This report presents the conclusions of OSHA's regulatory review of the Ethylene Oxide Standard under the requirements of both Sections 610 of the Regulatory Flexibility Act and Section 5 of Executive Order (EO) 12866. Under Section 610, this review examines whether the standard should be continued without change, rescinded, or amended to minimize any significant impact on a substantial number of small entities. OSHA has considered the continued need for the rule, comments and complaints received, complexity of the rule, whether the rule is duplicative and changes since its issuance. Under Section 5 of EO 12866, this review examines whether the standard has become unjustified or unnecessary as a result of changed circumstances, and whether the standard is compatible with other regulations or is duplicative or inappropriately burdensome in the aggregate.
This review also ensures that the regulation is consistent with the priorities and the principles set forth in EO 12866 and within applicable law, and examines whether the effectiveness of the standard can be improved. In order to assist OSHA in this review, OSHA requested public comments on these issues and held a public meeting (62 FR 28649, May 27, 1997).
The Section 610 Review of the EtO Standard indicates that:
- There is a continued need for the rule. Workers exposed to EtO in a range of industries continue to be at risk of cancer, genetic changes, reproductive effects, neurotoxicity, and sensitization. As part of this "Lookback" review, OSHA examined evidence submitted by commenters and from the scientific literature as it pertained to the findings of the 1984 risk assessment used to support the EtO Standard. Since the risk assessment was developed, the International Agency for Research on Cancer reclassified EtO as a known human carcinogen and the National Toxicology program reclassified EtO as one "known to be a human carcinogen." Based on the significant scientific information, OSHA finds that the potential carcinogenicity of EtO and the risk posed to workers continues to justify the need for the Standard. Comprehensive studies, compliance information, and public comments indicate that the Standard has been effective in reducing exposure to EtO thereby achieving the predicted health benefits. The public comments evidenced widespread support for continuance of the EtO Standard and endorsed its effectiveness. No commenter argued that the standard should be rescinded.
- The evidence indicates that the EtO Standard has not had a negative economic impact on the industries affected by the standard, generally, or on small businesses in those industries. Most of the small businesses affected by the EtO Standard are hospitals, medical device manufacturers, and spice manufacturers. There are no indications that the regulation of occupational exposure to EtO has impaired the economic well being of businesses in any of these sectors or has disproportionately affected small businesses.
- The rule is not unduly or unreasonably complex. Although most commenters did not directly address the issue of whether the standard was considered to be unduly or unreasonably complex, a number of comments at the public meeting and submitted to the Docket requested clarification of the requirements of the standard. Difficulty in understanding or interpreting some requirements of the standard was reported in the hospital sector, which contains the majority of affected small entities. OSHA intends to issue compliance assistance and outreach materials to aid employers' understanding of the standard.
- The EtO Standard does not overlap with other regulations. Four major federal regulatory entities in addition to OSHA currently regulate various aspects of EtO use and transport. The only potential regulatory conflict raised by one commenter during this Lookback review involved an Environmental Protection Agency standard under the Clean Air Act for EtO-using commercial sterilization and fumigation operations. Commercial sterilization and fumigation operations using one ton or more of EtO per year are required to use emission control technology to comply with EPA standards. The two agencies' rules do not actually conflict and no employers have stated that they have not been able to comply with both.
- Technological improvements have improved worker safety. OSHA's independent research, comments received, and the technical literature indicate that significant technological developments have occurred since the promulgation of the standard. Improvements in sterilizer technology, the growth in number and use of alternative sterilants and sterilizing processes, and use of contract sterilizers to perform EtO sterilization have contributed to an observed reduction in occupational exposure to EtO. None of the comments received by OSHA indicated that technological feasibility problems prevented affected businesses from complying with the EtO standard.
- The standard encouraged the development of improved sterilizers, which achieved compliance with the standard and cost less than other sterilizers. The newer equipment costs about half the cost of the older equipment with add-on controls. This reduced costs for all employers including small businesses.
- A 1995 Congressional Office of Technology Assessment study completed after the standard took effect concluded that the Feasibility Study, which OSHA performed before issuance of the standard, was accurate and well done.
An Executive Order 12866 review of the Standard indicates that:
- The EtO Standard remains both justified and necessary. As discussed in OSHA's Section 610 analysis, EtO poses significant health and safety risks to workers exposed to the substance. While the standard has resulted in dramatic reductions in occupational exposures to EtO, OSHA continues to document overexposures and non-compliance in the workplace. A study of Massachusetts hospitals demonstrated that enforcement actions were necessary before they came into compliance with the standard.
- The EtO Standard is compatible with other OSHA standards and is not inappropriately burdensome in the aggregate. No public comment questioned the compatibility of the EtO standard with any OSHA or state standard.
- The EtO Standard is compatible with E.O. 12866. The Executive Order essentially espouses a regulatory system that efficiently and effectively protects health and safety without imposing unacceptable or unreasonable costs on society. The regulations that are produced must be consistent, sensible, and understandable. The Lookback review has amassed numerous comments supporting the standard's effectiveness in reducing occupational exposures to EtO. In addition, the industries that use EtO appear to be familiar with the standard and have adopted improved technology, use of substitutes, and other methods to improve efficiency. No evidence was submitted to the Docket or identified by OSHA in the course of this Lookback review to suggest that the standard was imposing either a significant impact on a substantial number of small entities or that it was causing an excessive compliance burden.
- The EtO Standard is effective in achieving its mission. Uniform support for retaining the EtO standard is in the public record for this Lookback review.
Conclusions and Recommendations
Based on analyses performed for this Lookback review, OSHA concludes that the EtO Standard should be continued without change and does not need to be revised or rescinded to minimize economic impacts on small entities. OSHA's original conclusions concerning the health risks of exposure to EtO have been supported by new epidemiological and health risk studies released since the promulgation of the standard in 1984. Based on exposure monitoring data from several sources indicating that occupational exposure to EtO has fallen markedly since the EtO standard went into effect, workers are being protected. OSHA enforcement program data documenting that overexposures and accidental releases of EtO continue to occur at workplaces that are not in compliance with the standard underscore the continuing need for the standard. Both employer and employee representatives indicate a continued need for
the standard. OSHA has therefore concluded that the EtO Standard continues to be needed to protect workers health and safety. Accordingly, OSHA finds that there is no need at this time to modify the rule through a rulemaking process to make it more effective or less burdensome.
As a result of this Lookback review and the comments received from participants in the process, OSHA is considering enhancing the following compliance assistance materials to assist the regulated community in complying with the standard:
- Enhancing compliance assistance materials clarifying and explaining the exposure monitoring requirements of the standard, particularly as these relate to the standard's emergency alert requirements and to operations potentially involving overexposures to the excursion limit.
- Enhancing compliance assistance materials explaining the standard's medical surveillance requirements, particularly as these relate to the taking of occupational histories and the standard's triggers for medical surveillance.
- Reviewing OSHA's existing compliance interpretations as these relate to tank changing operations and the use of respiratory protection in these operations, and developing new materials addressing these topics if necessary.
- Making more widely available the 2000 NIOSH Hazard Alert entitled "Preventing Worker Injuries and Deaths from Explosions in Industrial Ethylene Oxide Sterilizer Facilities" to address the risk of explosions.
EXECUTIVE SUMMARY
CHAPTER I
CHAPTER II
Overview
Industry Profile
Employer Compliance Experience With the Standard
Conclusions
CHAPTER III
Overview
Information on Adverse Health Effects and Quantitative Risk Assessment
New Health Information Available Since the 1984 Standard
IARC and NTP Classifications of EtO as a Known Human Carcinogen
OtheR New Information Relating to Carcinogenicity
Exposure Trends Indicate that the Standard is Effective
Accidental EtO Releases in Hospitals and Other Health Care Service Establishments
Employers and Employees Support Continuance of the Standard
Summary
References for the Health Effects and Risk Assessment Section
CHAPTER IV
Overview
Summary of Docket Comments
CHAPTER V
Overview
Existing Technology and Changes in Technological Conditions
Industries that Use EtO as a Sterilant or Fumigant
EtO Production, Usage, and Substitution Trends
Economic Changes
CHAPTER VI
Industrial Sectors Containing Affected Small Entities
Public Comments Addressing Small Entities
Impacts on Affected Small Entities
Conclusion
CHAPTER VII
Findings
OSHA's Responses to Concerns Raised by Stakeholders
Findings Concerning Specific Public Comments and Complaints
Findings Concerning Complexity of the Standard
Findings Concerning Duplication, Conflict or Overlap
Findings Concerning Changes in Technological and Economic Conditions
Outcome of the Executive Order 12866 Review
Outreach and Compliance Assistance Activities OSHA is Considering in Response to this Lookback Review
Appendix A
LIST OF TABLES
| Table II-1 |
Number of American Hospital Association Registered Hospitals and Total Number of Beds By Type of Ownership and Type of Hospital 1980 and 1995 |
| Table II-2 |
Distribution of Community Hospitals by Bed Size Category |
| Table II-3 |
Number of Commercial Sterilization Facilities Using Ethylene Oxide |
| Table II-4 |
Top Five Most Frequently Cited EtO Standard Provisions, By Size-Class of Firm Inspected, Using Citation Data from 1985-1997 |
| Table III-1 |
EtO Exposure Monitoring Data from All Industries Collected by OSHA Before and After Issuance of the Final Ethylene Oxide Standard |
| Table III-2 |
EtO Exposure Monitoring Data Collected by OSHA Before and After Issuance of the Ethylene Oxide Standard, by Establishment Size |
| Table III-3 |
EtO Personal Exposure Monitoring Results from Hospital Studies |
| Table III-4 |
EtO Exposure Monitoring Data from Hospitals and Other Health Care Service Establishments Collected by OSHA Before and After Issuance of the EtO Standard |
| Table III-5 |
EtO Exposure Monitoring Data from the Medical and Pharmaceutical Products Sterilizing Industries Collected by OSHA Before and After Issuance of the EtO Standard |
| Table V-1 |
Advantages and Disadvantages of Different Methods of Sterilization (As Alternatives to EtO Sterilization) |
OSHA wishes to thank those on its staff who helped with this project, especially John Martonik, Joanna Dizikes Friedrich, Marthe Kent, Chuck Gordon, and Kathy Condit.
The Occupational Safety and Health Administration (OSHA) has conducted a review of its Ethylene Oxide (EtO) Standard under Section 610 of the Regulatory Flexibility Act1 and Section 5 of Executive Order (EO) 12866 on Regulatory Planning and Review.
The purpose of a review under Section 610 of the Regulatory Flexibility Act:
"(S)hall be to determine whether such rule should be continued without change, or should be rescinded, or amended consistent with the stated objectives of applicable statutes to minimize any significant impact of the rules on a substantial number of small entities."
"The Agency shall consider the following factors:
- The continued need for the rule;
- The nature of complaints or comments received concerning the rule from the public;
- The complexity of the rule;
- The extent to which the rule overlaps, duplicates or conflicts with other Federal rules, and, to the extent feasible, with State and local governmental rules; and
- The length of time since the rule has been evaluated or the degree to which technology, economic conditions, or other factors have changed in the area affected by the rule."
The review requirements of Section 5 of EO 12866 require agencies:
"To reduce the regulatory burden on the American people, their families, their communities, their State, local, and tribal governments, and their industries; to determine whether regulations promulgated by the [Agency] have become unjustified or unnecessary as a result of changed circumstances; to confirm that regulations are both compatible with each other and not duplicative or inappropriately burdensome in the aggregate; to ensure that all regulations are consistent with the President's priorities and the principles set forth in this Executive Order, within applicable law; and to otherwise improve the effectiveness of existing regulations."
To carry out these reviews, OSHA asked the public for comments on all issues raised by these provisions (62 FR 28649). Specifically, OSHA requested comments on: the impacts of the rule on small businesses; the benefits and utility of the rule in its current form and, if amended, in its amended form; the continued need for the rule; the complexity of the rule; and whether, and to what extent, the rule overlaps, duplicates, or conflicts with other Federal, State, and local government rules. OSHA also asked for comments on new developments in technology, economic conditions, or other factors affecting the ability of covered firms to comply with the EtO Standard. Furthermore, OSHA asked for comments on alternatives to the rule that would minimize significant impacts on small businesses, while achieving the objectives of the Occupational Safety and Health Act.
All documents and comments received relevant to the review, transcripts of the meeting, and documents discussed in this report are available at the OSHA Docket Office, Docket No. H-200C, Room N-3625, U.S. Department of Labor, 200 Constitution Avenue, N.W., Washington, DC 20210, Telephone (202) 693-2350.
CHAPTER II
REGULATORY ENVIRONMENT SURROUNDING THE STANDARD |
OVERVIEW
In conducting this Lookback review of the Ethylene Oxide (EtO) standard under Section 610 of the Regulatory Flexibility Act and Section 5 of Executive Order 12866, OSHA developed updated estimates of the number of affected employers and employees currently covered by the standard and examined the compliance experience of affected employers. This chapter presents a profile of the affected employers by industry grouping; analyzes recent OSHA enforcement and consultation program data; presents field and State-plan State enforcement officials' perspectives on key EtO-related compliance issues; and describes information on industry compliance obtained from a review of the occupational safety and health literature and other relevant studies. Thus, this chapter provides a description of the regulatory environment in which the standard is currently being implemented.
INDUSTRY PROFILE
The EtO standard applies to all occupational exposures to ethylene oxide occurring in facilities covered by the OSH Act. The standard covers general industry employment under §1910.1047, shipyard employment under §1915.1047, construction employment under §1926.1147, and marine terminal and longshoring operations under §§1917.1 and 1918.1, respectively.
According to the Chemical Economics Handbook, approximately 8.2 billion pounds of EtO were produced in the U.S. in 1999. Nearly all (99.2%) of the EtO consumed in the U.S. was domestically produced; only 1.7 million pounds of EtO were imported in 1999. It was projected that 7.8 billion pounds would be produced in 2003 (CEH, Oct. 2003). Most of the EtO was used as a process input by various chemical manufacturing industries; for example, only 8 to 9 million pounds of the 7.3 billion pounds was used in fumigant or sterilant applications in 19962. The industry profile that follows has, therefore, been broadly divided into two groupings: (1) chemical manufacturing industries (e.g., EtO producers and ethoxylators) and (2) industries that use EtO as a sterilant or fumigant (e.g., hospitals and commercial sterilizers such as
medical products manufacturers, spice manufacturers, and contract sterilizers).
Chemical Manufacturing Industries
Ethylene Oxide Producers
Producers manufacture EtO by the direct oxidation of ethylene. Producers of ethylene oxide are classified in SIC 2869, Industrial Organic Chemicals, Not Elsewhere Classified. The Small Business Administration (SBA) small firm definition for this industry is 1,000 employees.
According to the CEH, the U.S. ethylene oxide production industry has operated at annual capacity rates generally in the 84-89% range since 1985. Furthermore, this industry has added over 3 billion pounds of capacity since 1988. This capacity increase resulted from the addition of two new plants as well as significant capacity improvements made to six other existing plants. Domestic production of EtO has risen from 6.0 billion pounds in 1984 to 8.2 billion pounds in 1999. The economic situation of this industry has generally been favorable during these years.3
In 1984, in the Final Regulatory Impact Analysis for the Ethylene Oxide Standard, OSHA estimated that there were 13 domestic producers operating 16 EtO production facilities [Docket H-200, Ex. 164]. In 1999, there were 10 domestic producers of ethylene oxide operating 13 EtO production facilities. In 2003, there still were the 10 domestic producers, but they had consolidated production in 12 facilities. These producers are: BASF Corporation, Dow Chemical, Eastman Chemical Company, Equistar Chemical, Formosa Plastics Corporation, Huntsman Corporation, Old World Industries, PD Glycol, Shell Chemical Company, and Sunoco Inc.4 All of these companies are considered large firms, according to the SBA criterion for this industry. BASF, Equistar, Shell Chemical, and Sunoco are the four largest companies selling to the merchant market. Although there are fewer
companies and facilities producing EtO than was the case in 1984, this is the result of mergers and consolidations within the chemical industry rather than a decrease in demand for EtO. As noted above, EtO production has increased substantially in this period.
