend on whether the food does, or does not, support the
growth of L. monocytogenes.
A. Food that supports the growth of L.
monocytogenes
We recommend that you establish and follow a corrective
action plan that describes the steps to be taken, and assigns responsibility
for taking those steps, to ensure
that no finished RF-RTE food that supports the growth of L. monocytogenes
enters commerce if:
- The food bears or contains greater than or equal to 0.04 cfu of
L. monocytogenes per gram of food; or
- The food has been prepared, packed, or held under insanitary
conditions whereby it may have become contaminated with filth, or whereby it may
have been rendered injurious to health.
We recommend that such a corrective action plan include the
following steps:
- Segregate and hold any food that may have contacted the
contaminated food-contact surface until you have determined and taken
appropriate corrective actions. For example, segregate and hold any food
that was in contact with the contaminated food-contact surface since the
last time that a test was negative for Listeria species or L.
monocytogenes on that food-contact surface or since the last time that
food-contact surface was cleaned and sanitized;
- Determine and take appropriate corrective action for any
food that may have contacted the contaminated food-contact surface.
Examples of recommended corrective actions include:
- Treating the food with a listericidal control measure;
- Reprocessing the food so that the food:
- Does not support the growth of L. monocytogenes; and
- Contains less than 100 cfu of L. monocytogenes
per gram of food;
- Diverting the food to use by animals (as long as such
diversion would not adulterate the food for animals);
- Diverting the food to a use in which it will not be
consumed;
- Destroying the food;
- Recalling finished RF-RTE food that has been distributed.
B. Food that does not support the growth of L.
monocytogenes
We recommend that you establish and follow a corrective
action plan that describes the steps to be taken, and assigns responsibility
for taking those steps, to ensure that no finished RF-RTE food that does not
support the growth of L. monocytogenes enters commerce if:
- The food bears or contains greater than or equal to 100 cfu of L.
monocytogenes per gram of food; or
- The food has been prepared, packed, or held under insanitary
conditions whereby it may have become contaminated with filth, or whereby it
may have been rendered injurious to health.
We recommend that such a corrective action plan include the
following corrective actions:
- Segregate and hold any food that may have contacted the
contaminated food-contact surface until you have determined and taken
appropriate corrective actions. For example, segregate and hold any food
that was in contact with the contaminated food-contact surface since the
last time that a test was negative for Listeria species or L.
monocytogenes on that food-contact surface or since the last time the
surface was cleaned and sanitized;
- Determine and take appropriate corrective action for any
food that may have contacted the contaminated food-contact surface.
Examples of recommended corrective actions include:
- Treating the food with a listericidal control measure;
- Testing samples of the food to determine the number of L.
monocytogenes, and not distributing any food that has greater than or
equal to 100 cfu of L. monocytogenes per gram of food;
- Diverting the food to use by animals (as long as such diversion
would not adulterate the food for animals);
- Diverting the food to a use in which it will not be
consumed;
- Destroying the food;
- Recalling finished RF-RTE food that has been distributed.
C. Records of Corrective Actions
We recommend that you make and keep a record of all
corrective actions taken.
D. Applicable Sections of 21 CFR Part 110
Sections of 21 CFR part 110 that are applicable to
corrective actions regarding food if you detect L. monocytogenes on a
critical food-contact surface (or if you assume that Listeria species
that you detect on a critical food contact surface is L. monocytogenes) include
21 CFR 110.80, which requires that all reasonable precautions be taken to
ensure that production procedures do not contribute contamination from any
source and that chemical, microbial, or extraneous-material testing procedures
be used where necessary to identify sanitation failures or possible food
contamination.
Sections of 21 CFR part 110 that are applicable to
corrective actions regarding food if you detect L. monocytogenes in food
(or if you assume that Listeria species that you detect in food is L.
monocytogenes) include 21 CFR 110.80 and 110.80(b)(9).
XXI. What Training is Recommended?
We recommend that you provide training in employee health
and hygienic practices for all employees and contractors who enter production
and storage areas (e.g., employees who conduct production, maintenance,
quality assurance, quality control, or warehousing operations), including
employees and contractors who are temporary or seasonal. We also recommend
that the training be conducted before the employee performs job activities,
with refresher training on an annual basis.
