ISSN: 1080-6059
Volume 14, Number 9–September 2008
Dispatch
Monkey Malaria in a European Traveler Returning from Malaysia
Anu Kantele, ![]() Hanspeter Marti, Ingrid Felger, Dania Müller, and T. Sakari Jokiranta
Hanspeter Marti, Ingrid Felger, Dania Müller, and T. Sakari Jokiranta
Author affiliations: Helsinki University Central Hospital,
Helsinki, Finland (A. Kantele); University of Helsinki, Helsinki (A. Kantele,
T.S. Jokiranta); Swiss Tropical Institute, Basel, Switzerland (H. Marti, I.
Felger, D. Müller); and Helsinki University Central Hospital HUSLAB, Helsinki (T.S.
Jokiranta)
Suggested citation for this article
Abstract
In 2007, a Finnish traveler was infected in Peninsular
Malaysia with Plasmodium knowlesi, a parasite that usually causes
malaria in monkeys. P. knowlesi has established itself as the fifth Plasmodium species that can cause human malaria. The disease is potentially
life-threatening in humans; clinicians and laboratory personnel should become
more aware of this pathogen in travelers.
Traditionally, only 4 Plasmodium species have been known to cause malaria in humans: P. falciparum, P. vivax, P. ovale, and P. malariae, although >26 Plasmodium species are known to circulate among primate populations (1). Some of these species have been implicated in symptomatic human malaria after experimental or accidental infection (2). Only a few reports of naturally acquired monkey malaria in humans are currently available (1,3–9). The lack of data may be because light microscopy has been used as the sole diagnostic method and an atypical Plasmodium species may have been misidentified as one of the 4 traditional Plasmodium species causing human malaria.
P. knowlesi was first described in 1931 in a long-tailed macaque imported from Singapore to India; in 1932, P. knowlesi was experimentally shown to be infectious to humans (10). The first natural infection of P. knowlesi in humans was reported in 1965 in a man returning to the United States after a visit to Peninsular Malaysia (11). Subsequently, in 1971, there was a report of a presumed natural infection in a citizen of Malaysia (6). Despite extensive studies in Malaysia in the 1960s (2), no other reports were published on naturally acquired P. knowlesi infections in humans until 2004, when Singh et al. studied PCR-negative P. malariae cases in the Kapit division in Sarawak, Malaysia (3). A different PCR analysis showed that P. knowlesi caused 58% of the 208 malaria cases studied. Further cases reported from China (4), Thailand (5), Philippines (8), and Singapore (12) show that P. knowlesi infections in humans are not found exclusively in Malaysia. Recently, Cox-Singh et al. reported that P. knowlesi is widely distributed among inhabitants of Malaysia (7).
The Study
A 53-year-old Finnish man was admitted to a local hospital in Finland in March 2007 with fever after 4 weeks of travel in Peninsular Malaysia. He had not taken any antimalarial prophylaxis. In Malaysia, he spent 2 weeks in Kuala Lumpur and made a few day trips to surrounding rural areas. Thereafter, he traveled by car to the northwestern coast and stayed for 5 days in the jungle ≈80 km south of Ipoh. While in this area, he slept in a house without mosquito screens or nets and did not use any repellents; he did not report any mosquito bites. The last week of his travel was spent in the Langkawi Beach area where he stayed at a high-quality hotel. During his trip he occasionally had some minor abdominal problems, but these symptoms subsided spontaneously after his return to Finland. High fever (38.8°C axillary temperature) occurred 3 days after his return to Finland but abated quickly. On the fourth day, the fever returned and he sought medical care at a local hospital. Laboratory tests showed the following results: C-reactive protein 2.0 mg/dL (normal range <1.0 mg/dL), hemoglobin 15.2 g/dL (normal range 13.4–16.7 g/dL), leukocyte count 2.6 × 109/L (normal range 3.4–8.2 × 109/L), and thrombocytes 143 × 109/L (normal range 150–360 × 109/L). Blood smear was positive for Plasmodium organisms, and the causative agent was identified as P. falciparum with levels of parasitemia <1.0%. The patient was admitted to the hospital and given intravenous (IV) quinine dihydrochloride and oral doxycycline.