In the Regulatory Impact Analysis for the 1988 excursion limit rulemaking, OSHA estimated that there were approximately 1,046 employees exposed to EtO during its production [53 FR 11421]. This estimate was taken from a report by Heiden Associates that was submitted to OSHA by the Ethylene Oxide Industry Council (EOIC) during the 1988 rulemaking [Docket H-200B, Ex. 205-6B].5 In this report, Heiden Associates estimated that, on average, there were 80.5 affected employees per EtO production unit. Heiden Associates also provided other data indicating that there were approximately 1.1 EtO production units per EtO production facility. In order to update the estimate of EtO production workers, OSHA first multiplied these estimates to derive an average total of 88.6 workers per facility. OSHA then multiplied the 1999 number of production facilities (13)
by an average of 88.6 employees per facility. OSHA estimates that in 1999 there were approximately 1,151 employees currently employed in EtO production and, therefore, potentially exposed to EtO. This represents about a 9 percent increase in the number of employees potentially exposed to EtO in this industry compared with the number potentially exposed in1988.
Ethoxylators
Ethoxylators are chemical manufacturing firms that use EtO as a chemical feedstock to make chemical products such as ethylene glycol, glycol ethers, ethanolamines, surfactants, and other specialty chemicals. A total of 57 percent of all EtO is used to produce ethylene glycols. Many of the 10 current EtO producers listed above manufacture these products in addition to producing pure EtO. Producers of ethylene glycol and glycol ethers are classified in SIC 2869, Industrial Organic Chemicals, Not Elsewhere Classified. The SBA small firm definition for this industry is 1,000 employees. Producers of surfactants are classified in SIC 2843, Surface Active Agents, Finishing Agents, Sulfonated Oils, and Assistants. The SBA small firm definition for this industry is 500 employees. Most ethoxylators would thus be large companies according to the SBA definition.
In the 1984 Final Regulatory Impact Analysis, OSHA estimated that there were 50 ethoxylator firms in the United States in addition to those EtO producers then engaged in operating ethoxylation units [Docket H-200, Ex. 164]. The EtO producers listed above still produce nearly all of the ethylene glycol, glycol ethers, and ethanolamines generated by ethoxylation processes.6 Ten EtO producer firms operate 11 ethoxylation facilities manufacturing one or more of these products. In addition, one specialty chemical manufacturer that does not produce EtO produces glycol ethers at a single facility. Additional firms engage in the manufacture of surfactants and other specialty chemicals via ethoxylation processes. To update the estimate of the number of ethoxylator firms currently in operation, in addition to those engaged in the primary production of EtO,
OSHA reviewed recent CEH reports containing information on producers of EtO-based surfactants.
EtO is used as a process input during the production of three different types of surfactants: alcohol ether sulfates, alcohol ethoxylates, and alkylphenol ethoxylates. These surfactant compounds are used to manufacture shampoos, light-duty liquid dish detergents,7 heavy-duty laundry detergent powders and liquids, and specialty cleansers.8 In 1994, the CEH reported that there were 12 firms, with a total of 29 facilities, engaged in producing alcohol ether sulfates.9 In 1996, there were 19 firms, with a total of 29 facilities, engaged in producing alcohol ethoxylates and 3 additional firms engaged in producing alkylphenol ethoxylates.10 After adjusting this total for double-counting, OSHA estimates that there are approximately 21 stand-alone
ethoxylator firms engaged in the production of surfactants through ethoxylation reactions and that these 21 firms operate a total of 46 surfactant ethoxylation facilities. (In addition, 4 EtO producer firms operate a total of 9 surfactant ethoxylation facilities; 3 of the 9 facilities are presumably facilities already counted above, because they are sited at locations where ethylene glycol, glycol ethers, and ethanolamines are manufactured.) Therefore, OSHA estimates that there are at a minimum approximately 22 firms uniquely covered by the ethoxylator industry grouping (the 21 surfactant producers plus the 1 glycol ether manufacturer).
In the Regulatory Impact Analysis for the 1988 EL rulemaking, OSHA estimated that there were approximately 1,436 employees exposed to EtO during ethoxylation processes [53 FR 11421]. This estimate was also taken from the Heiden Associates report on EtO production and ethoxylation operations cited above [Docket H-200B, Ex. 205-6B]. Heiden Associates estimated that, on average, there were 37.8 affected employees per EtO ethoxylation unit and also provided data indicating that there were approximately 1.6 ethoxylation units per ethoxylation facility. Therefore, in order to update the estimate of ethoxylation facility workers, OSHA first multiplied these estimates to derive an average of 60.4 workers per facility. OSHA next added the number of ethoxylation facilities producing ethylene glycol, glycol ethers, and ethanolamines (12) to the number of ethoxylation facilities producing surfactants
(52),11 to get the total number of ethoxylation facilities believed to be currently in operation (64). OSHA then multiplied this number (64) by the estimated average of 60.4 employees per ethoxylation facility, to obtain an estimate of 3,866 employees engaged in ethoxylation processes, more than twice the number estimated to be potentially exposed in this process in1988.
Industries That Use EtO as a Sterilant of Fumigant
During the 1984 and 1988 EtO rulemakings, OSHA concluded that industries using EtO as a sterilant or fumigant would also be affected by the EtO standard. In the regulatory impact analyses accompanying the two rulemakings, OSHA identified the following industries where EtO was used as a sterilant or fumigant as industries that would be impacted by the standard: hospitals, medical products sterilizers, and spice manufacturers.12 In the 1988 Regulatory Flexibility and Impact Analysis, OSHA retained its 1984 estimates of approximately 6,300 hospitals, 125 medical products sterilizers, and fewer than 30 spice manufacturers [53 FR 11421]. However, in that analysis, OSHA provided new, and lower, estimates of the number of workers exposed to EtO during sterilization and fumigation operations. In 1988, OSHA estimated that 65,246 workers were exposed to
EtO during EtO sterilization and fumigation operations, with approximately 63,000 of these workers exposed to EtO in the hospital sector [53 FR 11421].
In conducting this Lookback review, OSHA has determined that EtO sterilization continues to be used in all of the industries identified in the 1984 and 1988 rulemakings. OSHA further concludes that EtO use for sterilization purposes is still most significant in the hospital industry and that the largest number of exposed workers is employed in that industry. The following discussion concerning industries using EtO as a sterilant or fumigant has therefore been divided into two groupings: (1) hospitals, and (2) commercial sterilizers (including all other industries identified by OSHA as currently engaged in significant EtO sterilization and fumigation operations).
A large number of EtO sterilization systems are currently in use in the hospital and commercial sterilization industries. Estimates from an article in Health Industry Today indicated that there were approximately 12,500 EtO sterilization systems in use in the U.S. in 1995.13 Nevertheless, OSHA also found evidence indicating that the number of hospitals using EtO sterilization systems has declined since 1984 (see the discussion below at pp. II-7 to II-9) and that much of the EtO sterilization of spices and, to a lesser extent, the sterilization of medical devices, has shifted from the manufacturers of these items to contract sterilizers (see the discussion at pp. II-13 to II-15). Some evidence was also found suggesting that there are several ongoing minor uses of EtO in sterilization and fumigation operations (e.g., laboratory sterilization operations,
veterinary clinic sterilization operations, and fumigation of beekeeping equipment).
Hospitals
Hospitals use EtO as a sterilant for reusable and single-use items that would be damaged by steam sterilization or other sterilization processes. Examples of items requiring EtO sterilization include catheters, cardiac pulse generators, orthopedic prostheses, and other implantable medical devices [Docket H-200C, Ex. 2-2]. The use of EtO sterilization systems is common, but not universal, in the hospital setting. Two recent studies of EtO use in the hospital sector (discussed below) contain data suggesting that between 44% and 60% of U.S. hospitals have on-site EtO sterilization operations. Most hospitals are classified in SIC 8062, General Medical and Surgical Hospitals and SIC 8069, Specialty Hospitals. Table II-1 presents details on the number of American Hospital Association (AHA)-registered hospitals and the total number of beds, by ownership and hospital type, in 1980 and 1995.
The SBA small firm definition for profit-making enterprises in the hospital sector is $5 million in revenues. However, since most hospitals are either non-profit organizations or are owned by municipal governments, special size criteria apply. The Regulatory Flexibility Act defines a "small organization" as an organization that is independently owned and operated and is not dominant in its field. Furthermore, the SBA has defined a "small governmental jurisdiction" as an establishment that serves a population of less than 50,000. Table II-2 presents statistics on the distribution of community hospitals by size in order to provide some indication of the size distribution of entities in the hospital sector most likely to be affected by the EtO standard.
In the 1984 Regulatory Impact Analysis, OSHA estimated that there were 6,237 hospitals currently using EtO sterilizers [Docket H-200, Ex. 164]. The estimate of the number of affected hospitals was derived from the findings of a 1977 study by Z. R. Glaser.14 In that study, Glaser estimated that there were 8,100 hospitals in the United States. Glaser also presented an estimate indicating that 77 percent of hospitals belonging to the American Hospital Association (AHA) used EtO sterilizers. The estimated number of hospitals affected by the EtO standard was therefore generated by multiplying the estimated percentage of EtO-using hospitals by the total number of U.S. hospitals (8,100 hospitals x .77 = 6,237 EtO-using hospitals).
Table II-1
Number of American Hospital Association Registered Hospitals and Total Number of Beds
By Type of Ownership and Type of Hospital 1980 and 1995
| Ownership and Type of Hospital |
Number of
AHA-Registered Hospitals |
Percentage of Total AHA- Registered Hospitals |
Total Number of Beds |
| 1980a |
1995b |
1980 |
1995 |
1980 |
1995 |
| Federal |
359 |
299 |
5.2 |
4.8 |
117,000 |
77,079 |
| Non-federal, psychiatric, TB, and other respiratory disease hospitals |
545 |
660 |
7.8 |
10.5 |
217,000 |
110,471 |
| Non-federal, long-term general and other special hospitals |
157 |
112 |
2.3 |
1.8 |
39,000 |
18,765 |
| Hospital units of state/local govt. or nonprofit institutions |
74 |
26 |
10.0 |
0.4 |
4,000 |
1,550 |
| Community hospitals (non-profit) |
3,322 |
3,092 |
47.7 |
49.1 |
692,000 |
609,729 |
| Community hospitals (for-profit) |
730 |
752 |
10.5 |
12.0 |
87,000 |
105,737 |
| Community hospitals (state/local govt.) |
1,778 |
1,350 |
25.5 |
21.5 |
209,000 |
157,270 |
| Total |
6,965 |
6,291 |
100.1c |
100.1 |
1,365,000 |
1,080,601 |
aSource: JRB Associates, "Economic and Environmental Impact Study of Ethylene Oxide," Final Report, prepared for U.S. Department of Labor, OSHA, April, 1983.
bSource: Hospital Statistics, AHA, 1996-1997 edition, Chicago, 1996. Data are from the 1995 annual survey of hospitals.
cTotal is greater that 100 percent because of rounding. |
Table II-2
Distribution of Community Hospitals a by Bed Size Category
Hospital Size
(Number of Beds) |
Number of Community Hospitals |
Percent of Community
Hospitals |
Total Number of Beds |
| 1980b |
1995c |
1980 |
1995 |
1980 |
1995 |
| 6-24 |
259 |
278 |
4.4 |
5.4 |
4,932 |
5,085 |
| 25-49 |
1,029 |
922 |
17.7 |
17.8 |
37,478 |
34,352 |
| 50-99 |
1,462 |
1,139 |
25.1 |
21.9 |
105,278 |
82,024 |
| 100-199 |
1,370 |
1,324 |
23.5 |
25.5 |
192,892 |
187,381 |
| 200-299 |
715 |
718 |
12.3 |
13.8 |
172,390 |
175,240 |
| 300-399 |
412 |
354 |
7.1 |
6.8 |
139,434 |
121,136 |
| 400-499 |
266 |
195 |
4.5 |
3.8 |
117,724 |
86,459 |
| 500+ |
317 |
264 |
5.4 |
5.1 |
218,259 |
181,059 |
| Total |
5,830 |
5,194 |
100.0 |
100.1d |
988,387 |
872,736 |
aCommunity hospitals, as defined by the American Hospital Association (AHA), are non-federal acute-care facilities.
bSource: JRB Associates, "Economic and Environmental Impact Study of Ethylene Oxide," Final Report, prepared for U.S. Department of Labor, OSHA, April, 1983.
cSource: Hospital Statistics, AHA, 1996-1997 edition, Chicago, 1996. Data are from the 1995 annual survey of hospitals.
dTotal is greater than 100 percent because of rounding. |
In order to update the estimate of the number of EtO-using hospitals, OSHA reviewed recent AHA statistics concerning the number of AHA-registered hospitals and also reviewed the findings of two recent studies concerning EtO use in hospitals. Table II-1 indicates that, in 1995, there were a total of 6,291 AHA-registered hospitals--nearly a 10 percent decrease from the 6,965 hospitals registered in 1980. Most of these hospitals (5,194, or 82.5%) are community hospitals; these hospitals are defined by the AHA as non-federal, acute-care facilities. These statistics were used in conjunction with the data discussed below from studies of EtO use in the hospital sector in two states, Massachusetts and Colorado. As of 2002, there were 5,794 registered hospitals according to the AHA Resource Center, hospitalconnect.com, 10/13/04, continuing the slow consolidation of
hospitals.
The first of two studies used by OSHA to estimate the current use of EtO in the hospital sector was the 1993 Massachusetts Hospital EtO Health and Safety Study conducted by Dr. Anthony LaMontagne [Docket H-200C, Ex. 2-9K].15 This study surveyed all 159 hospitals in the Commonwealth of Massachusetts and found that 92 of the 154 responding hospitals reported using EtO as a sterilant at their hospitals. Dr. LaMontagne therefore estimated that approximately 96 hospitals, or 60 percent of all hospitals in Massachusetts, used EtO in 1993. The Massachusetts Hospital Study also produced several other noteworthy findings, including the conclusions that (1) EtO-using hospitals are typically acute-care facilities, (2) EtO-using hospitals are more likely to be privately operated than non-using hospitals, and (3) EtO-using hospitals are somewhat larger on average
than non-using hospitals [Docket H-200C, Ex. 2-9K]. With regard to the type of facility, 87 of the 92 EtO-using hospitals (95 percent) were acute-care facilities, while only 12 of the 62 non-using hospitals (19 percent) were acute care facilities. Taking hospital ownership into consideration, the study found that 76 of the 92 EtO-using hospitals were privately operated (83 percent), while only 33 of the 62 non-using hospitals (53 percent) were private hospitals. Finally, with regard to hospital size, the study found that EtO-using hospitals had an average of 253 patient beds versus 158 patient beds for hospitals that were non-users [Ex. 2-9K]. This information suggests that smaller hospitals may be somewhat less likely to use EtO and thus to have compliance responsibilities related to the EtO standard.
A second study, "Survey of Chemical Use in Colorado Hospitals," conducted in the fall of 1995 by Colorado Hospitals for a Healthy Environment (CHHE), also provided OSHA with useful data on the prevalence of EtO-using hospitals.16 CHHE surveyed 96 Colorado hospitals ranging in size from small rural institutions to large urban health care complexes about their chemical usage patterns. Fifty-two hospitals responded to this survey. CHHE commented that the 52 responding hospitals provided a "good representation of hospitals across Colorado." Furthermore, a significant number of the respondents to the CHHE survey were relatively small hospitals.17 Of the 52 respondents to the CHHE survey, 19 respondents (or 37 percent) reported using EtO, 29 respondents reported that they did not use any EtO,
and there were 4 non-respondents to this question. The CHHE report did not present a distribution of EtO-using hospitals by ownership type or size class. However, the findings of the CHHE survey suggest that it would be reasonable to estimate that a maximum of 44% of Colorado hospitals are engaged in EtO use (19 EtO users + 4 non-respondents/52 respondents).
The findings of the Massachusetts Hospital EtO Health and Safety Study, in conjunction with the findings of the CHHE survey, suggest that the percentage of hospitals using EtO has declined significantly from the mid-1970's to the mid-1990's (77% of hospitals in 1977 versus a range of 44% to 60% during the mid-1990s). To derive an updated estimate of the number of such hospitals, OSHA averaged the 44% and 60% estimates to derive an estimated average of 52%. OSHA then multiplied this percentage by 6,291, the total number of AHA-registered hospitals in 1995, to derive an estimate of 3,271 EtO-using hospitals nationwide.
This estimated decrease in the number of EtO-using hospitals is explained, in part, by certain demographic trends occurring in the hospital sector. First, the total number of non-federal, acute-care community hospitals declined from 5,830 hospitals in 1980 to 5,194 hospitals in 1995 (see Table II-2). Since the findings of the Massachusetts Hospital Study indicated that acute-care facilities are particularly likely to use EtO, this reduction in the number of non-federal acute care community hospitals may have contributed to a decrease in the percentage of hospitals using EtO. Second, while the number of AHA-registered hospitals declined by almost 10 percent between 1980 and 1995, the number and percentage of smaller hospitals among AHA-registered hospitals increased (see Table II-2). This may also have had some significance for trends in EtO use in the hospital sector, since the Massachusetts
Hospital Study found that, on average, EtO-using hospitals tend to be somewhat larger than non-using hospitals. There are no indications that costs of complying with the EtO Standard, which is a tiny fraction of the costs of operating a hospital, contributed to the decrease in number of hospitals.