We recommend that personnel who supervise or are otherwise
responsible for the operations listed below successfully complete training in
the application of the principles of the practices recommended in this guidance
to the control of L. monocytogenes in RF-RTE food.
- Establishing effective listericidal and listeristatic
control measures and ensuring that such control measures consistently
operate as intended;
- Collecting and testing environmental samples and samples
of finished RF-RTE foods;
- Determining and taking corrective actions; and
- Establishing and using sanitation standard operating
procedures and conducting associated monitoring.
Sections of 21 CFR part 110 that are applicable to training
include the recommendations in 21
CFR 110.10(c) and the requirements in 21 CFR 110.10(d).
XXII. What Analytical Methods Can I Use to Detect Listeria
Species, and to Detect, Confirm, or Enumerate L. monocytogenes?
We recommend that you use the procedures described in
Appendix 4 for preparing environmental samples for analysis. We recommend that
you use the procedures described in FDA's Bacteriological Analytical Manual Online
(BAM), Chapter 10 - "Listeria monocytogenes," "Detection and Enumeration
of Listeria monocytogenes in Foods" (Ref. 22) for preparing food samples
for analysis.
We recommend that you use the analytical methods in FDA's BAM
(Ref. 22) to:
- Detect the presence of Listeria species or L.
monocytogenes in environmental samples;
- Detect the presence of Listeria species or L.
monocytogenes in RF-RTE Foods that support the growth of L.
monocytogenes; and
- Confirm whether Listeria species is L.
monocytogenes.
We recommend that you use ISO 11290-2:1998(E) "Microbiology
of food and animal feeding stuffs - Horizontal method for the detection and
enumeration of Listeria monocytogenes - Part 2: Enumeration method" to
enumerate L. monocytogenes in RF-RTE foods that do not support its
growth. (ISO 11290-2:1998/Amd. 1:2004(E) "Microbiology of food and animal
feeding stuffs - Horizontal method for the detection and enumeration of Listeria
monocytogenes - Part 2: Enumeration method AMENDMENT 1: Modification of the
enumeration medium" amends ISO 11290-2:1998(E). The amendment uses ALOA agar
instead of PALCAM agar. If ALOA agar is not commercially available in the United States, we recommend that you use PALCAM according to ISO 11290-2:1998(E)). ISO
methods are available from the International Organization for Standardization 
If you use analytical methods other than those provided in
the BAM or by ISO, we recommend that you use written methods that have
equivalent or greater sensitivity for detecting and enumerating Listeria
species and L. monocytogenes and for confirming the presence of L.
monocytogenes.
XXIII. What Records Should I Make and Keep?
We recommend that you make and keep the following records:
- Documentation of listeristatic control measures;
- Documentation of listericidal control measures;
- Your list of ingredients and other raw materials that are
reasonably likely to be contaminated with L. monocytogenes;
- Any Certificate of Analysis or Certificate of Conformance
(i.e., supplier's guarantee) that you rely on to control L.
monocytogenes in ingredients;
- A written plan that you establish and implement for
collecting and testing environmental samples;
- A written plan that you establish and implement for
sampling and testing a finished RF-RTE food;
- Any written analytical method (other than the methods
provided in FDA's BAM or by ISO) that you use to detect or enumerate Listeria
species or L. monocytogenes, including the sensitivity of the
method;
- The results of any tests that you conduct to detect or
enumerate Listeria species or L. monocytogenes;
- Documentation of any corrective actions taken;
- A written sanitation standard operating procedure; and
- Sanitation control records.
We recommend that these records include:
- Your name and address;
- The date and time of the activity that the record
reflects;
- Actual values observed, when applicable;
- The signature or initials of the person performing the
operation, and the date of the signature or initials; and
- Where appropriate, the identity of the finished food and
the production code, if any.
We recommend that you enter processing and other information
on records at the time that it is observed.
We recommend that you retain records for 2 years and review
and update, as needed, your written plans at least once a year.