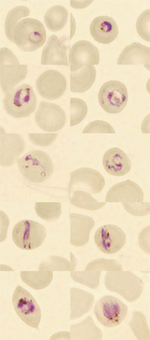 |
Figure. Microscopic findings in the thin blood smears of a patient with Plasmodium knowlesi malaria. Early ring forms are shown in the first row, later trophozoites in the second and third rows... |
On day 2 of the patient's hospital stay, fever returned and he was transferred to the Helsinki University Central Hospital (Department of Infectious Diseases at Aurora Hospital). Blood smears obtained there showed Plasmodium parasites that were considered atypical, and the laboratory reported suspicion of a co-infection (P. falciparum and P. malariae) (Figure). The IV quinine dihydrochloride was replaced with oral quinine hydrochloride, and doxycycline was continued. During treatment, the patient experienced an attack of hypoglycemia (electrocardiogram and blood pressure was normal during this attack), transient mild visual and hearing loss, and transient lymphopenia (a low of 0.46 × 109/L). He received quinine hydrochloride and doxycycline for a total of 10 days.
Because identification of the Plasmodium species was difficult, a blood sample was drawn for PCR analysis on day 2 of hospitalization. First, a nested PCR was performed according to a standard protocol with rOva1 and rPLU2 primers (template DNA purified in Basel from 200 μL of erythrocytes by QIAamp DNA Mini Blood Kit (QIAGEN, Helsinki, Finland) (13,14), but the reaction did not yield any amplification product. Nested PCR was repeated with an alternative primer pair (rPLU6 and rPLU2) (14) derived from a conserved region of the 18S rRNA marker gene, and an amplicon was obtained. Failure of PCR amplification has been reported for some P. ovale isolates (15); therefore, a P. ovale infection was suspected, and the patient was given primaquine phosphate for 14 days as an outpatient to eradicate possible liver hypnozoites. The PCR product was subjected to direct nucleotide sequencing (GenBank accession no. FJ009511) and found to be identical to 2 P. knowlesi sequences previously submitted to GenBank, 1 human isolate from Malaysian Borneo (AY327556) and a Macaca mulatta isolate from Columbia (U72542). Six other published P. knowlesi sequences differ from our sequence only by 1 nucleotide (99% identity). In contrast, a number of differences were seen between our sequence and the P. ovale sequences (15). The sequence from our case showed only 50% identity to the ova1e primer; therefore, we concluded that our patient was infected with P. knowlesi. During the 12-month follow-up period, the patient showed no signs of relapse.
Conclusions
We suggest that P. knowlesi infection should be considered in malaria patients who have a history of a travel to forested areas in Southeast Asia, especially if P. malariae malaria is diagnosed or atypical plasmodia are seen with microscopy. The asexual stages of various species of P. knowlesi can easily be misidentified as P. malariae in light microscopic examination (Figure) (3,7,10). Because most laboratories diagnose malaria by light microscope examination only, numerous cases of P. knowlesi malaria may have been misdiagnosed as ordinary P. malariae malaria; monkey malaria may be more widespread among humans than was previously thought. As the disease is potentially dangerous, a proper identification of the malaria species is crucial. If PCR assays for malaria detection are used, PCR primers specific for P. knowlesi (3) should be included to provide valuable diagnostic information.
P. knowlesi has established itself as the fifth species of Plasmodium that causes human malaria (3,7,12). Because the disease is potentially life-threatening in humans, laboratory clinicians and physicians (especially those taking care of travelers) should become more aware of this disease; it is easily misdiagnosed as a less severe form of malaria.
Acknowledgments
We thank the patient for allowing us to publish his case, Heli Siikamäki for helpful discussions, and personnel of the Unit of Parasitology, Helsinki University Central Hospital Laboratory (HUSLAB), for recognizing the atypical nature of Plasmodium parasites in the patient's thin blood smears.
The research of T.S.J. is financially supported by the Academy of Finland (projects 201506 and 202529), the Helsinki University Central Hospital Funds, and the Sigrid Jusélius Foundation; the work of A.K. is supported by the Finnish Medical Foundation and the special Finnish governmental subsidy for health sciences research.
Dr Kantele is an infectious diseases specialist in the Division of Infectious Diseases, Helsinki University Central Hospital. She is also a scientist in the Department of Microbiology and Immunology, Helsinki University. Her research has focused on immune responses to infections and vaccines, and recently she has become interested in travel medicine and tropical diseases.