In the 1984 Regulatory Impact Analysis, OSHA estimated that there were, on average, 10 directly exposed workers per EtO-using hospital facility. OSHA therefore estimated that there were 62,370 employees directly exposed to EtO in the hospital sector (6,237 hospitals x 10 directly exposed workers per hospital) [Docket H-200, Ex. 164].18 In order to update this estimate, OSHA used data from the Massachusetts Hospital Study, which reported that EtO-using sterilization departments in Massachusetts hospitals employed 12 workers on average [Docket H-200C, Ex. 2-9K]. OSHA multiplied this estimate of the average number of workers times the estimated number of EtO-using hospitals to calculate a total estimate of 39,252 workers exposed to EtO during hospital sterilization operations.19 This represents a decline of
nearly 40 percent in the number of potentially exposed sterilization workers since 1984. However, a recent study indicates the EtO use for sterilization has stabilized or increased because of technological improvements. Also, some of the EtO sterilization which had been performed by hospitals and medical device manufacturers was being performed by contract sterilizers because of their lower costs. See K. Hemmerich, et al., Sterilization Methods Stand the Test of Time, MDDI, Aug. 2004, devicelink.com. This is based on 1997 hospital numbers. The Hospital Statistics 2004 estimates that there were 4% fewer hospitals in 2002. However, the hospitals averaged more employees and probably used more contractor sterilizers, so it would be difficult to estimate any further changes in the numbers of exposed workers.
Commercial Sterilizers
Firms that perform EtO sterilization as part of a production process are commonly referred to as commercial sterilizers. Commercial sterilizers can generally be classified into two groups: in-house sterilizers and contract sterilizers. In-house sterilizers produce goods requiring sterilization and sterilize them as part of their production process. (Some other types of entities that have been known to use EtO sterilization, such as laboratories, museums, and libraries, are also similar to in-house sterilizers because, although they do not produce the goods needing sterilization, sterilization is a small part of their routine operations.)20 Contract sterilizers are companies that specialize in offering sterilization and/or fumigation services to clients. Contract sterilizers do not, in general, produce any of the goods being sterilized. The trend
toward the use of contract sterilizers has continued throughout the 90s, as more and more companies focus on their core business and contract out other services that they need.
During the 1988 rulemaking, OSHA examined the use of ethylene oxide in two industries where commercial sterilization processes were performed: the medical products sterilization and spice manufacturing industries. As indicated above, at that time, OSHA used estimates from the 1984 rulemaking of 125 medical products sterilizers and fewer than 30 spice manufacturers [FR Vol. 53, No. 66, 4/6/88, p. 11421]. During the process of updating this industry profile, OSHA found very little current data on industries where commercial sterilization operations occur. Some of the best publicly available data identified by OSHA was found in a 1993 Environmental Protection Agency (EPA) background information document. This document was produced to support an EtO air emissions standard for commercial sterilization operations that was promulgated in 1994 (the National Emission Standards for Hazardous Air
Pollutants (NESHAP) for Ethylene Oxide Commercial Sterilization and Fumigation Operations).21 The information in this EPA document is more current than the data OSHA relied on in the 1988 rulemaking, and it also presents more recent estimates of the number of affected small entities.22
The EPA document estimates that, in 1988, there were 188 facilities engaged in commercial sterilization operations. EPA grouped these facilities into seven major categories: medical equipment suppliers (62 facilities); other health-related suppliers (24 facilities); pharmaceutical manufacturers and other drug-related manufacturers (39 facilities); spice manufacturers and other food-related manufacturers (23); museums and libraries (13); research, testing, and animal breeding laboratories (10); and contract sterilizers (17). Table II-3 presents these estimates, along with EPA's estimates of the number of these facilities that belonged to firms meeting the EPA definition of "small entity."23 (In later research, EPA also identified the fumigation of privately owned beekeeping equipment by state government Departments of Agriculture as a
limited use of EtO as a fumigant.)
Table II-3
Number of Commercial Sterilization Facilities Using Ethylene Oxide |
| Industry Group |
SIC Codesa |
Number of Facilities |
Number of Small Entitiesb |
| Medical Equipment Suppliers |
3841, 3842 |
62 |
6 |
| Other Health-Related Suppliers |
3079, 3693, 5086, 2821, 2211 2879, 3069, 3569, 3677, 3999 |
24 |
5 |
| Pharmaceutical Manufacturers |
2834, 5122, 2831, 2833 |
39 |
2 |
| Spice Manufacturers |
2099, 5149, 2034, 2035, 2046 |
23 |
3 |
| Contract Sterilizers |
7389, 7218, 8091 |
17c |
12 |
| Museums and Libraries |
8411, 8231 |
13 |
4 |
| Laboratories |
0279, 7391, 8922, 8071, 7397 |
10 |
2 |
Environmental Protection Agency, OAR/OAQPS, Ethylene Oxide Emissions from Commercial Sterilization/Fumigation Operations--Background Information for Proposed Standards (Pub. No. EPA-435/D-93-016), March 1993, Tables 8-2; 8-20.
aSICs are listed in order of frequency of establishments reporting that SIC.
bBased on EPA's definition of small entities as those having annual sales of less than $3.5 million.
cWhile EPA was collecting the data, one contract sterilizer ceased use of EtO. |
In order to update the industry profile for commercial sterilizers, OSHA relied heavily on the findings of this EPA research. However, OSHA chose to revise the groupings used by the EPA to reflect the groupings used by OSHA in the EtO rulemakings. Thus, the EPA profile of commercial sterilizers that follows is broken into the following groupings: (1) medical and pharmaceutical product manufacturers, (2) spice manufacturers, (3) contract sterilizers, and (4) other sterilization and fumigation applications.
Medical and Pharmaceutical Product Manufacturers
Medical products manufacturers use EtO to sterilize equipment, medical devices, and supplies that might be damaged by other sterilization methods. Some examples include x-ray equipment and catheters. A breakdown of the sterilization methods used for medical products in 1995 indicated that EtO was used as the sterilization method 52 percent of the time, while gamma radiation was used 46 percent of the time. Steam sterilization comprised less than 2 percent, and other methods 0.1 percent, of all methods used.24 Most EtO-using medical products manufacturers are found in SIC 3841, Surgical and Medical Instruments and Apparatus, and SIC 3842, Orthopedic, Prosthetic, and Surgical Appliances and Supplies. However, these manufacturers are found in a number of other SICs as well, reflecting the diversity of products that can be sterilized using EtO. The
SBA small business size definition for both SICs 3841 and 3842 is 500 employees.
A few pharmaceutical products manufacturers use EtO to sterilize products such as bulk powders, ingredients used in ophthalmic ointments and suspensions, and packaging materials. These uses seem to be in decline. In particular, according to the FDA, use of EtO in bulk powder sterilization appears to have been declining because of difficulties related to verifying the sterility of the product after sterilization cycles have been completed.25 Most pharmaceutical manufacturers are typically found in SIC 2834, Pharmaceutical Preparations. The SBA small business size definition for this industry is 750 employees.
According to Bureau of Census data, in 1994, there were approximately 2,817 firms and 3,138 establishments in the main SICs for medical products sterilization (SICs 3841 and 3842);26 2,479, or 88 percent, of these firms employ fewer than 500 employees. With regard to pharmaceutical firms, according to the Census data, there were approximately 592 companies and 707 establishments in SIC 2834 in 1994. Four hundred eighty-five, or 82 percent, of these firms employ fewer than 500 employees (employee size breakdowns to match the SBA size definition of 750 employees were not available from the SBA's data). Only a small subset of medical and pharmaceutical products manufacturers appears to perform EtO sterilization in house, probably because medical and pharmaceutical product manufacturers typically make significant use of contract sterilization services.
According to the findings of a survey conducted by CMR and MDS Nordion in 1996, a total of 87 percent of health care manufacturers were using contract sterilization services for their products.27 In addition, health care manufacturers also have the option to use other sterilization methods such as gamma radiation and e-beam sterilization technologies (see Chapter V for more details).
In 1984, OSHA estimated that there were 125 medical products sterilization firms (including both medical product manufacturers and contract sterilizers of medical products) [Docket H-200, Ex. 164]. In the 1988 STEL rulemaking, OSHA used an estimate from a second Heiden Associates report submitted to OSHA by the Ethylene Oxide Industry Council (EOIC) to revise its estimates of the number of affected workers in medical products sterilization; this estimate of the number of workers was based on an estimate of 95 medical product sterilizing facilities.28
OSHA has concluded that the EPA data presented above in Table II-3 are the best data available for updating the number of affected firms in the medical products and pharmaceutical manufacturing sectors. These data are particularly useful because they permit contract sterilizers to be identified and discussed as a separate industry grouping. As shown in Table II-3, in 1988, approximately 86 medical products manufacturing facilities nationwide used EtO to sterilize medical equipment and other health-related products and 39 pharmaceutical facilities used EtO to sterilize pharmaceutical products. In 1988, EPA also identified 11 medical product manufacturing facilities and 2 pharmaceutical manufacturing facilities as facilities owned by small businesses, based on a sales level small business size criterion of $3.5 million.
In the Regulatory Impact Analysis for the 1988 rulemaking, OSHA estimated that there were approximately 1,814 employees exposed to EtO during medical products sterilization processes [FR Vol. 53, No. 66, 4/6/88, p. 11421]. This estimate was based on an average of 19.1 exposed employees per facility taken from the Heiden Associates report on EtO use in the medical products industry [Docket H-200B, Ex. 205-6]. This estimate remains the best estimate of the average number of workers exposed per medical product sterilization facility. OSHA therefore multiplied the number of medical products manufacturing facilities (86) times 19.1 workers per firm to obtain an updated estimate of 1,643 exposed employees employed by medical products manufacturers. In the absence of more specific data, OSHA is using the same estimate of 19.1 exposed employees per facility to estimate employment in the pharmaceutical
manufacturing industry. To derive the number of employees exposed in this industry, OSHA multiplied the number of pharmaceutical manufacturing facilities (39) times 19.1 workers per firm to yield an adjusted estimate of 745 EtO exposed employees employed by pharmaceutical manufacturers.
Spice Manufacturers
Spice manufacturers use EtO to fumigate spices to prepare them for sale. EtO is used to reduce the microbial load and to kill pathogens on select spices such as black pepper, paprika, and cassia. Each year, approximately 15% to 22% of the total sales volume of spices sold in the U.S. (imported and domestic) is fumigated with around 500,000 pounds of EtO [Docket H-200C, Exs. 2-8; 2-8C]. Fumigation is either conducted in-house with state-of-the-art equipment or by contract fumigators [Docket H-200C, Ex. 2-8].
Spice manufacturers are typically classified in SIC 2099, Food Preparations, Not Elsewhere Classified. This SIC has an SBA size class definition of 500 employees. In the Regulatory Flexibility Analysis for its 1994 NESHAP on commercial sterilizers, the EPA concluded that only a few EtO-using spice manufacturing facilities were small businesses, based on a sales threshold of $3.5 million. As shown in Table II-3, the EPA determined that, in 1989, there were 3 EtO-using spice manufacturing facilities owned by small businesses.
In 1984, OSHA reported that there were approximately 25 spice manufacturers and 150 employees exposed to EtO in this industry [Docket H-200, Ex. 164]. However, the structure of the spice manufacturing industry has changed dramatically since the 1984 rulemaking. The downsizings, consolidations, and increase in foreign ownership that have occurred in the spice manufacturing industry since 1989 have apparently led to a substantial decrease in the number of EtO-using spice manufacturers, although exact statistics are not available [Docket H-200C, Ex. 2-8C].29 None of these changes are related to the use of EtO as a sterilant.
In 1989, the American Spice Trade Association (ASTA), the major trade association for the spice industry, reported that 16 ASTA spice trade companies used EtO in their own facilities [Docket H-200C, Ex. 2-8C]. (It is possible that these companies operated more than one facility, because EPA reported that there were 23 facilities using EtO in the spice manufacturing sector in 1989 (see Table II-3).) In 1989, ASTA also reported that approximately 22 percent of the total U.S. sales volume of spices had been treated with EtO during that year [Docket H-200C, Ex. 2-8C].
In a 1995 survey, ASTA resurveyed the 16 ASTA member companies that had used EtO in 1989 and reported that "many" of them now use outside contractors to treat their spices [Docket H-200C, Ex. 2-8C].30 Though OSHA is not aware of a more recent survey, a more recent article indicates the growth of contract sterilization because of lower costs, Hemmerich, 2004, supra p. II-9. The 1995-1996 ASTA survey results also indicated that approximately 15 percent of the spices handled by the 16 companies surveyed were treated with EtO. (However, since this finding applied to only 70 percent of its members' total sales volume of spices, and because EtO manufacturers have reported a constant level of EtO use by the spice industry, ASTA concluded that this finding did not necessarily suggest a decline in the total volume of spices sterilized with EtO.)
Approximately 94% of EtO-treated spices produced by the 16 ASTA survey respondents were treated by the largest contract sterilizer [Docket H-200C, Ex. 2-8C]. EPA also submitted comments indicating that spices sent to contract sterilizers are usually handled by one of four large contract fumigators [Docket H-200C, Ex. 2-8]. Thus, although the exact numbers are not available, the number of spice manufacturing firms using EtO to sterilize spices in-house is likely to be very small, and thus very few small spice manufacturing firms are potentially affected. Similarly, most of the employees exposed during fumigation of spices with EtO are likely to be employed by contract sterilizers rather than by the spice manufacturers themselves.
Contract Sterilizers/Fumigators
Contract sterilizers sterilize and fumigate products for manufacturing sector clients. These firms may use any of a variety of sterilization technologies, including EtO sterilization, gamma irradiation, e-beam sterilization, and steam sterilization. Some contract sterilizing firms specialize in only one sterilization method, while other firms can provide multiple sterilization methods. Some examples of products commonly processed by contract sterilizers include medical products, pharmaceuticals, food products, spices, cosmetics, seeds, and packaging. According to one estimate, the U.S. market for contract sterilization was approximately $170 million in 1996.31
Contract sterilizers are typically classified in SIC 7389, Business Services, Not Elsewhere Classified. This SIC currently has an SBA size class definition of $5.0 million dollars in annual sales. In the Regulatory Flexibility Analysis for its 1994 NESHAP on commercial sterilizers, the EPA concluded that contract sterilization firms were often small firms. As shown in Table II-3, the EPA estimated that 12 of 16 contract sterilizers identified during the process of developing the Regulatory Flexibility Analysis were small businesses according to EPA's criterion of $3.5 million in sales (one of the original 17 contract sterilizers identified by EPA had discontinued use of EtO by the time the analysis was concluded).
According to the findings of a survey conducted by CMR and MDS Nordion in 1996, a total of 87 percent of health care manufacturers are currently using contract sterilization services for their products.32 In addition, as described above, many of the spice manufacturers who sell EtO-treated spices now utilize contract sterilization services. Information gathered during this Lookback review suggests that medical device and spice manufacturers are increasingly turning to the use of contract sterilizers and fumigators to concentrate on their core businesses. For example, the CMR and MDS Nordion survey indicated that manufacturers of single-use medical devices had increased the volume of devices sterilized by contract sterilizers from 50% to 55% over a two-year period between 1994 and 1996.
Most of the publicly available information concerning the contract sterilization industry focuses on the sterilization of medical products, since sterilization of these products represents a significant portion of most contract sterilizers' businesses. (To take just one example, in 1997, one large contract sterilizing company that performs both gamma radiation and EtO sterilization reported that 80 percent of its business involves medical device sterilization.)33 According to an article in Plastics News, approximately 50 to 55 percent of the contract sterilization of medical devices utilizes EtO, 40 to 45 percent utilizes gamma radiation, and approximately 5 percent utilizes electron-beam sterilization techniques.34
According to an article published in the Biomedical Market Newsletter, there were 152 FDA-registered establishments engaged in contract sterilization of medical devices nationwide in 1996.35 This number of establishments includes all contract sterilizers of medical devices, regardless of the type of sterilization process performed, and may also include contract manufacturers who both manufacture and sterilize medical devices under contract to other firms (any such contract manufacturers using EtO would also have been included above under medical products manufacturers). The number of contract sterilizing facilities using EtO is likely to be much smaller than the number of FDA-registered contract sterilizing establishments, although precise statistics are not available.