XXIV. References
We have placed the following references on display in the
Division of Dockets Management, Food and Drug Administration, 5630 Fishers Lane, rm. 1061, Rockville, MD 20852. Unless otherwise specified, we placed the
references in Docket No. 2007D-0494. You may see them at that location between
9 a.m. and 4 p.m., Monday through Friday.
- U.S. Food and Drug Administration, and U.S. Food Safety and Inspection Service. 2003. Quantitative
Assessment of the Relative Risk to Public Health from Foodborne Listeria
monocytogenes Among Selected Categories of Ready-to-Eat Foods. Available
in Docket No. 1999N-1168, Vols. 23 through 28, and at Quantitative Assessment of Relative Risk to Public Health from Foodborne Listeria monocytogenes Among Selected Categories of Ready-to-Eat Foods
- National
Advisory Committee on Microbiological Criteria for Foods. 1991. Listeria
monocytogenes. Recommendations by The National Advisory Committee on
Microbiological Criteria for Foods. International Journal of Food
Microbiology 14 (3-4): 185-246. Available in Docket No. 1999N-1168, Volume
0015 bkg1 (12 of 17), Tab 287.
- Fenlon DR, Wilson J, and Donachie W. 1996. The incidence and level of Listeria monocytogenes
contamination of food sources at primary production and initial processing. Journal
of Applied Bacteriology 81 (6): 641-650. Available in Docket No.
1999N-1168, Volume 0011 bkg1 (08 of 17), Tab 134.
- Fenlon DR. 1999. Listeria
monocytogenes in the natural environment. eds ET Ryser and EH Marth, 21-37.
New York: Marcel Dekker, Inc. Available in Docket No. 1999N-1168, Volume
0009 bkg1 (06 of 17), File name bkg0001_5_a.pdf.
- Tompkin RB, Scott
VN, Bernard DT, Sveum WH, and Gombas KS. 1999. Guidelines to Prevent
Post-Processing Contamination from Listeria monocytogenes. Dairy,
Food and Environmental Sanitation 19 (8): 551-562.
- Tompkin RB.
2002. Control of Listeria monocytogenes in the food-processing
environment. Journal of Food Protection 65 (4): 709-725.
- Moretro T, and
Lansgrund S. 2004. Listeria monocytogenes: Biofilm Formation and
Persistence in Food Processing Environments. Biofilms 1: 107-121.
- Gall K, Scott VN,
Collette R, Jahncke M, Hicks D, and Wiedmann M. 2004. Implementing Targeted
Good Manufacturing Practices and Sanitation Procedures to Minimize Listeria
Contamination of Smoked Seafood Products. Food Protection Trends 24 (5):
302-315.
- Kornacki JL. 2005.
Controlling Listeria in the Food Processing Environment. Food Technology
59 (11): 36-42.
- Scott VN,
Wiedmann M, Collette R, Jahncke ML, and Gall K. 2005. Guidelines for Listeria
Testing of Environmental, Raw Product and Finished Product Samples
in Smoked Seafood Processing Facilities. Food Protection Trends 25 (1):
23-24.
- Lou Y, and
Yousef AE. 1999. Characteristics of Listeria monocytogenes important to
food processors. In Listeria, Listeriosis, and Food Safety, eds ET Ryser
and EH Marth, 131-224. New York: Marcel Dekker, Inc. Available in Docket No.
1999N-1168, Volume 0014 bkg1 (11 of 17), Tab 245.
- Institute of Food Technologists. 2001. Evaluation and Definition of Potentially Hazardous Foods. IFT/FDA Contract No. 223-98-2333.
- Slutsker L, and
Schuchat A. 1999. Listeriosis in humans. In Listeria, Listeriosis and Food
Safety, eds ET Ryser and EH Marth, 75-95. New York: Marcel Dekker, Inc. Available
in Docket No. 1999N-1168, Volume 0018 bkg1 (15 of 17), Tab 389.
- Barnes R, Archer
P, Strack J, Istre GR, and Centers for Disease Control and Prevention. 1989.