References
- Rich SM, Ayala FJ. Progress in malaria research: the case for phylogenetics. Adv Parasitol. 2003;54:255–80. PubMed DOI
- Garnham PCC. Malaria parasites and other haemosporidia. Oxford (UK): Blackwell Scientific Publications; 1966.
- Singh B, Kim Sung L, Matusop A, Radhakrishnan A, Shamsul SS, Cox-Singh J, et al. A large focus of naturally acquired Plasmodium knowlesi infections in human beings. Lancet. 2004;363:1017–24. PubMed DOI
- Zhu HM, Li J, Zheng H. Human natural infection of Plasmodium knowlesi [in Chinese]. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 2006;24:70–1.
- Jongwutiwes S, Putaporntip C, Iwasaki T, Sata T, Kanbara H. Naturally acquired Plasmodium knowlesi malaria in human, Thailand. Emerg Infect Dis. 2004;10:2211–3.
- Fong YL, Cadigan FC, Coatney GR. A presumptive case of naturally occurring Plasmodium knowlesi malaria in man in Malaysia. Trans R Soc Trop Med Hyg. 1971;65:839–40. PubMed DOI
- Cox-Singh J, Davis TM, Lee KS, Shamsul SS, Matusop A, Ratnam S, et al. Plasmodium knowlesi malaria in humans is widely distributed and potentially life threatening. Clin Infect Dis. 2008;46:165–71. PubMed DOI
- Luchavez J, Espino F, Curameng P, Espina R, Bell D, Chiodini P, et al. Human infections with Plasmodium knowlesi, the Philippines. Emerg Infect Dis. 2008;14:811–3.
- Ng OT, Ooi EE, Lee CC, Lee PJ, Ng LC, Pei SW, et al. Naturally acquired human Plasmodium knowlesi infection, Singapore. Emerg Infect Dis. 2008;14:814–6.
- Knowles R, Das Gupta BM. A study of monkey malaria and its experimental transmission to man (preliminary report). Ind Med Gaz. 1932;67:301–20.
- Chin W, Contacos PG, Coatney GR, Kimball HR. A naturally acquired quotidian-type malaria in man transferable to monkeys. Science. 1965;149:865. PubMed DOI
- Fleck F. Monkey malaria could represent a new human strain. Bull World Health Organ. 2004;82:392–3. DOI: 10.1590/S0042-96862004000500017
- Snounou G, Singh B. Nested PCR analysis of Plasmodium parasites. Methods Mol Med. 2002;72:189–203.
- Snounou G, Viriyakosol S, Zhu XP, Jarra W, Pinheiro L, do Rosario VE, et al. High sensitivity of detection of human malaria parasites by the use of nested polymerase chain reaction. Mol Biochem Parasitol. 1993;61:315–20. PubMed DOI
- Win TT, Jalloh A, Tantular IS, Tsuboi T, Ferreira MU, Kimura M, et al. Molecular analysis of Plasmodium ovale variants. Emerg Infect Dis. 2004;10:1235–40.
Figure
Suggested Citation for this Article
Kantele A, Hanspeter M, Felger I, Müller D, Jokiranta TS. Monkey malaria in a European traveler returning from Malaysia. Emerg Infect Dis [serial on the Internet]. 2008 Sep [date cited]. Available from http://www.cdc.gov/EID/content/14/9/1434.htm
DOI: 10.3201/eid1409.080170
Please use the form below to submit correspondence to the authors or contact them at the following address:
Anu Kantele, Helsinki University Central Hospital, Department of Medicine, Division of Infectious Diseases, Aurora Hospital, Building 5, 3rd Floor, Post Office Box 348, FIN-00029 HUS, Helsinki, Finland; email: anu.kantele@hus.fi
Please contact the EID Editors at eideditor@cdc.gov
The opinions expressed by authors contributing to this journal do not necessarily reflect the opinions of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.
This page posted August 26, 2008
Centers for Disease Control and Prevention, 1600 Clifton Rd, Atlanta, GA 30333, U.S.A
Tel: (404) 639-3311 / Public Inquiries: (404) 639-3534 / (800) 311-3435