As shown above in Table II-3, EPA estimated that there were 17 contract sterilizer facilities where EtO was being used. One facility discontinued using EtO during EPA's rulemaking process, leaving 16 EtO-using contract sterilization facilities. OSHA believes that these are the best available data on the number of EtO-using contract sterilization facilities and that it is a reasonable estimate of the number of affected facilities, since OSHA's review indicates that EtO use in the contract sterilization industry is highly concentrated. (As described above, most EtO-treated spices are handled by just four contract sterilization firms [Docket H-200C, Ex. 2-8].)
Information on contract sterilizing firms suggests that the number of potentially exposed workers per facility is likely to be small. One large contract sterilizing firm that offers both gamma radiation and EtO sterilization employs 500 people and operates 11 facilities (45 employees per facility).36 Another contract sterilizer that specializes in EtO sterilization and identifies itself as one of the largest sterilization contractors in the U.S. employs 150 people and operates 6 facilities (25 employees per facility).37
In 1984 and 1988, the estimated number of employees of contract sterilization firms engaged in medical products sterilization was included in the total medical products sterilization estimates [Docket H-200, Ex. 164]. In the Regulatory Impact Analysis for the 1988 rulemaking, OSHA used an estimated average of 19.1 exposed employees per facility (taken from the Heiden Associates report on EtO use in the medical products industry) to estimate the number of workers affected at firms engaged in medical products sterilization [Docket H-200B, Ex. 205-6]. This estimate remains the best estimate of the average number of workers exposed at contract sterilization facilities. OSHA therefore multiplied the number of contract sterilization facilities (16) times 19.1 workers per firm to obtain an updated estimate of 306 EtO exposed employees employed by contract sterilizing firms. However, a recent
article indicates that there is some growth in the contract sterilization industry because of its lower costs, Hemmerich, 2004, supra. P. II-9.
Other Sterilization and Fumigation Applications
EPA research conducted after the promulgation of EPA's 1994 EtO air emissions standard indicates that museums and libraries have largely discontinued EtO use [Docket H-200C, Ex. 2-8]. EPA found that routine use of EtO to treat museum specimens was discontinued because standard aeration times of EtO-treated specimens could not be established, making it impossible to determine when the articles could be safely handled. Library items may still be sent to contract sterilizer facilities for EtO fumigation as a last resort to treat major outbreaks of mold [Ex. 2-8]. OSHA therefore assumes that there are no longer any entities using in-house EtO sterilizers in these industry sectors.
The use of EtO as a sterilant and fumigant in laboratory and beekeeping applications appears to be very minor. EPA reported in 1988 that 10 laboratory firms used EtO at that time. Four of these firms were commercial laboratory rat and mice breeders who used EtO to sterilize plastics, equipment, and articles used to operate the animal breeding facilities. The remaining facilities appeared to be specialized research laboratories. The fumigation of beekeeping equipment is also a minor use of EtO. Only two State Departments of Agriculture currently hold the EPA registrations required to perform fumigation of beekeeping equipment [Docket H-200C, Ex. 2-8].
Summary
The industry definitions used in the data sources relied on to make estimates of the number of workers exposed to EtO at the time of the 1984-1988 rulemakings and in the sources used to estimate the potentially exposed workforce for this Lookback review have some differences. For example, OSHA apparently included pharmaceutical product manufacturers in the medical products manufacturing group in 1984, and current data separate these two groups. Although these differences make comparisons somewhat difficult, OSHA believes that the number of potentially exposed workers has declined from about 66,000 in the 1984-1988 period to about 46,000 in the late '90s, a drop of approximately 30 percent. This change does not indicate any loss of employment from the EtO standard. Some employers who were not experts or larger users of EtO sterilization switched to contract sterilizers and others may
have switched to alternatives. The affected industries continue to grow. Few employees outside of contract sterilizers, spent the majority of their time sterilizing.
EMPLOYER COMPLIANCE EXPERIENCE WITH THE STANDARD
As part of this Lookback review, OSHA analyzed the compliance experience of affected firms to aid in determining whether the standard was imposing a significant impact on them. During the review, special attention was given to information concerning the compliance experience of small businesses and other entities. OSHA evaluated three major sources of information on the compliance experience of affected firms in addition to the public comments submitted to the docket for this review [Docket H-200C]. These sources were: (1) an analysis of OSHA enforcement and consultation program data, (2) input provided by OSHA enforcement and State-plan State officials, and (3) a review of the occupational safety and health literature relating to the EtO standard.
Analysis of Enforcement and Consultation Program Data
Enforcement Program Data
Since the effective date of the EtO standard in February 1985, OSHA has conducted several hundred inspections during which worker exposures to ethylene oxide were monitored. During this period, three OSHA regions (Regions II, III, and VIII) also conducted local emphasis programs targeting EtO hazards. In recent years, OSHA enforcement staff has also responded to reports of EtO releases in hospitals as well as to reports of fires and explosions occurring at EtO-using facilities in other industries.
The EtO standard is not frequently cited. For this review, OSHA analyzed the patterns in recent enforcement data in two ways. The first analysis evaluated data on the industries inspected and the numbers of inspections where §1910.1047 was cited in FY 1996, 1997 and 2000. The second analysis consisted of an analysis of patterns found in the distribution of citation data by size class of the firms cited for the period in which the EtO standard has been in effect.
Analysis of Industries Inspected
OSHA analyzed enforcement data to determine whether facilities in covered sectors continued to be cited for non-compliance and to examine patterns in OSHA enforcement activity by the size class of firms inspected. In Fiscal Year 1996, across all size classes of business, there were 12 federal inspections in which §1910.1047 was cited. Nine of the inspections occurred at firms employing more than 250 employees, and three occurred at firms employing between 20 and 99 employees. Eight inspections occurred in SIC 8062, General Medical and Surgical Hospitals. There was one inspection in each of the following four SICs: (1) SIC 2821, Plastics Materials, (2) SIC 3841, Surgical and Medical Instruments and Apparatus, (3) SIC 0742, Veterinary Services, and (4) SIC 8011, Doctors' Offices.
In Fiscal Year 1997, across all firm size classes, there were 13 federal inspections where §1910.1047 was cited. Eight of these inspections occurred at firms employing more than 250 employees. One inspection occurred at a firm employing between 100 and 250 employees, 1 at a firm with between 20 and 99 employees, and 3 at firms with 1 to 19 employees. The data on the industries inspected indicated that there were 3 inspections in SIC 8069, Specialty Hospitals, and 2 in SIC 8062, General Medical and Surgical Hospitals. In addition, there was one inspection in each of the following eight SICs: (1) SIC 2865, Cyclic Organic Crudes and Intermediates, (2) SIC 2869, Industrial Organic Chemicals, Not Elsewhere Classified, (3) SIC 3599, Industrial and Commercial Machinery and Equipment, Not Elsewhere Classified, (4) SIC 3841, Surgical and Medical Instruments and Apparatus, (5) SIC 3842, Orthopedic,
Prosthetic, and Surgical Appliances and Supplies, (6) SIC 8099, Health and Allied Services, Not Elsewhere Classified, (7) SIC 8711, Engineering and Architectural Services, and (8) SIC 8733, Noncommercial Research Organizations. An update of the citation history for EtO in FY 2000 shows that the EtO standard was cited 10 times during Federal inspections of facilities in SIC 80 in that year.
Because of the small size of this data set, only limited conclusions can be drawn. In these three fiscal years, the industries in which the EtO standard has been cited are largely the same industries that OSHA identified as affected during the rulemaking process: the chemical manufacturing industries and the industries where EtO is used as a sterilant or fumigant (e.g., the hospital sector and the medical product manufacturing industries). In addition, the firms that received citations during the last few years were mainly large firms. In SIC 80, which includes hospitals and other health service providers and is the industry with the largest number of EtO-exposed employees, Federal OSHA cited this standard between 6 and 10 times a year in 1996, 1997, and 2000.
Analysis of Violations Cited
OSHA also conducted an analysis of Federal OSHA citation data from 1985 through 1997 in order to determine which provisions of the standard were most frequently violated and to evaluate the most common compliance problems experienced by firms of different sizes. Table II-4 presents citation data for the top five most frequently cited provisions of the standard by firm size class. Across all three size classes, the most commonly cited provisions were §1910.1047 (h)(1) and (j)(3). Paragraph (h)(1) requires a written emergency plan, and paragraph (j)(3) requires employee training on EtO hazards. As shown in Table II-4, the majority of the citations issued (74%) were issued to large employers--employers with more than 500 employees.
Table II-4
Top Five Most Frequently Cited EtO Standard Provisions,
By Size-Class of Firm Inspected, Using Citation Data from 1985-1997 |
| Rank |
Top Five Provisions Cited (and Number of Citations), by Firm Size* |
| Firms With 1-99 Employees |
Firms With 100-500 Employees |
Firms With 500+ Employees |
| 1. |
|
|
|
| 2. |
|
|
|
| 3. |
|
|
|
| 4. |
|
(d)(2), (f)(1),
(g)(3), (h)(2) |
(17) |
|
|
| 5 |
|
---------------------- |
|
(c)(1) Permissible Exposure Limit--8 hour TWA PEL
(d)(1) Exposure Monitoring--general exposure monitoring requirement
(d)(2) Exposure Monitoring--representative sampling requirement
(f)(1) Methods of Compliance--engineering control and work practices requirement
(g)(1) Respiratory Protection--general respirator requirement
(h)(1) Emergency Situations--general written plan requirement
(h)(2) Emergency Situations--means of alerting employees requirement
(j)(1) Communication of EtO Hazards--general signs and labels requirement
(j)(3) Communication of EtO Hazards--training requirement
(k)(2) Recordkeeping--exposure monitoring recordkeeping requirement |
Sources: OSHA IMIS Enforcement Database; 29 CFR 1910.1047
*The total number of citations issued, by firm size class, from 1985 to 1997 were: 332 (firms with 1-99 employees), 270 (firms with 100-499 employees), and 1,677 (firms with 500 or more employees), for a total of 2,279 citations.
Consultation Program Data
Since 1984, OSHA consultation program staffs have conducted several hundred consultation visits that involved assessments of ethylene oxide exposure levels, with a large percentage of these visits occurring in 1984 and 1985, immediately following the promulgation of the standard. Most of these 1984-85 visits were in the hospital sectors in SIC 80. The remaining visits occurred in other health care settings such as medical doctors' offices and at medical product and device manufacturing establishments. The exposure data collected during consultation visits, like the exposure data collected during compliance visits, indicate that occupational EtO exposures were typically quite low following the promulgation of the standard (see Chapter III).
Review of the Literature on Employer Compliance Experience
As part of this Lookback review of the EtO standard, OSHA reviewed the occupational safety and health literature as well as other relevant studies to find and evaluate information on the compliance experience of affected businesses, entities, and industries. During this review, OSHA did not find any evidence of recent research on the compliance experience of employers in the chemical manufacturing industries (EtO producers and ethoxylators). The limited information in the existing literature suggests that the chemical manufacturing firms affected by the standard have continued to use conventional engineering controls and work practices to comply with the standard [Docket H-200C, Exs. 2-6G; 2-9J]. Similarly, most of the articles concerning EtO use as a sterilant or fumigant addressed the potential for EtO use reduction or substitution rather than employers' compliance experience. All
of the directly relevant literature identified by OSHA during this Lookback review addressed compliance issues in the hospital sector. This is consistent with OSHA's conclusion that the hospital sector continues to be the industry most affected by the standard.
Many of the works related to hospital sector compliance that were identified by OSHA were journal articles authored by Dr. Anthony LaMontagne of the New England Research Institutes and his various co-authors (as cited below). These journal articles presented information on aspects of hospital sector compliance drawn from the findings of a 1993 study known as the Massachusetts Hospital EtO Health and Safety Study. A summary of the findings of this comprehensive study is provided below. In addition to these articles, OSHA also reviewed the findings of two additional studies. The first study, "Ethylene Oxide: A Case Study in Hazard Identification, OSHA Regulation, and Market Response," was prepared by Meridian Research, Inc., under contract to OSHA in 1992. The second study, "Gauging Control Technology and Regulatory Impacts in Occupational Safety and Health--An Appraisal
of OSHA's Analytic Approach," was prepared by the Congressional Office of Technology Assessment in 1995. Brief discussions of these studies are provided below.
The Massachusetts Hospital EtO Health and Safety Study
The Massachusetts Hospital EtO Health and Safety Study evaluated the compliance patterns of nearly all (96 percent) of the EtO-using hospitals in the Commonwealth of Massachusetts during the period 1985 to 1992.38 Although the study results indicated widespread awareness and implementation of the EtO standard in the hospital sector and demonstrated that the standard has been successful in reducing worker exposures, some of the study's detailed findings suggested that there were portions of the standard with which there has been delayed or incomplete compliance over the period from 1985 to 1992. The findings also indicated that sterilizing department managers experience some difficulty understanding the regulatory intent of portions of the exposure monitoring, emergency alert, and medical surveillance provisions. The study also concluded that OSHA
enforcement efforts in Massachusetts had led to improved compliance with the EtO standard.
Exposure Monitoring Findings from the Massachusetts Hospital Study
The Massachusetts Hospital EtO Health and Safety Study found that, by 1993, almost all EtO-using hospitals in Massachusetts had conducted personal monitoring to characterize worker exposure and to monitor compliance with the EtO standard's permissible exposure limits. By 1993, 95% of hospitals (87 of 92) had performed monitoring for the action level and 87% (80 of 92) had performed monitoring for the excursion limit.39 However, the study findings indicate that most hospitals did not comply with this requirement until after the 1985 personal exposure monitoring implementation deadline and that some hospitals had not yet complied with the initial monitoring requirements as of 1993.40 The authors concluded that OSHA enforcement activities had stimulated compliance with the exposure monitoring requirements in
Massachusetts.
Most hospitals that monitored did so more frequently than the standard would have required them to do, and they also tended to monitor periodically regardless of the results of their exposure monitoring findings. Most occupational exposure measurements collected by the hospitals that provided their exposure monitoring results to Dr. La Montagne were quite low. Of the exposure monitoring data collected by surveyed hospitals during 1990 to 1992, only 0.4% of samples exceeded the action level (47/10,981) and only 0.7% exceeded the excursion limit (64/8,916). This finding indicates that occupational exposures are generally very low in hospitals and that employers are able to comply with the PELs. However, the study also reported that exposures above the action level or excursion limit were measured at a number of hospitals, and that such exposures continued to occur during 1990 to 1992 (the
most recent period evaluated).41
Emergency Situation Findings from the Massachusetts Hospital Study
The Massachusetts Hospital Study found significant compliance with the emergency situation requirements of the standard. Ninety-four percent of EtO-using hospitals (85 of 90) had implemented a written emergency response plan by 1993.42 Furthermore, by 1993, 66% of EtO-using hospitals (59 of 90) in Massachusetts had elected to install EtO alarms to comply with the emergency alert requirement.43,44 (EtO alarms are not required by the standard, although they are a common means of compliance with the emergency alert requirement.) Here again, the Hospital Study findings show that the installation of alarms occurred later than the 1985 compliance deadline for the EtO standard and peaked following a series of OSHA inspections and citations in Massachusetts hospitals.45
Despite a high degree of compliance with the PELs and the EtO exposure monitoring and emergency situation requirements, a number of Massachusetts hospitals experienced accidental releases during the period studied. Approximately two-thirds of the responding hospitals (58 of 90) reported evacuations of the sterilization department or area in response to a real or suspected EtO leak or an EtO alarm at some time during 1985 to 1993. Fifty percent of these hospitals (46 of 92) reported accidental releases during the period 1985 to 1993 and 33% of hospitals (32 of 90) reported accidental releases during the period 1990 to 1992.46
Medical Surveillance Findings from the Massachusetts Hospital Study
Hospital sector compliance with the medical surveillance requirements of the EtO standard was extensively studied during the Massachusetts Hospital EtO Health and Safety Study. The Hospital Study results indicated that medical surveillance had been provided one or more times at 62% (57 of 92) of EtO-using hospitals between 1985 and 1993. During the period from 1990 to 1992, 53% (48 of 90) of EtO-using hospitals reported providing surveillance. The study investigators also found that 11% of the hospitals (10 of 92) failed to provide medical surveillance in situations where the requirement should have been triggered.47 Medical surveillance was most commonly provided on a single occasion (typically in response to a potential overexposure occurring during an accidental release of EtO).