Epidemiologic notes and reports listeriosis associated with consumption of
turkey franks. Morbidity and Mortality Weekly Report 38: 267-268. Available
in Docket No. 1999N-1168, Volume 0007 bkg1 (04 of 17), Tab 21.
- Shank FR, Elliot
EL, Wachsmuth IK, and Losikoff ME. 1996. US position on Listeria
monocytogenes in foods. Food Control 7 (4-5): 229-234. Available in
Docket No. 1999N-1168, Volume 0018 bkg1 (15 of 17), Tab 374.
- Sorrells KM, Enigl DC, and Hatfield JR. 1989. Effect of pH, Acidulant, Time, and Temperature on the
Growth and Survival of Listeria monocytogenes. Journal of Food
Protection 52 (8): 571-573.
- Petran RL, and
Zottola.E.A. 1989. A Study of Factors Affecting Growth and Recovery of Listeria
monocytogenes Scott A. Journal of Food Science 54 (2): 45-460.
- Tienungoon S,
Ratkowsky DA, McMeekin TA, and Ross T. 2000. Growth limits of Listeria
monocytogenes as a function of temperature, pH, NaCl, and lactic acid. Applied
and Environmental Microbiology 66 (11): 4979-4987.
- Leistner L. 1995.
Principles and applications of hurdle technology. In New Methods of Food
Preservation, ed GW Gould, 1-21. London: Blackie Academic &
Professional.
- Scott VN, Swanson
KMJ, Freier TA, Pruett WP, Sveum WH, Hall PA, Smoot LA, and Brown DG. 2005.
Guidelines for Conducting Listeria monocytogenes Challenge Testing of
Foods. Food Protection Trends 25 (11): 818-825.
- Doyle ME, Mazzotta AS, Wang T, Wiseman DW, and Scott VN. 2001. Heat resistance of Listeria
monocytogenes. Journal of Food Protection 64 (3): 410-429.
- U.S. Food and Drug Administration. 2003. Chapter 10. Listeria monocytogenes. Detection and
Enumeration of Listeria monocytogenes in Foods. In Bacteriological
Analytical Manual Online.
XXV. List of Appendices
- Appendix 1. Schematics Relevant to Recommended Plant Design
- Appendix 2. Recommended Schedules for Routine Cleaning and Sanitizing
- Appendix 3. Recommended Procedures for Collecting Environmental Samples
- Appendix 4. Recommended Procedures for preparing environmental samples for analysis
Appendix 1. Schematics Relevant to Recommended Plant Design
Figure 1.
Air Flow
| Recommended | Not Recommended |
|---|
 |  |
| Raw Area has "Negative Air Pressure" | Raw Area has "Positive Air Pressure" |
Figure 2.
Separation of Raw and RTE Areas by Partitions
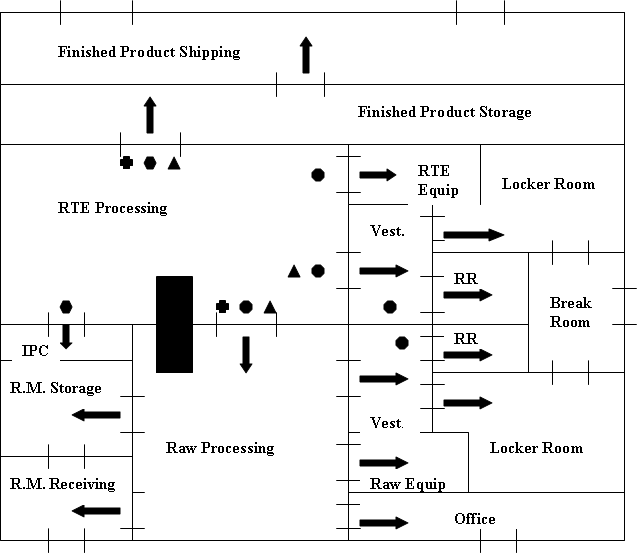 |
Legend
 | Direction of air flow |
|---|
 | Hand wash station |
|---|
 | Footbath |
|---|
 | Equipment decontamination |
|---|
 | Product cooker |
|---|
| Vest. | Vestibule |
|---|
| Equip | Equipment cleaning |
|---|
| IPC | In-process cooler |
|---|
| RR | Restroom |
|---|
| R.M. | Raw material |
|---|
Figure 3.
Separation of Raw and RTE Areas by Air Flow
 |
Legend
 | Direction of air flow |
|---|
 | Hand wash station |
|---|
 | Footbath |
|---|
 | Equipment decontamination |
|---|
 | Product cooker |
|---|
| Vest. | Vestibule |
|---|
| Equip | Equipment cleaning |
|---|
| IPC | In-process cooler |
|---|
| RR | Restroom |
|---|
| R.M. | Raw material |
|---|
Appendix 2. Recommended schedules for routine cleaning and sanitizing
Recommended Schedules for Routine Cleaning and Sanitizing for
Food-Contact Surfaces Used with RF-RTE foods (2005 Food Code)
| Temperature of the Room Where the Surface is Located |
Frequency of Cleaning* |
|---|
| Less than or equal to 5 °C (Less than or equal to 41 °F) |
Less than or equal to 24 hours |
| 5-7 °C (41-45 °F) |
Less than or equal to 20 hours |
| 7-10 °C (45-50 °F) |
Less than or equal to 16 hours |
| 10 -13 °C (50-55 °F) |
Less than or equal to 10 hours |
| Greater than 13 °C (Greater than 55 °F) |
Less than or equal to 4 hours |
Recommended Schedules for Routine Cleaning and Sanitizing for
Surfaces, Areas or Equipment that do not Contact RF-RTE Foods (Tompkin et al. 1999)
| Type of Surface or Area |
Frequency of
Cleaning* |
|---|
| Drains and floors |
Daily |
| Pallets |
Daily |
| Waste containers |
Daily |
| Cleaning tools (e.g., mops, brushes) |
Daily |
| Condensate drip pans |
Weekly |
| Motor housings, overhead piping, external surfaces of enclosed processing systems |
Monthly |
| Ceilings and walls** |
Monthly |
| Freezers (e.g., spiral, blast, tunnel) containing exposed RRTE foods*** |
Semi-annually |
| Interiors of Ice Makers*** |
Semi-annually |
| Surfaces that have a potential to become a niche for L. monocytogenes
contamination (e.g., when employees touch the equipment during operation,
or where there may be build-up of moisture or product residue) |
Daily |
*If the results of environmental monitoring or product testing indicates a problem with contamination,
we recommend that you increase the frequency of cleaning.
**If surfaces such as ceilings or walls have a potential to become a niche for L. monocytogenes contamination,
we recommend that you increase the frequency of cleaning to a daily basis.
***If the manufacturer of the equipment recommends
cleaning on a more frequent basis, we recommend that you increase this
frequency to match the recommendations of the manufacturer.
Appendix 3. Recommended Procedures for collecting environmental samples
- Reagents and supplies
- Gloves (sterile)
- Sponges (non-microbiocidal, sterile)
- Plastic bags (sterile) to hold sponges (e.g., tomacher bag, Whirl-Pak®, or equivalent)
- Swabs (cotton tipped applicators; non-microbiocidal and sterile)
- Sterile containers (with lids) to hold swabs (e.g., screw-capped or snap-capped plastic tubes),
or sterile commercial swab-container devices with neutralizing broth
- Scissors (sterile)
- Beakers to hold 100 ml (sterile)
- Bottles (with lids) to hold 100 ml (sterile)
- Dey-Engley (D/E) neutralizing broth (Commercially available, or prepared according to the formula below)
- Tryptone, 5 g
- Yeast extract, 2.5 g
- Dextrose (glucose) 10.0 g
- Sodium thioglycollate, 1.0 g
- Sodium thiosulfate, 6.0 g
- Sodium bisulfite, 2.5 g
- Polysorbate 80, 5.0 g
- Lecithin (soy bean), 7.0 g
- Bromcresol purple, 0.02 g
- Distilled water 1.0 L
- Final pH 7.6 ± 0.2 at 25 degrees C (77 degrees F)
- Collecting Samples from Surfaces (Including both Food Contact Surfaces and Non-food-contact Surfaces)
The two methods that we recommend to collect samples are
"surface sponging" and "swabbing." In general, the preferred method of
sampling is surface sponging, but swabs may be more appropriate for surfaces
that could be described as "nooks and crannies."