The Hospital Study found that many EtO-using hospitals offered routine, periodic EtO surveillance. In general, however, the provision of medical surveillance was most strongly related to the occurrence of accidental exposure situations. Eighty-nine percent of the 52 hospitals reporting worker exposure to accidental EtO releases also reported providing medical surveillance.48 In addition, the medical surveillance content of EtO health and safety training was strongly related to the provision of medical surveillance. However, the action level was found not to be an important trigger of medical surveillance.49 Of the nine hospitals (9 of 82) where the 30-day action level trigger was exceeded on at least one occasion, only five hospitals provided EtO medical surveillance. Other OSHA medical surveillance triggers
(pre-employment and termination examinations, EtO-related symptoms, and reproductive concerns) were not found to be significant determinants associated with the provision of EtO medical surveillance.50
The study investigators concluded that there was low awareness on the part of hospitals about the standard's requirement to provide medical surveillance once the action level has been exceeded for 30 or more working days.51 The study investigators also documented that approximately half of the 92 sterilization department managers interviewed during the study cited difficulty in understanding OSHA EtO medical surveillance requirements as a moderate to severe barrier to providing EtO medical surveillance.
A survey of EtO medical surveillance providers was also undertaken as part of the Massachusetts Hospital EtO Health and Safety Study. This portion of the study found that nearly all of the 37 health care providers who responded to the survey provided a medical history, physical exam, and complete blood count test as part of EtO medical surveillance (as required by the EtO standard). However, the study also found that only 78% of providers (29 of 37) reported collecting the required occupational histories and only 76% (28 of 37) performed leukocyte differentials as required.52 (These failures to perform procedures required by the standard occurred even though many of the medical surveillance providers (20 of 34) reported that they used OSHA outreach materials as an information source for guidance in providing EtO medical surveillance.) A number
of the surveyed providers (10 of 37) reported observing symptoms that were potentially related to EtO and providing medical surveillance to these workers. Most of the reports of symptoms were related to accidental EtO overexposures. Dr. LaMontagne extrapolated from these data to estimate that one or more workers at 19% of EtO using Massachusetts hospitals had experienced acute EtO-related health effects over the time period evaluated by the Massachusetts Hospital Study.
Training Findings from the Massachusetts Hospital Study
Ninety-eight percent (90 of 92) of the EtO-using hospitals in Massachusetts had provided EtO training to sterilization department workers by 1993 (regardless of whether measured occupational exposure levels would actually have required such training) [Ex. 2-9K]. The Hospital Study also found that sterilization workers were typically offered an average of 1.4 hours of EtO health and safety training per year and that there was generally good coverage of the various training topics required by the standard [Ex. 2-9K].
However, hospitals were slow in implementing the standard's training requirement. Only 58% had provided training by the end of 1985, when the requirement became effective. Another potential problem that the Hospital Study identified in these training efforts was the failure to provide training to maintenance workers exposed to EtO in the course of their work (only 54% of hospitals provided training to such workers). (OSHA notes that the standard requires the training of workers (including maintenance workers) only if they are potentially exposed to EtO at or above the EL or excursion limit.) The Hospital Study also found that many training programs did not provide complete coverage of the required medical surveillance topics during training.
The Meridian Research Report
In 1992, OSHA commissioned Meridian Research, Inc. ("Meridian") to study the effectiveness of the EtO standard. In the resulting report, "Ethylene Oxide: A Case Study in Hazard Identification, OSHA Regulation, and Market Response," Meridian used a qualitative case-study approach to evaluate how hospital sector employers responded to the technological challenges presented by the 1984 EtO standard.53 This report examined exposures to EtO in the hospital sector both before and after the promulgation of the EtO standard, identified the measures implemented by hospitals to reduce employee exposures, and assessed the responses of equipment manufacturers in designing retrofit controls and new equipment to comply with the standard. As part of this study, Meridian evaluated a number of studies and other data sources (e.g., OSHA's IMIS
database) that contained historical EtO exposure data and grouped the largest exposure data sets by period, concluding that there had been substantial decreases in worker exposure between the period before the promulgation of the standard (1979 to 1984) and the period following the standard (1985 to 1992). Meridian concluded, based on the available exposure data up through 1992, that in the post-1984 period, "approximately 90 percent of hospitals achieved TWAs below 1 ppm, the majority had TWAs below 0.5 ppm, and a substantial number of hospitals were achieving TWAs of 0.1 ppm."
In its report, Meridian also concluded that, within seven years after promulgation of the 1984 standard, equipment manufacturers had made significant improvements in sterilizing equipment technologies. The improved sterilizers that these manufacturers developed included built-in exposure control features (e.g., built-in aerators), unlike earlier models, which required the purchase of add-on controls to achieve compliance with the EtO standard. These improved sterilizers were also less costly than the earlier models. In 1992, sterilizers featuring built-in exposure controls were being sold at prices that were approximately half the price of older models of equipment featuring add-on engineering controls.
The Office of Technology Assessment Report
In 1995, the Congressional Office of Technology Assessment (OTA) issued a report, "Gauging Control Technology and Regulatory Impacts in Occupational Safety and Health--An Appraisal of OSHA's Analytic Approach," that included a review of the findings of OSHA's 1984 Regulatory Impact Analysis for the EtO standard.54 OTA's review was limited to evaluating the conclusions made by OSHA with regard to the feasibility of implementing the standard in the hospital sector. In its report, OTA concluded that "OSHA's analyses for this rulemaking correctly gauged the feasibility of the tightened PEL and other compliance requirements and correctly anticipated most of the specific characteristics (engineering controls, work practice changes, and their unit costs) of the control measures implemented." OTA also indicated that "Within a year
and a half after promulgation, the vast majority of hospitals were operating with ethylene oxide (EtO) exposure levels in compliance with the new PEL." However, although OTA concluded that OSHA had accurately estimated the unit costs for the control technologies that OSHA assumed the hospital sector would use, OTA found that "the sector's actual overall spending appears to have at least modestly exceeded the agency's estimate." OTA concluded that these increased costs were the result both of additional spending on modifications to existing ventilation systems not anticipated by OSHA in the 1984 Regulatory Impact Analysis and of actions taken by many hospitals to reduce exposures to a level substantially below the OSHA PEL, even though the standard did not require them to do so.
CONCLUSIONS
Data and information compiled and analyzed for this Lookback review indicate that ethylene oxide production and use has increased by about 25 percent since 1984, when the final EtO standard was issued. The number of workers estimated to be potentially exposed to EtO, however, has declined since then, falling from approximately 66,000 in 1984-1988 to about 46,000 in the mid-1990's. Most of this decline has occurred in the hospital sector, although this sector continues to have the largest EtO-exposed population (about 39,000 workers). The hospital sector also accounts for almost all of the small facilities (using the SBA's size criteria) affected by the standard. The factors principally responsible for this decline in the number of potentially exposed workers include mergers, consolidations, and firm closures in many sectors (e.g., hospitals, pharmaceuticals and chemicals), unrelated
to the EtO standard, the switch to contract sterilizers rather than hospitals having some staff operating sterilizer for a small part of the day, and shifts to other sterilizing technologies (see chapter V for additional details).
Studies of the EtO standard conducted for OSHA, published in the scientific literature, and performed for the Congressional Office of Technology Assessment indicate that the standard has been successful in reducing the exposures of workers using existing control methods. In addition, OSHA's compliance experience indicates that few employers have been cited for exceeding the standard's PELs and that the technology to achieve these limits is effective and widely used. These compliance program findings provide additional evidence that the standard is technologically and economically feasible for most employers. Although some OSHA Federal and State-plan compliance personnel indicated that some small employers experience difficulty in complying with the standard's exposure monitoring and emergency alert requirements, they felt that additional compliance assistance materials, rather than revisions
to the standard, would be helpful for smaller employers. (See chapter VII, Conclusions, for OSHA's responses to the comments received during this Lookback review.)
CHAPTER III
CONTINUED NEED FOR AND EFFECTIVENESS OF THE RULE |
OVERVIEW
Section 610 of the Regulatory Flexibility Act requires that OSHA consider whether there is a continued need for the Ethylene Oxide standard, and Executive Order 12866 requires OSHA to determine whether, to reduce regulatory burden, the standard should be modified or eliminated. During the original rulemaking, OSHA determined that the EtO standard was needed to prevent the occurrence of adverse health effects among employees exposed to ethylene oxide and that the standard would be effective in achieving that goal. Information gathered by OSHA and comments submitted to Docket H-200C during the regulatory review process support both of these earlier determinations.
The evidence available to OSHA can be grouped into four main issues demonstrating the continued need for, and effectiveness of, the standard: (1) OSHA's original conclusions concerning the health risks of exposure to EtO have been supported by the results of new animal and epidemiological studies released since the promulgation of the standard in 1984; (2) exposure monitoring data from several sources indicate that occupational exposure to EtO has fallen markedly since the EtO standard went into effect; (3) OSHA compliance program data indicate that overexposures and accidental releases of EtO continue to occur at workplaces that are not in compliance with the standard; and (4) both employer and employee representatives indicate a continued need for the standard. OSHA has therefore concluded that the EtO standard is needed, has been effective in achieving employee protection, and should
not be rescinded or revised at this time.
INFORMATION ON ADVERSE HEALTH EFFECTS AND QUANTITATIVE RISK ASSESSMENT
This section examines evidence pertaining to the adverse health effects (health hazards) and quantitative risk assessment portions of the preamble to the 1984 OSHA EtO standard and assesses whether sufficient new health-related evidence has since become available to change OSHA's conclusions about the risks associated with occupational exposure to EtO. Accordingly, the following analyses review the methodology and conclusions presented in 1984 and assess the more recent evidence on the adverse health effects from exposure to EtO.
The 1984 OSHA quantitative risk assessment procedures relied on carcinogenicity results from EtO rodent bioassays to estimate human cancer risks, since, at that time, OSHA concluded that the rodent data were the best available for risk assessment purposes. However, in the intervening years more than a half-dozen new cohort studies on occupational exposure to EtO and cancer have been published, and these human studies further support the need for the Standard. The section below provides a brief summary of the several new studies in workers.
OSHA's 1984 Risk Assessment Findings
Health Hazard Assessment
In 1984, OSHA concluded that "EtO can cause several serious adverse health effects" (49 FR 25737). These included the "suggest(ion) that EtO may cause cancer of the blood (leukemia), as well as other organs in humans. In addition, EtO exposure causes mutations, increases the rate of chromosomal aberration and sister chromatid exchange, and causes other undesirable changes in the DNA of mammalian cells....EtO exposure has also been associated with an increased risk of spontaneous abortion among pregnant women and is capable of causing other adverse reproductive effects in both men and women. Exposure to high concentrations of EtO causes central nervous system depression and other neurological effects, which are thought to be reversible with cessation of exposure. In addition, exposure to EtO gas causes sensitization and irritation of human tissues, including the eyes and
respiratory tract." (49 FR 25738).
Carcinogenicity was the health effect most emphasized in the 1984 preamble, and OSHA's conclusion that EtO is a potential occupational carcinogen was based mostly on findings from long-term animal studies in different species administered EtO by different routes of exposure. Although cancers had been induced at site of first contact in oral (gavage - rat) and subcutaneous injection (mice) studies, OSHA's strongest evidence for designating EtO a potential occupational carcinogen came from positive findings of tumors in two long-term inhalation studies in rats. These studies were the Bushy Run Research Center (BRRC- Snellings, et al. 1984) study and the National Institute for Occupational Safety and Health (NIOSH - Lynch et al. 1984) study. Both studies found that EtO exposure increased tumor incidence at multiple sites; the BRRC study found increases in mononuclear cell leukemias (MCL)
in female F344 rats, peritoneal mesotheliomas (PM) in male F344 rats, and brain gliomas in both sexes. The NIOSH rat inhalation study, using male F344 rats only, also found increased incidences of gliomas and PM; in addition it found increased risks of MCL. Although MCL is a common old-age tumor in this rat, gliomas are rare and PM are uncommon.
In addition to the evidence in animals, the evidence in human studies available in 1984 was based on three smaller epidemiologic studies, two (both by the same authors, Hogstedt et al. 1979a,b) of which were positive for increased leukemia mortality. In 1984, OSHA concluded, "Although OSHA believes that none of the available epidemiologic studies are in and of themselves definitive evidence of EtO's carcinogenicity, the Agency agrees that the two Hogstedt studies 'provide evidence of a possible association between occupational exposure to ethylene oxide and death from leukemia'."(49 FR 25740)
Quantitative Cancer Risk Assessment - Choice of Study, Tumor Site, Dose Metric and Dose-Response Model
In 1984, OSHA conducted a quantitative risk assessment only for carcinogenicity. The Agency's carcinogenicity risk estimates relied on data from the BRRC, using PM in male rats as the appropriate site-sex-species for animal-to-human extrapolation for risk modeling. Several reasons given for this choice related to a) the quality of the study, b) the statistical significance observed in the increase in neoplasms, c) the availability of concurrent control information, d) the fact that dose-response relationships were observed in both sexes, e) the specifics of the dosing regimen were well-documented, f) the availability of information on individual rats, and g) the fact that the study used an exposure route that was relevant to anticipated worker exposure (49 FR 25756).
OSHA concluded in 1984 that the human data were inadequate for quantitative risk assessment due to limitations in terms both of limited study size and exposure uncertainties (49 FR 25756), and that use of the MCL data in the rats was not the best choice of tumor site for animal to human extrapolation because of the high background rate of MCL in the F344 rat (49 FR 25756). Nevertheless, OSHA conducted comparison model fits and calculations for both MCL and PM.
The dose-metric used by OSHA both for animal dose-response and for the animal-to-human equivalence was mg/kg-day intake dose, where the ppm administered concentrations in the BRRC rat study were converted to mg/kg-day based on animal breathing volumes and time of animal exposure. The rat dose-response curve was fit using the rat's calculated intake doses based on the study's exposure conditions, which were 10 ppm, 30 ppm, or 100 ppm for 6-hours per day, 5 days per week over the 2-year study length (assumed to represent the rat's full life span). The lifetime risk to workers was then estimated by first calculating the human occupational intake dose on a mg/kg b.w.-day basis over an 8-hr. day, using human breathing volumes. Total intake dose was based on a 45-year occupational exposure for 46 weeks per year, and adjusted to the rat lifetime daily equivalent intake dose units. Human risk
was then estimated by reading the corresponding risk from the dose-response curve in rats (49 FR 25760).
The dose-response model used to fit the BRRC data in 1984 is a version of a polynomial with parameters restricted to be non-negative; OSHA termed this the multistage model, which for these data is linear at low doses. This model provided an adequate fit to the data. OSHA used the maximum likelihood estimates of the parameters to generate its final estimates, and also provided 95% upper-limit estimates for the animal dose-response curve, which, when adjusted for human exposure conditions, were designated 95% upper-limit risk estimates for humans.
OSHA fit its multistage model to both the PM in the male rats and the MCL in the female rats. After presenting alternative estimates from other models, including a multistage-Weibull time-to-tumor model, OSHA reaffirmed the risk estimates it had presented at the time of the proposal (48 FR 17284): "OSHA predicted an excess lifetime risk for cancer from (45 year occupational) exposure to EtO at 50 ppm to be 634 to 1093 per 10,000 workers, with 95% upper confidence limits on the excess risk of 1,008 to 1,524 deaths per 10,000." (49 FR 25757). At a 1 ppm 8-hr. TWA exposure level, the estimated excess lifetime risks were "approximately 12 to 23 excess cancer deaths per 10,000 with 95% upper confidence limits of 21 to 33 excess deaths per 10,000." (49 FR 25757). OSHA chose 1 ppm as the final rule's PEL.
In 1988 OSHA amended the 1984 Final Standard to adopt an Excursion Limit (EL) of 5 ppm, averaged over a sampling period of 15 minutes (53 FR 11414-11438). The EL was not based on new health information or on information about dose-rate effects, but on OSHA's determination that such a limit was feasible and would further reduce risk under some exposure circumstances.
NEW HEALTH INFORMATION AVAILABLE SINCE THE 1984 STANDARD
Since 1984, much new information on the health effects of EtO has become available. However, the most relevant information for evaluating the appropriateness of OSHA's PELs for EtO relates to EtO's carcinogenicity. This section discusses some of that new information. For hazard identification, three areas are discussed: 1) new animal inhalation studies, 2) new human studies, 3) classification of EtO as a known human carcinogen by the International Agency for Research on Cancer (IARC, 1994), and 4) the National Toxicology Program's (NTP's) decision, in its 9th report on carcinogens, to classify EtO as a substance "known to be a human carcinogen." For quantitative risk assessment three areas are discussed: 1) choice of most appropriate animal data set, including proper animal dose-metric and proper animal-to-human species conversion, 2) choice of best human study, and 3) EPA's
comments recommending that OSHA lower its PEL to 0.1 ppm. For each of these areas, pertinent comments in the Docket are also discussed.