To the extent practical, samples should represent a constant
area and be from areas as large as possible. We recommend that you choose an
area that is 30-100 square centimeters (5-15 square inches) in size for
sponging and an area that is 10-13 square centimeters ( 1.5-2 square inches) for
swabbing.
When using a sponge to collect samples, add 10 ml D/E
Neutralization Broth to a bagged sponge just before sampling. Allow the sponge
to absorb the liquid. Squeeze off excess before removing the sponge, so it
does not drip. When using a swab that is not pre-packaged in a neutralization
broth, soak the tip of the swab in D/E Neutralization Broth and press out excess before removing the swab, so it does
not drip, just before sampling.
We recommend that you wear sterile gloves and wipe the area
with a D/E moistened sponge or swab using a systematic technique that thoroughly reverses
direction between successive strokes. Place the sponge in a sterile plastic
bag, such as a Stomacher bag. Place the swab
tip back into its original container with the pre-filled neutralization broth.
Separate the handle from the swab tip, either by snapping it off or by cutting
it off using sterile scissors. Discard the swab handle.
You should store the used sponge or swab at approximately 5 degrees
C (41 degrees F), including immediately after use and during transport to the
testing laboratory.
- Collecting Liquid Samples (Including Floor Drain Effluents)
We recommend that you use a sterile beaker or similar
container to collect 110 ± 5 ml of liquids such as drainage effluents,
standing water, melt water from thawed processing ice, and vacuum or drip pan
condensate. We recommend that you immediately transfer the collected sample
into a sterile screw-capped bottle and then chill and store the bottle at 5 degrees
C (41 degrees F), including during transport to the testing laboratory.
Appendix 4. Recommended Procedures for preparing environmental samples for analysis
Laboratory analysis of samples should only be
conducted by persons with appropriate microbiological training or experience.
Listeria monocytogenes infection can cause serious illness and death,
including fetal death. We recommend that pregnant women and persons who are
immunocompromised because of illness, medication, or advanced age avoid working
with this organism. Contaminated equipment and media should be sterilized
before disposal or reuse.
- Reagents, supplies, and equipment
- Gloves (sterile)
- Scissors (sterile)
- Plastic bags (sterile) (e.g., Stomacher bag, Whirl-Pak®, or equivalent)
- Pipettes (sterile) and 10 ml pipetting device
- Vortex (or similar device for agitating)
- Bottles with lids to hold 100 ml (sterile)
- Tubes with lids (sterile) to hold 10 ml
- Microbiological filters, 25-50 mm diameter (1-2 inches), 0.45 micron pore-diameter
- Pre-filter - if there is a need to remove large
particulates, use a pre-filter that is more coarse than the filters (e.g.,
sterile glass wool or cheesecloth with a diameter of 6.4 cm (2.5 inches)
- Disposable filter/funnel units may be useful
- Microbiological filter holder, water trap, water-driven air pump
- Scalpels (sterile or capable of being flame-sterilized with 70% v/v ethanol)
- Forceps/tweezers (sterile or capable of being flame-sterilized with 70 % v/v ethanol)
- Ethanol solution, aqueous, 70% v/v (for flame sterilizing equipment such as forceps and scalpels)
- Dey-Engley (D/E) neutralizing broth (Commercially available, or prepared according to the formula below)
- Tryptone, 5 g
- Yeast extract, 2.5 g
- Dextrose (glucose) 10.0 g
- Sodium thioglycollate, 1.0 g
- Sodium thiosulfate, 6.0 g
- Sodium bisulfite, 2.5 g
- Polysorbate 80, 5.0 g
- Lecithin (soy bean), 7.0 g
- Bromcresol purple, 0.02 g
- Distilled water 1.0 L
- Final pH 7.6 ± 0.2 at 25 degrees C (77 degrees F)
- Buffered Listeria Enrichment Broth base (BLEB) (includes sodium pyruvate) (see FDA's BAM, Chapter 10, M52)
- Stomacher or sterile blender
- Samples collected from sponges or swabs (Composite)
A common technique we recommend is to combine analytical portions from several samples
and analyze the mixture of the portions (which is referred to as a
"composite"). If there is a positive result from the composite, the analysis
could be repeated for reserve analytical portions from each individual sample
that was combined to form the composite.
To prepare a 5-sponge
composite, squeeze or press liquid broth from the sponges from each of the 5
samples. Massage, blend, or stomach for 2 minutes. For each sample, pipette 5
ml of the broth into a sterile tube with lid, and retain it at 5 degrees C (41 degrees F) as a
reserve for analysis if the composite is
positive for Listeria species. Combine the remaining portions of each
sample, including the sponges, to form the composite that will be used for
analysis. With sterile forceps remove each of the 5 sponges and place into 225
ml of BLEB.
To prepare a 5-swab
composite, shake and then press the liquid from the swab for each sample.
Pipette 5 ml of broth into a sterile tube with lid for each sample, and retain
it at 5 degrees C (41 degrees F) as a
reserve for analysis if the composite is positive for Listeria species. Combine the remaining
portions of each sample, including the swabs, to form the composite that will
be used for the analysis.
If you homogenize using a Stomacher, add 225 ml of BLEB to
the individual or
composited sponges/swab tips in transport fluid in a sterile plastic bag
suitable for use in a Stomacher. Place in the Stomacher for 2 minutes. If you
use a blender, add 225 ml of BLEB to the individual or composited sponges/swab tips and
transport fluid in sterile blender jar. Blend for 2 minutes.
- Samples collected from liquids
We recommend that as soon as possible after sample
collection, you filter 100 ml of the collected liquid through one or more
sterile 0.45 micron pore-diameter filters. If particulate content is high (e.g.,
judging from the sample turbidity), we recommend that you pass the liquid
through a sterile glass pre-filter before the 0.45 micron filter. Rinse the retentate on the filter plus any pre-filter
with 5-10 ml of D/E broth to remove any residual inhibitory substances. If necessary, excise the filters from the
funnel devices, using sterile scalpels. Put each
filter and the pre-filter, if any, in a sterile
bag (if you will use a Stomacher) or in a sterile container (such as a blender
jar, if you use a blender).
Note: If composites are made
from the filters, cut filter and any pre-filter in half using sterile
instruments. Use one half of each filter to form a composite and retain the
other half at 5 degrees C (41 degrees F) as
a reserve for analysis if the composite is positive for Listeria species.
Add 225 ml of BLEB to the individual or composited filters/pre-filters. Stomach or blend for 2 minutes.
D. Sample analysis
We recommend that you incubate the analytical portions
prepared in BLEB at 30 degrees C (86 degrees F) for 4.0 hours to pre-enrich for
Listeria species. We also recommend that you then add the selective
agents, i.e., acriflavin, sodium nalidixate, and cycloheximide (BAM; M52), and
continue incubating at 30 degrees C (86 degrees F) for another 20.0 hours for a
total incubation time of 24 hours. If using FDA's BAM, the applicable section
for you to refer to is Section D. Isolation procedure.
Descriptions
Figure 1 is a graphic representation showing that the recommended air flow should have negative pressure in the raw processing area rather than in the RTE processing area.
Figure 2 is one of two figures with schematic representations showing examples of plant design, including schematic representations related to recommendations for air flow, product flow and the use of partitions in the design of the plant. In Figure 2, the raw processing area is separated from the RTE processing area by a partition. This differs from Figure 3, in which there is no partition, but there is additional positive air pressure in the RTE processing area.
The rest of both figures show recommendations to:
- Place partitions between:
- The office area and the raw processing area;
- Areas designated for receiving raw materials, storing raw materials, processing raw foods, and the in-process cooler;
- The raw processing area and the area designated for cleaning equipment used in processing raw foods;
- The raw processing area and a vestibule separating that area from rest rooms and locker rooms designated for use by employees when they work in the raw processing area;
- The in-process cooler and the RTE processing area;
- The RTE processing area and the area designated for cleaning equipment used in processing RTE foods;
- The RTE processing area and a vestibule separating that area from rest rooms and locker rooms for use by employees when they work in the RTE processing area;
- The RTE processing area and the finished product storage area;
- The finished product storage area and the finished product shipping area; and
- Locker rooms and a break room.