Hazard Identification
In cancer hazard identification, three areas are considered: 1) new animal studies, 2) new human studies, and 3) the IARC classification. Other new information is also briefly summarized.
New Animal Studies
The major finding in the animal studies area since 1984 is the finding that EtO causes cancers at multiple sites in a second species (mice) in inhalation studies. The NTP (1987) inhalation study exposed groups of 50 male and 50 female BC63F1 mice to EtO levels of 0, 50 or 100 ppm EtO for 6 hrs/day, 5 days per week for 2 years. Findings of significantly increased alveolar/bronchiolar carcinomas both in male (6/50 control, 10/50 low dose, and 16/50 high dose, p<.05 trend test), and female (0/49 control, 1/48 low dose, and 7/49 high dose, p<.01) mice, plus malignant lymphomas (9/49 control, 6/48 low-dose, and 22/49 high dose, p<.05 trend test), uterine adenocarcinomas (0/47 control, 1/47 low dose, and 5/49 high dose, p<.05 trend test), and mammary gland tumors (1/49 control, 8/48 low-dose, p<.05 pairwise test) in female mice significantly add to the body of evidence that
EtO is likely to be a human carcinogen.
A second study, reported by IARC (1994), found increased multiplicity and incidence of lung tumors in a screening assay of groups of 30 female strain A/J mice exposed to 0, 70, or 200 ppm EtO for up to 6 months.
New Human Studies
Although there were few human studies on the carcinogenicity of EtO available in 1984, there are now cancer mortality or incidence data on more than 33,000 workers involving more than 800 cancer deaths from 12 EtO production or use cohorts in 5 countries (Shore et al. (1993), Teta (1997)). The larger studies are summarized below; for an in-depth description and summary of these as well as additional studies, see IARC (1994) and Shore et al. (1993).
The largest of the study groups is the NIOSH cohort of 18,254 U.S. sterilant workers in 14 plants with 343 cancer deaths, an average follow-up of 16 years, and an average exposure duration of 4.8 years (Steenland et al., 1991, Ex. 2-10-R; Stayner et al., 1993, Ex. 2-10-Q). This same cohort, with minor differences in the criteria for study inclusion and with one year of additional follow-up, was also studied by Wong and Trent (1993), with essentially the same results. The findings in both papers were that the cohort had statistically significant lower mortality than the general population from all causes, all cancers, and non-malignant diseases. Among individual cancers, only non-Hodgkin's lymphoma was significantly increased, and only among men. A deficit was found among women. However, Steenland et al. found "positive trends for all cancers and hematopoietic cancer with latency,"
while Wong and Trent did not (Steenland and Stayner, 1993). In the earlier publication, Steenland et al. (1991) found that, while "there was no significant increase in mortality from any cause of death, ... an increase in mortality from all hematopoietic neoplasms (SMR = 1.55, 95% C.I. 1.02-2.26) was observed among males that was concentrated in the subcategories of lymphosarcoma-reticulosarcoma (SMR = 2.60, 95% C.I. 1.05-5.36) and non-Hodgkin's lymphoma (SMR =2.16, 95% C.I. 0.87-4.45)." (Stayner et al., 1993).
The Stayner et al. (1993) analysis discusses trends related both to latency and exposure. The study's detailed assessment of exposure allowed individual average and cumulative exposure to be estimated for each member in this cohort using a job-category/time exposure matrix; the average exposure was 5.5 ppm 8-hr. TWA, with an average cumulative exposure of 26.6 ppm-years. These individual exposure estimates allowed use of a Cox proportional hazards model for the trend analysis, which controlled for calendar year, age at risk, sex, and race. Using this model, the authors found statistically significant cumulative exposure-response trends for all hematopoietic cancer mortality (ICD 200-208) (p<0.05), and for "lymphoid cancer mortality (ICD 200, 202, 204)"(p<0.01). The same analysis also found non-statistically significant increases for both leukemia (p=0.15) and NHL (p<0.1).
No other exposure metrics showed significant results. The authors concluded that their findings "provide some support for the hypothesis that cumulative exposure to EtO increases the risk of mortality from hematopoietic neoplasms, particularly neoplasms of lymphoid origin."
The NIOSH study was subsequently updated by Steenland et al. (2004) to extend the mortality follow-up from 1987 to 1998. The analyses of the cohort of 18,235 men and women exposed to EtO focused on haematopoietic and breast cancer mortality. There was little evidence of increased cancer mortality in the EtO-exposed cohort overall compared to the general population. However, there were positive exposure-response trends observed in males for haematopoietic cancer (particularly lymphoid tumors) and in females for breast cancer.
In a separate study, Steenland et al. (2003) examined the relationship between EtO exposure and the incidence of breast cancer among 7,576 women who were employed for at least one year in the NIOSH study cohort. Breast cancer incidence was ascertained using interview, death certificates, cancer registries, and medical records. The standardized incidence ratio (SIR) for incident breast cancer in the whole cohort using external referent rates was 0.87. The authors believed that SIRs were underestimated because the breast cancer incidence in the whole cohort was under-ascertained due to incomplete response and lack of coverage of state cancer registries. In internal nested case-control analyses of those with interviews, controlling for reproductive risk factors, a positive exposure-response was found with the log of the cumulative exposure with a 15-year lag.
One other cohort, studied by Hagmar et al., also contained individual cumulative EtO exposure estimates and dose-response analyses, but both the size of the cohort and the level of exposure are much smaller. Hagmar et al. (1991, 1995) studied cancer incidence (40 cases) in 2,170 Swedish workers in two plants that produce disposable medical equipment sterilized with EtO. The median duration of EtO exposure was 5.6 years, but the median cumulative exposure was low, only 0.13 ppm-years, and the median follow-up time of 11.8 years was short. Nevertheless, the authors found a non-statistically significant increase in leukemia, both with cumulative exposure and with time since start of exposure to EtO. Among the 930 subjects with at least 0.14 ppm-years exposure to EtO, a standardized incidence ratio (SIR) = 7.14 (95% C.I. 0.87-1.99) which was, however, based only on two cases, was found.
For all lymphohematopoietic cancers the SIR = 1.78 (95% C.I. 0.62-1.19), based on six cases.
A third study, by Teta et al. (1993), involved follow-up of a group initially studied by Greenberg et al., which analyzed the mortality of a cohort of 1,896 Union Carbide workers (UCC, 110 cancer deaths) with average exposure duration of 5.4 years but with an average follow-up observation of 27.2 years. The average exposure levels were estimated to have decreased from 14 ppm 8 hr. TWA, 1940-56; to 5-10 ppm, 1957-1973; to <1 ppm 1974-1988. Peaks of several hundred ppm were hypothesized but not taken into account in the analyses. The results of dose-response analyses were reported only for several cancers among workers in departments using or producing EtO. No dose-response trends were observed for total malignancies, pancreatic, leukemia and aleukemia, and brain/nervous system cancers, based on 110, 4, 8, 5, and 5 deaths, respectively. Although the UCC study found a lower than expected
ratio (SMR=0.59) for total lymphatic and hematopoietic cancer deaths, based on 7 observed deaths, there was an increased ratio (SMR=1.06) for the 5 leukemia or aleukemia deaths, and an SMR>2.5 for the group exposed at least 10 years in departments using or producing EtO. The overall SMR of 0.79 (95% C.I. 0.71-0.87) was significantly below expected death rates.
A fourth study, by Bisanti et al. (1993), involved 1,971 Italian chemical workers but had limited EtO exposure information. This study also found an increased SMR=2.50 (95% C.I. 0.91-5.44; 6 observed deaths) for all hematopoietic cancer deaths, which included a statistically significant SMR=6.82 (95% C.I. 1.86-17.45; 4 observed deaths) for lymphosarcoma/ reticulosarcoma. For a well-defined group of 637 subjects licensed only to handle EtO, they found a SMR of 7.00 (95% C.I. 2.37 -16.37; 5 deaths) for all hematopoietic cancers, which included a SMR of 16.93 (95% C.I. 3.49 - 49.53; 3 deaths) for lymphosarcoma, and a SMR of 6.50 (95% C.I. 0.79 -23.49) for leukemia-aleukemia. Mortality from all causes was decreased both in the total cohort and in the EtO-licensed only cohort, while all cancer mortality was increased by 30-40%.
Two other relatively large studies reported opposing results on hematolymphopoietic cancers. Gardner et al. (1989) reported on 2,876 British EtO-exposed workers from four chemical manufacturing companies (1,471 workers) and 1,405 workers from eight hospital sterilization units. There was little information either on duration of exposure or length of follow-up, although exposures before 1977 were reported probably to be greater than 5 ppm, and less after 1977. The authors' major finding was an overall SMR of 1.89 (95% C.I. 0.76 - 3.90; 7 observed deaths) from leukemia and NHL. The excess was slightly greater among the chemical manufacturing companies' workers, whose SMR was 2.2, but neither SMR was statistically significant. However, a cohort study by Kiesselbach et al. (1990) of 2,658 German chemical workers from six chemical companies in eight plants found no increases in mortality
from cancers of the lymphatic and hematopoietic tissues (five observed and five expected). The exposure information in this study was only qualitative.
In an attempt to combine information from the 10 distinct cohorts that had been studied at that time, Shore et al. (1993) calculated separate meta-analysis estimates of the pooled SMRs for the individual cancer mortalities for leukemia, non-Hodgkin's lymphoma, stomach cancer, pancreatic cancer, and cancer of the brain and nervous system. They also examined these cancers for trends by latency, by duration, and by intensity or frequency of exposure, but there was "no attempt to weight the studies according to quality." The pooled SMR for each type of cancer was merely estimated by the (3 observed deaths in all the studies) ÷ (3 expected deaths in all the studies). Because the largest study groups (Steenland et al., 1991, also published by Wong and Trent, 1993) also had the largest number of observed and expected deaths, this study accounted for between one-third and two-thirds
of both the numerator and denominator totals. Based on this analysis of pooled total deaths, Shore et al.'s conclusions were similar to those of Trent and Wong, but less indicting of EtO for lymphoid cancers than are those of Stayner et al. (1993). Stayner et al., unlike Shore et al., developed exposure histories for the cohort and was able to analyze for, and find, significant exposure-response trends by cumulative exposure, while Shore's analysis did not have this sensitivity. Shore et al. concluded that their analyses "did not indicate a risk from EtO for pancreatic cancer (SMR = 0.98), brain and nervous system cancer (SMR = 0.89), or total cancer (SMR = 0.94). Although the current data do not provide consistent and convincing evidence that EtO causes leukemia or NHL, the issues are not resolved and await further studies of exposed populations." Although these authors also
found a "suggested increase in the overall SMR for stomach cancer (SMR = 1.28, 95% C.I. 0.98 - 1.65)," their (dose-response) "analyses by intensity or duration of exposure or cumulative exposure did not support a causal association."
In their OSHA docket submission of August 8, 1997, the Ethylene Oxide Industry Council (EOIC, Ex. 2-6) refers to the conclusions of Shore et al. (1993) and to an update of Shore et al.'s analysis by Teta, partially presented at the OSHA public meeting on this Lookback study. The update includes two additional studies, the above- discussed Hagmar et al. (1995) study and a study by Olsen et al. (1997), which examined relationships between EtO and pancreatic and hematopoietic cancer mortality among 1361 men employed by Dow Inc. in the ethylene or propylene production process at one of three U.S. plants. These latter authors found a small non-statistically significant increase in hematopoietic cancer mortality (SMR of 1.29, 95% C.I. 0.62 - 2.38) among a group with an overall SMR = 0.90. Dr. Teta (EOIC, Ex. 2-6) also presented risk ratios for several cancers from three studies according to
sex, concluding that "females do not appear to be more sensitive (than males)."
IARC AND NTP CLASSIFICATIONS OF ETO AS A KNOWN HUMAN CARCINOGEN
In 1987 the International Agency for Research on Cancer (IARC) classified EtO in sub-group 2A, a designation of probably carcinogenic to humans, based on sufficient evidence in animals and limited evidence in humans. Upon updating its classification based on additional information, the IARC (1994, referenced in Ex. 2-6 and 2-10) concluded, "that EtO is carcinogenic to humans (Group 1)." (page 139). This conclusion was based on a total weighing of the evidence, which included a finding of limited evidence for carcinogenicity in humans, sufficient evidence for carcinogenicity in experimental animals, and the following supporting evidence:
"(i) induces a sensitive, persistent dose-related increase in the frequency of chromosome aberrations and sister chromatid exchange in peripheral lymphocytes and micronuclei in bone-marrow cells of exposed workers;
(ii) has been associated with malignancies of the lymphatic and hematopoietic system in both humans and experimental animals;
(iii) induces a dose-related increase in the frequency of hemoglobin adducts in exposed humans and dose-related increases in the numbers of adducts in both DNA and hemoglobin in exposed rodents;
(iv) induces gene mutations and heritable translocations in germ cells of exposed rodents; and
(v) is a powerful mutagen and clastogen at all phylogenetic levels."
In comments submitted to OSHA, the EOIC included a letter to Dr. Paul Kleihues, dated 3/25/1994, which it had sent to the IARC working group (Ex. 2-6-C), detailing criticism of the reasoning used by IARC to upgrade EtO from a Group 2A to Group 1 classification. Specifically, the EOIC argued that 1) although EtO is genotoxic, "because a relevant mechanism has not been defined for EtO it is not possible to conclude that the genotoxic changes which have been observed will lead to cancer in humans"; 2) the IARC draft wrongly attributed the excess of lymphatic and hematopoietic cancers in a study of UCC chlorhydrin production unit workers to EtO exposure, even though the subgroup with the excess "had little potential exposure to EtO"; and 3) the IARC draft gave too much weight to the NIOSH epidemiology study of sterilizer workers, particularly the Stayner et al. (1993)
results instead of a study the EOIC considers as relevant, i.e., the UCC chemical workers, which has a longer follow-up period than the sterilizer workers. The EOIC's letter also pointed out that IARC's conclusion about EtO's carcinogenicity is stronger than that of Stayner et al.
NTP Classification of EtO as Reasonably Anticipated to be a Human Carcinogen
In the Eighth Report on Carcinogens (1990), the NTP classified EtO as "reasonably anticipated to be a human carinogen." The NTP found at that time that there was "sufficient evidence for the carcinogenicity of ethylene oxide in experimental animals." The NTP relied on the findings of tumors in mice and rats of both sexes in making its finding. The NTP's classification affirmed OSHA's 1984 findings on the carcinogenicity of EtO in animals and its potential carcinogenicity in occupationally exposed workers.
In its 9th Report on Carcinogens (2000), the NTP upgraded its classification of EtO from Reasonably Anticipated to be a Human Carcinogen (first listed in its 4th Annual Report, 1985) to its highest category, Known to be a Human Carcinogen ", based on sufficient evidence of carcinogenicity from studies in humans, involving a combination of epidemiological and mechanistic investigations which indicate a causal relationship between exposure to EtO and human cancer." (pg. III-35). In addition to summarizing the animal biossays and the newer epidemiology evidence, also presented in Section I.C.1.2 above, the NTP provided a summary of the evidence, concluding that it supported a known human carcinogen classification. This is discussed further below.
OTHER NEW INFORMATION RELATING TO CARCINOGENICITY
The IARC evaluation of EtO (1994) includes additional information, particularly on the genotoxicity of EtO. IARC considered the new genotoxicity studies highly supportive of its conclusion that EtO should be classified as a known human carcinogen. NTP (2000) made similar note of this evidence, and included several additional studies, concluding, "For direct-acting mutagenic chemicals (e.g. EtO), increases in chromosome aberration frequency appear to be a good predictor of increased human cancer risk. Thus, all measurable genotoxic endpoints that are considered to be indicators of chemical carcinogenesis have been observed in both human and experimental animals exposed to EtO." (pg. III-36). These newer studies showing genotoxicity at all phylogenetic levels generally support OSHA's 1984 conclusions that "EtO exposure causes mutations, increases the rate of chromosomal
aberration and sister chromatid exchange, and causes other undesirable changes in the DNA of mammalian cells." (49 FR 25738).
New Information on Reproductive Effects
Several new studies that have become available since 1984 provide additional evidence that EtO exposure is associated with an increased risk of spontaneous abortion among pregnant women and is capable of causing other adverse reproductive effects in both men and women (49 FR 25738). All but one of these studies have been in experimental animals (e.g., Generoso et al., 1986; Generoso et al., 1987; Rutledge et al., 1989; Katoh et al., 1989; and Mori et al., 1991). In addition, a questionnaire mail survey of 4,856 dental assistants by Rowland et al. (1996) reported age-adjusted relative risks of 2.1 to 2.7 for spontaneous abortions (p<0.05), preterm births, and post-term births. In its comments to OSHA, the EOIC (Ex. 2-6) critiqued the Rowland et al. study, especially the accuracy of its results, because they were based on responses to a questionnaire survey. The EOIC also pointed out
that "there are no usable quantitative exposure data" in the Rowland et al. study.
EPA Comments on OSHA's 1984 Quantitative Risk Assessment
EPA's Office of Pesticide Programs provided comments (Ex. 2-8) recommending that OSHA lower its PEL for EtO to 0.1 ppm, based on EPA's own quantitative risk assessment of EtO, which estimates risks to workers about 10 times higher than OSHA's. Although EPA's risk assessment used the same rat inhalation studies as OSHA did for animal-to -human quantitative risk extrapolation, EPA used a different dose-equivalence assumption -- (mg/body mass2/3) vs. OSHA's use of (mg/body mass). EPA also used the total number of tumor bearing animals to estimate human risk, while OSHA estimated risks for each tumor type separately. Finally, EPA used the upper-limit risk estimate while OSHA relied on maximum likelihood estimates of risk. Thus, EPA's recommendation that OSHA lower the EtO PEL is based not on new risk assessment information or studies but on different animal-to-human extrapolation methodologies.
Conclusion on Health Effects
New scientific information supports OSHA's conclusion that EtO is a potential occupational carcinogen. EPA's comments, based on its own risk assessment of EtO, provide further support to OSHA's 1984 conclusions on the potential occupational carcinogenicity of EtO. The EtO Standard is necessary to prevent adverse health effects associated with EtO exposure.
EXPOSURE TRENDS INDICATE THAT THE STANDARD IS EFFECTIVE
The overall trend observed in EtO exposure monitoring data following the promulgation of the EtO standard suggests that the standard has been effective in decreasing employee exposure to EtO in many industries. This conclusion is based on the results of exposure studies documented in the literature and on an analysis of exposure monitoring data from OSHA's Integrated Management Information System (IMIS)55 database. OSHA's review of the findings of a number of large-scale hospital exposure studies as well as an analysis of IMIS exposure monitoring data collected from hospital and other health care service establishments demonstrate that the EtO standard has been particularly successful in decreasing occupational EtO exposure in the health care sector, the industry with the largest number of EtO-exposed employees. An analysis of data contained in
the IMIS database also suggests that the standard has decreased exposures in the medical and pharmaceutical products sterilizing industries.
OSHA's analysis of exposure monitoring data across all industries shows that there has been a decline in occupational EtO exposure levels since the promulgation of the EtO standard (see Table III-1). As shown in Table III-1, prior to the February 19, 1985 compliance deadline for the standard, approximately 81 percent of the 165 personal 8-hour TWA samples in the IMIS database were at or below the 1984 PEL of 1 ppm. In the period following the 1985 compliance deadline and extending through 1996, approximately 90 percent of the 767 personal 8-hour TWA samples in the IMIS database were at or below the 1984 PEL of 1 ppm.
OSHA also reviewed exposure monitoring data across all industries by establishment size. The purpose of this analysis was to examine the compliance patterns of small firms and to compare the results with the compliance patterns for large firms.56 As shown in Table III-2, despite the limited amount of sampling data available from the period prior to the promulgation of the rule, the exposure data collected from small establishments reflect a similar pattern to that found in the overall data set. These data suggest that exposure levels occurring at small establishments have also declined since the promulgation of the rule. In addition, in each of the three small establishment size categories shown in Table III-2 (fewer than 50 employees, 50 to 99 employees, and 100 to 499 employees), more than 80 percent of exposure measurements collected from small
establishments were at or below the PEL in the period following the deadline for compliance with the rule. In comparison, approximately 95 percent of the 394 exposure measurements collected from large establishments (establishments with 500 or more employees) were at or below the PEL in the period following the compliance deadline.
Exposure Trends in the Health Care Sector
The findings of numerous published studies in the occupational safety and health literature as well as data from OSHA's IMIS database indicate that hospitals and other health care sector employers have been successful in reducing EtO exposures and in achieving compliance with the PEL. A number of hospital sector exposure studies and sources of exposure monitoring data dating from the late 1970s through 1984 indicate that, while some hospital sector employers were able to reduce occupational EtO exposures to or below 1 ppm at that time, workers at many hospitals still had exposures well above 1 ppm during the period preceding the promulgation of the EtO Standard.57,58 Much greater success in exposure control was achieved after promulgation of the standard.59,60,61,62
Table III-1
EtO Exposure Monitoring Data from All Industries Collected by
OSHA Before and After Issuance of the Final Ethylene Oxide Standard
| |
1979 to Pre-1985 Deadline |
Post-1985 Deadline to 1996 |
| 8-Hour TWA |
Number of Samples
n=165 |
Percent of Samples |
Number of Samples
n=767 |
Percent of Samples |
| 10.01 ppm+ |
11 |
6.6% |
10 |
1.3% |
| 5.01 to 10 ppm |
5 |
3.0% |
13 |
1.7% |
| 1.01 to 5 ppm |
15 |
9.1% |
54 |
7.0% |
| 1.0 ppm or less |
134 |
81.2% |
690 |
90.0% |
Source: OSHA IMIS database.
Table III-2
EtO Exposure Monitoring Data Collected by OSHA Before and After
Issuance of the Ethylene Oxide Standard, by Establishment Size
| |
< 50 Employees |
50-99 Employees |
100-499 Employees |
| |
Pre-1985 |
Post-1985 |
Pre-1985 |
Post-1985 |
Pre-1985 |
Post-1985 |
| 8-Hour TWA |
Percent of all Samples
n=22 |
Percent of all Samples
n=122 |
Percent of all Samples
n=8 |
Percent of all Samples
n=59 |
Percent of all Samples
n=57 |
Percent of all Samples
n=172 |
| 10.01 ppm+ |
18.1%
n=4 |
3.3%
n=4 |
12.5%
n=1 |
15.3%
n=9 |
17.5%
n=10 |
8.7%
n=15 |
| 5.01 to 10 ppm |
9.1%
n=2 |
0.8%
n=1 |
0%
n=0 |
0%
n=0 |
5.3%
n=3 |
5.2%
n=9 |
| 1.01 to 5 ppm |
9.1%
n=2 |
11.5%
n=14 |
12.5%
n=1 |
15.3%
n=9 |
17.5%
n=10 |
8.7%
n=15 |
| 1.0 ppm or less |
63.6%
n=14 |
84.4%
n=103 |
75.0%
n=6 |
83.1%
n=49 |
66.7%
n=38 |
83.7%
n=144 |
Source: OSHA IMIS database.
The Meridian Research Report
A detailed discussion of many of the early published hospital EtO exposure studies as well as other sources of EtO exposure data can be found in the 1992 report prepared for OSHA by Meridian Research entitled "Ethylene Oxide: A Case Study in Hazard Identification, OSHA Regulation, and Market Response" (previously discussed in Chapter II).63 Based on an analysis of the literature, Meridian concluded that the use of EtO in hospitals was largely uncontrolled during the late 1970s. During this period, the majority of employees' 8-hour TWA exposures ranged from 1 ppm to 10 ppm, and approximately 5 to 10 percent of all full-shift exposures exceeded OSHA's then existing standard of 50 ppm.64 Meridian characterized the period from 1978 to 1984 as one in which hospitals focused greater attention on controlling
exposures by designing and retrofitting engineering controls, upgrading ventilation systems, and improving employee work practices. However, despite these efforts by employers, a significant percentage of employee exposures (approximately 20 to 30 percent) remained in the range of 1 to 5 ppm.65 Finally, in the years immediately following the promulgation of the EtO Standard (1985 to 1992), a significant reduction in employee exposures to EtO occurred. Meridian concluded that, during the post-promulgation period, not only had more than 90 percent of hospitals achieved compliance with the new 8-hour TWA 1 ppm PEL for EtO, but also that the majority of hospitals had reduced their employee exposures to well below 0.5 ppm, and that a substantial number had managed to achieve TWAs of 0.1 ppm.66 The hospital studies
are consistent with the results of several other lookbacks, that some employers start reducing exposure or reduce risks when OSHA proposes a new standard and do not wait until it has been made final. Other employers do not act until the standard is made final, or enforcement actions are taken.
Findings from Large-Scale Hospital Exposure Studies and the IMIS Database
The success of health care sector employers in reducing EtO exposures and achieving compliance with the PEL is best illustrated by examining the findings of the largest exposure data studies available from both before and after the promulgation of the EtO standard. Table III-3 presents the distributions of exposure monitoring results from four large-scale hospital exposure studies conducted prior to the promulgation of the standard and two large-scale studies conducted during the period following the compliance deadline for the 1984 standard. Each of these studies reported data from more than ten hospitals.
Table III-3
EtO Personal Exposure Monitoring Results from Hospital Studies
| Hospital EtO Exposure Studies |
Years Covered by Study |
Percent of Samples At or Below 1.0 ppm |
Percent of Samples Above 1.0 ppm |
| Studies Conducted Prior to 1985 |
| Hospital Council of Southern California |
1978 |
27.2% |
70.8% |
| Hospital Council of Southern California |
1979-1982 |
64.7% |
35.3% |
| Emergency Care Research Institute |
1981-1982 |
34.6% |
65.4% |
| Hospital Council of Southern California |
1982-1983 |
77.6% |
22.4% |
| Later Studies |
| Gschwandtner et al. |
1985 |
91.0% |
9.0% |
| LaMontagne and Kelsey |
1990-1992 |
99.6% |
0.4% |
Sources: Docket H-200, Exs. 4-14; 4-53; 11-152; Gschwandtner et al. "Compliance with the EtO Standard in the United States," pp. 38-41; LaMontagne et al., "Evaluating OSHA's Ethylene Oxide Standard: Employer Exposure-Monitoring Activities in Massachusetts Hospitals from 1985 through 1993," pp. 1119-1125.
The studies of hospital EtO exposure levels conducted by the Hospital Council of Southern California (HCSC) and the Emergency Care Research Institute (ECRI) during the late 1970s and early 1980s indicated that, while some employers were capable of reducing exposures to 1 ppm, many employers were not. As shown in Table III-3, the range of results found in these early large-scale studies showed a minimum of 29.2 percent of samples to be at or below 1 ppm in a 1978 HCSC study and a maximum of 77.6 percent of samples to be at or below 1 ppm in a 1983 HCSC study. Later studies conducted after the promulgation of the rule by Gschwandtner et al. (using 1985 data) and LaMontagne et al. (using 1990 to 1992 data) showed significantly lower exposures (more than 90 percent of exposures below 1 ppm) and suggested that most employers were capable of complying with the standard by the time the EtO
standard's compliance deadline had been reached. Brief descriptions of these studies follow.
During 1978, HCSC conducted 27 surveys in 23 hospitals [Docket H-200, Ex. 4-14].67 At that time, HCSC estimated worker exposures by averaging instantaneous readings collected with a vapor analyzer during various steps of sterilization processes. HCSC found that only 29.2 percent of the sites surveyed had estimated exposures less than 1 ppm, that 62.6 percent of the sites had exposures between 1 ppm and 50 ppm, and that 8.3 percent of the sites surveyed had exposures in excess of the then-current OSHA standard of 50 ppm.
HCSC conducted a second round of site surveys from January 1979 to February 1982 [Docket H-200, Ex. 4-14]; 232 surveys were conducted at this time. One hundred fifty of these surveys, or 64.7 percent, yielded exposure monitoring results below 1 ppm. An additional 28 percent of the surveys showed exposures between 1 ppm and 4.9 ppm. No exposures greater than 50 ppm were observed.
A third round of site surveys was conducted by HCSC from March 1982 to March 1983 [Docket H-200, Ex. 11-122]. One hundred forty three surveys were conducted during this round. One-hundred eleven of these surveys, or 77.6 percent, yielded exposure monitoring results below 1 ppm. An additional 21 percent of the surveys showed exposures between 1 ppm and 4.9 ppm. No exposures greater than 20 ppm were observed.
ECRI also conducted a large-scale hospital exposure study during the period preceding the issuance of the standard [Docket H-200, Ex. 4-53]. ECRI conducted site visits to 26 hospitals during a six-month period during late 1981 or early 1982. An infrared spectrophotometer operating in a continuous sampling mode was used to measure EtO levels.
The sampling results from these visits showed much higher exposures than the two roughly contemporaneous (1979-1982 and 1982-1983) HCSC studies. Only 34.6 percent of the 26 samples were below 1 ppm. However, no data above 50 ppm were recorded. (It is important to note that these measurements are not personal readings; their relevance to employee exposures is therefore unclear.)
A 1986 study by Gschwandtner et al. provides information on exposure levels occurring at hospitals during the period immediately following the promulgation of the EtO standard.68 This study summarized the analytical results from a large set of passive dosimeter devices (personal exposure monitoring badges) that had been submitted to a single device manufacturer for analysis. These devices had been submitted by approximately 1,000 hospitals, making this an extremely large, and presumably representative, set of EtO exposure monitoring results. Ninety-one percent of the badge monitoring results were below 1 ppm, and 82 percent of the results were below 0.5 ppm.
The most comprehensive large-scale study of hospital exposure levels is the Massachusetts Hospital EtO Health and Safety Study. This study of the compliance patterns of nearly all of the EtO-using hospitals (92 hospitals, or 96 percent) in Massachusetts found even lower EtO exposure monitoring results at hospitals during the period 1990 through 1992. During this study, 8-hour TWA exposure data were collected from 75 of the 92 participating hospitals, and short-term excursion level exposure data were collected from 72 of the 92 hospitals. Of the exposure monitoring data collected by surveyed hospitals during 1990 to 1992, only 0.4 percent of samples exceeded the 0.5 ppm action level (47/10,981) and 0.7 percent exceeded the 5 ppm excursion limit (64/8,916). This finding suggests continued improvement in the control of EtO exposures and confirms that occupational exposures are now typically
well below the 8-hour TWA PEL in hospitals.69
In a related study of Massachusetts hospitals data, LaMontagne and Kelsey analyzed the determinants for (1) exceeding the EtO action level, (2) exceeding the EtO excursion limit, and (3) experiencing uncontrolled or accidental releases in the absence of personal monitoring.70 Of those hospitals that performed action level monitoring from 1990 to 1992, about one quarter exceeded the action level 1 or more times (17/75). Of those hospitals that performed excursion limit monitoring, about one fourth exceeded the excursion limit 1 or more times (17/72). During this period, 32 of 90 hospitals reported the occurrence of 1 or more uncontrolled releases. The analysis associated the use of positive pressure sterilizers with elevated risks of exceeding the excursion limit and experiencing accidental exposures. The study concluded that important design differences
between sterilizers that use chlorofluorocarbon and EtO gas mixtures and sterilizers that use 100% EtO likely account for the observed associations.
OSHA's IMIS database contains additional data confirming that most health care sector employers comply with the existing 1 ppm PEL and suggesting that exposures have decreased since the promulgation of the EtO Standard. Table III-4 shows the distribution of these data by exposure level. This set of EtO exposure monitoring data, which was collected from both hospitals and other similar health care service establishments (e.g., large clinics), generally supports the Gschwandtner et al. and LaMontagne et al. findings of a high degree of compliance with the 1 ppm PEL. (However, this data set shows a less dramatic decrease in exposures between the pre- and post-promulgation period than a review of the findings of the separate studies above would indicate.71)
Table III-4
EtO Exposure Monitoring Data from Hospitals and Other Health Care Service Establishments
Collected by OSHA Before and After Issuance of the EtO Standard
| |
1979 to Pre-1985 Deadline |
Post-1985 Deadline to 1996 |
| 8-Hour TWA |
Number of Samples
n=114 |
Percent of all Samples |
Number of Samples
n=490 |
Percent of all Samples |
| 10.01 ppm+ |
1 |
.9% |
2 |
.4% |
| 5.01 to 10 ppm |
1 |
.9% |
2 |
.8% |
| 1.01 to 5 ppm |
6 |
5.3% |
22 |
4.5% |
| 1.0 ppm or less |
106 |
93.0% |
462 |
94.3% |
Source: OSHA IMIS database.
Exposure Trends in the Medical and Pharmaceutical Products Sterilization Industries
During OSHA's review of the EtO exposure monitoring data in the IMIS database, OSHA determined that there were enough exposure data from the medical and pharmaceutical products sterilizing industries to warrant analyzing these data as a separate industry-specific data set. OSHA's analysis of this exposure data shows that the EtO standard had an effect on decreasing exposures in the medical and pharmaceutical products sterilizing industries, although the amount of data from the period prior to the 1985 compliance deadline is limited. Table III-5 shows the distribution of these data by exposure level.