- Place doorways between:
- The outside and the office area;
- The outside and the area designated for receiving raw materials;
- The areas designated for receiving and storing raw materials;
- The office area and the raw processing area;
- The raw processing area and the areas designated for receiving raw materials, storing raw materials, and cleaning equipment used in processing raw foods;
- The raw processing area and a vestibule leading to the rest rooms and locker rooms for use by employees when they work in the raw processing area;
- The RTE processing area and the in-process cooler;
- The RTE processing area and the areas designated for cleaning equipment used in the processing of RTE foods and for storing finished product;
- The RTE processing area and the vestibule leading to the rest rooms and locker rooms for use by employees when they work in the RTE processing area;
- The finished product storage area and the finished product shipping area;
- The finished product shipping area and the outside; and
- Locker rooms and a break room.
- Place the product cooker so that it spans the raw processing area and the RTE processing area and can be opened in both areas;
- Place hand washing stations:
- In the RTE processing area at the entrance to the raw processing area, the in-process cooler, the area designated for cleaning equipment used to process RTE foods, the area designated for finished product storage, and the vestibule leading to rest rooms and locker rooms; and
- In the vestibules leading to rest rooms and locker rooms.
- Place footbaths in the RTE processing area at:
- The entrance to the raw processing area;
- The entrance to the finished product storage area; and
- The vestibule leading to the rest rooms and locker rooms.
- Provide for equipment decontamination in the RTE processing area at:
- The entrance to the raw processing area; and
- The finished product storage area.
- Provide positive air pressure:
- In the office area near the entrance to the raw processing area;
- In the raw processing area near the entrance to the RTE processing area;
- In the raw material receiving area near the entrance to the raw processing area;
- In the raw material storage area near the entrance to the raw processing area;
- In the area designated for cleaning equipment used to process raw foods near the entrance to the raw processing area;
- In the in-process cooler near the entrance to the RTE processing area;
- In the finished product storage area near the entrance to the RTE processing area;
- In the finished product shipping area near the entrance to the finished product storage area;
- In the area designated for cleaning equipment used to process RTE foods near the entrance to the RTE processing area;
- In the rest rooms near the entrance to the vestibule; and
- In the locker rooms near the entrance to the vestibule.
Figure 3 is one of two figures with schematic representations showing examples of plant design, including schematic representations related to recommendations for air flow, product flow and the use of partitions in the design of the plant. Figure 3 shows additional positive air pressure at the juxtaposition between the RTE processing area and the raw processing area relative to the amount of positive air pressure in Figure 2, where the raw processing area is separated from the RTE processing area by a partition. The remaining recommendations in Figure 3 are the same as those in Figure 2.
(1)This guidance has been prepared jointly by the Office of Food Safety and the Office of Compliance (both in the Center for Food Safety and Applied Nutrition), and by the Office of Regulatory Affairs, at the U.S. Food and Drug Administration.
(2)Frankfurters and deli meats are under the jurisdiction of USDA/FSIS rather than FDA. However, we mention them for completeness.
(3)Intrinsic factors include chemical and physical factors that are normally within the structure of the food, e.g., pH and water activity. Extrinsic factors are those that refer to the environment surrounding the food, e.g., storage temperature.
(4) Because normal pasteurization will effectively eliminate L. monocytogenes, it is generally assumed that contamination of products such as pasteurized fluid milk is the result of post-pasteurization contamination (see Section V of Reference 1, p. 170).
(5)
American Society of Heating Refrigerating and Air-Conditioning Engineers. 2000. Standard 52.2-1999 -- Method of Testing General Ventilation Air-Cleaning Devices for Removal Efficiency by Particle Size.: ASHRAE. Available for purchase at
http://www.webstore.ansi.org/. 