Table III-5
EtO Exposure Monitoring Data from the Medical and Pharmaceutical Products Sterilizing
Industries Collected by OSHA Before and After Issuance of the EtO Standard
| |
1979 to Pre-1985 Deadline |
Post-1985 Deadline to 1996 |
| 8-Hour TWA |
Number of Samples
n=27 |
Percent of all Samples |
Number of Samples
n=137 |
Percent of all Samples |
| 10.01 ppm+ |
6 |
22.2% |
3 |
|
| 5.01 to 10 ppm |
2 |
7.4 |
7 |
|
| 1.01 to 5 ppm |
7 |
25.9% |
22 |
|
| 1.0 ppm or less |
12 |
44.4% |
105 |
|
Source: OSHA IMIS database.
Conclusion on Exposure Data
These exposure data indicate a continued need for the EtO Standard.
ACCIDENTAL ETO RELEASES IN HOSPITALS AND OTHER HEALTH CARE SERVICE ESTABLISHMENTS
Many documented EtO-related emergencies have involved accidental releases of EtO from sterilizing equipment used in hospitals and other health care service establishments. The limited data available concerning accidental EtO releases from EtO sterilization equipment suggest that such releases are not uncommon. EtO-related health effects have been observed in workers exposed to EtO during these accidental releases.
One source of information on accidental releases of EtO from hospital sterilization operations is the Massachusetts Hospital EtO Health and Safety Study [Ex. 2-9K]. This study documented that two-thirds of the responding hospitals (58 of 90) had reported evacuations of the sterilization department or area in response to a real or suspected EtO leak or an EtO alarm at some time during 1985 to 1993. Fifty percent of EtO-using Massachusetts hospitals (46 of 92) reported accidental releases during the period 1985 to 1993 and 33 percent (32 of 90) reported accidental releases during the period 1990 to 1992.72 As discussed above, a related survey of medical surveillance providers conducted as part of the Massachusetts Hospital EtO Health and Safety Study produced data suggesting that one or more workers at 19 percent of EtO-using Massachusetts hospitals
had experienced EtO-related health effects during the time period covered by the survey. Many of the EtO-related health effects observed by the surveyed medical surveillance providers were observed in workers who had been overexposed to EtO during accidental releases.
In a follow-up study of determinants for experiencing uncontrolled or accidental releases in the absence of personal monitoring in Massachusetts hospitals, LaMontagne and Kelsey found that 32 of 90 hospitals reported the occurrence of 1 or more uncontrolled EtO releases during the period 1990 to 1993.73 One or more workers were definitely or potentially exposed during accidental releases at most of these hospitals (29 of 32). In addition, 25 of these 29 had experienced evacuations in response to real or suspected leaks detected by EtO alarms or by other means. The authors found that the use of positive pressure sterilizers was associated with elevated risks of exceeding the excursion limit and experiencing accidental exposures. In a related study, LaMontagne and Kelsey noted that the Massachusetts Hospital study also showed that "personal
monitoring activities have failed to detect the widespread occurrence of accidental exposures during EtO leaks and spills.74
OSHA's IMIS accident investigation database contains further information on accidental releases occurring in hospitals and other health care services establishments. The IMIS database contains 25 records of incidents occurring between 1990 and 1996 that involved accidental exposure to EtO used in sterilization operations. Three of these accidents resulted from the use of incorrect work practices, nine occurred during sterilant gas tank changing, and 13 were the result of sterilizing equipment failures. These 25 incidents involved a total of 73 workers. Examples of these reported incidents follow:
- A sterilizer was overpressurized due to the mechanical failure of a pressure-sensing device. The overpressure condition ruptured the sterilizer's door seal, exposing 6 employees to EtO. The employees, who were preparing surgical instruments at the time of the accident, subsequently experienced eye irritation, headaches, and nausea.
- During the installation and testing of a new sterilizer, safety devices malfunctioned and EtO was released into the work area, setting off an EtO monitor. Employees were evacuated from the area. One employee re-entered the area, despite the alarm, to test for leaks. A second employee also re-entered the area without wearing a respirator (this employee had not received any training). While the two employees were testing for leaks with the sterilizer pressurized, the charge valves remained open. The sterilizer pressure exceeded the overpressure relief valve setting, releasing EtO into the face of the second employee.
- An EtO alarm was triggered by an EtO leak in an equipment room. A central sterile unit employee notified a maintenance employee and a nursing supervisor, who then entered the work area to evaluate the problem. The maintenance employee entered the equipment room with a hand-held detector that began to beep when lowered toward the sterilizer. The central sterile unit employee returned to the work area to get her purse. Following this, the central sterile department was evacuated. The nursing supervisor had not received any training on EtO or emergency procedures. All employees were later seen by a physician. Two employees experienced eye irritation.
- A supervisor was overseeing the aeration of surgical equipment following its sterilization in an EtO sterilizer. The supervisor was ordered by upper management to remove the load from the aerator after the load had completed only one hour of the eight-hour aeration cycle. (The supervisor was not normally responsible for removing sterilized goods from the aerator.) The supervisor experienced headaches and a burning (sensation) on exposed body parts.
- Two employees were changing EtO supply cylinders in the central supply room. The cylinders were connected through a valve to a supply line, another valve system, and the sterilizer chamber. One employee disconnected a supply line from an EtO cylinder without properly venting the system. Both employees heard hissing sounds from the lines. An EtO gas leak tester carried by one of the employees sounded an alarm. Both employees received medical treatment for symptoms, including shortness of breath, eye irritation, upper respiratory system irritation, blisters, and headaches. One employee missed two days of work because of this incident. Neither employee had been given adequate training on tank changing procedures. In addition, the employee who regularly performed tank changing duties was unavailable for this assignment on the day of the accident.
EtO-Related Explosions in Commercial Sterilization Facilities
Ethylene oxide is a highly reactive chemical. EtO-related explosions cause significant property losses, and the most severe of these accidents have resulted in worker fatalities and/or injuries.75,76
During the late 1980s and early 1990s, at least seven serious EtO-related explosions occurred at EtO production and ethoxylation facilities worldwide.77 One of these accidents occurred at an EtO production facility in the United States. EtO-related explosions have also recently occurred at U.S. plants in the commercial sterilization industry and sterilant gas packing industry. In 2000, NIOSH, in conjunction with the EPA and the Ethylene Oxide Sterilization Association, issued an Alert entitled "Preventing Worker Injuries and Deaths from Explosions in Industrial Ethylene Oxide Sterilization Facilities" because of the large number of explosions involving EtO sterilizers. NIOSH reports that, between 1994-1998, 10 EtO explosions occurred in different industrial sterilization facilities. In a single incident, 1 worker died and 70 were injured.
A few brief examples demonstrating the severity of such accidents are presented below:
- In 1988, an explosion occurred at a medical products manufacturing plant during the repair of an EtO sterilizer unit. The explosion was linked to residual EtO present in the sterilizer during the repair. This accident resulted in one worker fatality, 6 workers hospitalized with injuries, and one worker with an injury that did not require hospitalization.
- In 1991, an explosion occurred in the oxide removal system of an ethylene oxide recovery tower at an EtO production plant. This accident resulted in one worker fatality and 32 injuries. The explosion also resulted in the destruction of a refining column of the ethylene oxide plant, considerable damage to a nearby glycol ether unit and pipe racks, and slight damage to other units at the plant.78,79
- In 1997, an explosion occurred at a sterilant gas packing plant during an EtO canister filling operation. The cause of the explosion was determined to be ethylene oxide gas ignition in a tank storage room. This accident resulted in one fatality and the hospitalization of numerous employees for over-exposure. More than 70 persons were injured as a result of this accident.80
- In 1997, in an effort to correct an EtO overcharge in one of the vessels of the sterilization unit, the operator added dilution air to the vessel. When he opened the front door of the sterilizer, a dump valve at the back opened, allowing the EtO gas stream to bypass the scrubbers and come into contact with the emission control device. The explosion that followed severely damaged the sterilizer, the roof of the building, the walks, the ductwork, and the oxidizer. No workers were injured in the blast [EPA 1997, as cited in NIOSH 2000].
- In 1998, a facility that repackaged EtO experienced an EtO explosion during a test run on the inside of the thermal oxidizer. The inlet concentration of EtO had risen from 1,000 ppm to approximately 35,000 ppm just before the explosion. The blast damaged the thermal oxidizer bed and the production equipment [EPA 1998, as cited in NOISH 2000].
The NIOSH-EPA-EOSA document concludes that these commercial sterilization incidents occurred in facilities where an oxidizing emission control device (OECD) alone was used as the emissions control device, i.e., acidified-wet scrubbers were not used or were bypassed. If the back vent system of the sterilizer opens when the concentration of EtO in the chamber is at or above the lower flammable limit for EtO (approximately 3%, or 30,000 ppm EtO), the sterilant gas is likely to ignite on contact with the OECD. The report states that high EtO concentrations must be isolated from the oxidizer under all operating conditions and makes several engineering, work practice, and hazard analysis recommendations designed to prevent such occurrences in the future [NIOSH 2000].
EMPLOYERS AND EMPLOYEES SUPPORT CONTINUANCE OF THE STANDARD
OSHA's Lookback review of the EtO standard included a public meeting held on June 30, 1997 and a request for information [62 FR 28649] from interested persons, particularly small employers. The types of organizations expressing support for the standard during the regulatory review process included employers, a sterilant manufacturer, industry associations, employee representatives, professional organizations, and individuals, as well as EPA and NIOSH [Docket H-200C, Exs. 2-14; 2-4; 2-1; 2-6; 2-2; 2-7; 2-12; 2-15; 2-9; 2-8; 2-10; Tr. 6/30/97]. These commenters' support for continuing the standard was based primarily on the perceived effectiveness of the standard and commenters' recognition of the importance of having an enforceable federal standard to prevent overexposure to EtO.
Many of the employer and employee representatives who provided comments during the regulatory review process indicated for the continuance of the standard. Examples of their comments are provided below:
- The Ethylene Oxide Industry Council (EOIC) wrote: "EOIC supports OSHA's EO standard as technically and economically feasible...OSHA is to be commended for its EO standard...EOIC firmly supports the existing standard and believes that revisions are not needed either from the standpoint of safety, feasibility or burden. The EO standard should be retained in its present form" [Ex. 2-6]. Note: The Council refers to EtO as EO.
- The American Hospital Association (AHA) indicated that "the standard is working and has been effective in protecting employee health" and concluded that "the existing EtO standard is effective as written and requires no significant change in the context of this review" [Ex. 2-2].
- Griffith Micro Science commented that "the EtO standard provides increased protection to all workers working with ethylene oxide. The rule has significantly reduced the workplace EtO exposures to all employees in the EtO industry...." [Ex. 2-4].
- A representative of Clariant Corporation wrote that "Overall, I feel that the EtO standard is well conceived and well written" [Ex. 2-14].
- The Pennsylvania Engineering Company commented that "The OSHA rule on Ethylene Oxide has done an excellent job in assuring the safe use of Ethylene Oxide in the essential use in health care facilities and manufacturers who supply medical devices to health care facilities...we do not recommend making any changes in any part of the OSHA regulations" [Ex. 2-1].
- The AFL-CIO commented that "the EtO standard has achieved its goal" of protecting workers and stated further that OSHA must maintain the standard [Tr. 6/30/97, p. 10].
- The American Federation of State, County, and Municipal Employees (AFSCME) testified at the public meeting that the union was generally "pleased with the 1984/1988 EtO standard" and that the standard "has had a profound effect on improving the safety and health of AFSCME members" [Tr. 6/30/97, p. 30].
- The American Nurses Association (ANA) indicated that the standard is "essential in protecting the safety and health of health care workers who may come in contact with this chemical. The EtO Standard has met its goal of reducing the exposure of health care workers...without being unduly burdensome to the employer. It is absolutely critical that the Occupational Safety and Health Administration continues strong enforcement of the standard" [Ex. 2-7].
- The American Association of Nurse Anesthetists (AANA) encouraged OSHA to "retain the current requirements for Ethylene Oxide utilization" and to continue the standard [Ex. 2-13].
- The Massachusetts Nursing Association (MNA) indicated that it "believes that Ethylene Oxide...should continue to be regulated. The Massachusetts Nurses Association further believes that inspection and enforcement of this standard should receive high priority because of the number of workers with the potential for exposure" [Ex. 2-12].
- Ms. Betty Napier, an R.N. with a previous history of occupational exposure to EtO, also wrote to the docket in support of the continuance of the EtO standard. She indicated that she felt that the "exposure limits and monitoring should remain the same" [Ex. 2-15].
The support expressed by these commenters provides further evidence that the standard is effective and should be retained in its present form.
SUMMARY
OSHA believes that retaining the EtO standard will ensure that workers continue to be protected from the adverse health effects of overexposure to EtO. The toxicological and epidemiological studies discussed in this chapter have provided further evidence of the seriousness of the health effects associated with EtO exposure. Further, a review of OSHA's IMIS accident investigation database and the literature indicates that workers still face health risks associated with accidental releases and EtO overexposures and a recent NIOSH alert (2000) indicates that EtO use in sterilization operations continues to pose fire and explosion risks to workers in these facilities. The preponderance of the evidence assembled during this Lookback review, including the findings of studies in the literature, overall trends observed in the EtO exposure data, and the support of both employer and employee representatives
for the standard indicates that the EtO standard has had a significant effect on decreasing occupational EtO exposures and that there is a continued need for the standard. OSHA has therefore concluded that the standard is necessary and should continue in effect.
REFERENCES FOR THE HEALTH EFFECTS AND RISK ASSESSMENT SECTION
BIER IV. (1998). Health risks of radon and other internally deposited alpha-emitters. National Research Council. National Academy Press. Washington, D.C.
Bistanti, L., Megan, M., Rasceta, R., et al. (1993). Cancer mortality in ethylene oxide workers. Br. J. Ind. Med., 50: 31, 7-24.
Ethylene Oxide Industry Council. (1997). Comments of the EOIC concerning OSHA's review of EtO under the regulatory flexibility act. OSHA Docket No. H-200-C, Ex. 2-6.
Gardner, M.J., Coggon, D., Pannett, B., Harris, E. (1989). Workers exposed to ethylene oxide: a follow-up study. Br. J. Ind. Med., 46: 860-5.
Generoso, W.M., Cain K.T., Hughes, L.A., et al. (1986). EtO dose and dose-rate effects in the mouse dominant-lethal test. Environ. Mutagen. 8: 1-7.
Generoso, W.M., Rutledge, J.C., Cain, K.T., et al. (1987). Exposure of female mice to EtO within hours after mating leads to fetal malformation and death. Mutat. Res. 176: 269-274.
Greenberg, H.L., Oh, M.G. and Shore, R. (1990) Men Assigned to Ethylene Oxide Production or other Ethylene Oxide Related Chemical Manufacturing: A Mortality Study. Br. J. Ind. Med. 47, 221-230.
Hagmar, L., Welinder, H., Linden, K., Attewell, R., et al. (1995). An epidemiological study of cancer risk among workers exposed to ethylene oxide using hemoglobin adducts to validate environmental exposure assessments. Int. Arch. Occup. Environmen. Health, 63: 271-7.
Hagmar, L., Mikoczy, Z., and Welinder, H. (1995). Cancer incidence in Swedish sterilant workers exposed to EtO. Occupational and Environmental Medicine 52: 154-156.
Hogstedt, C., Rohlen, O., Berndtsson, B.S., Axelson, O., and Ehrenberg,L. (1979). A cohort study of mortality and cancer incidence in ethylene oxide production workers. Br. J. Ind. Med., 36, 276-280.
Hogstedt, C., Malmqvist, N., and Wadman, B. (1979b). Leukemia in workers exposed to EtO. J.Am. Med. Assoc., 241, 1132-33.
International Agency for Research on Cancer (IARC). (1994). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Some Industrial Chemicals. Vol. 60. Lyon, France.
Katoh, M.,Cachiero, N.L.A., Comett, C.V., Cain, K.T., et al. (1989) Fetal anomalies produced subsequent to treatment of zygotes with EtO or ethyl methanesulfonate are not likely due to the usual genetic causes. Mutat. Res. 210: 337-344.
Kiesselbach N., Ulm, K., Lange, H.J., and Korallus, U. (1990). A multicentre mortality study of workers exposed to ethylene oxide. Br. J. Ind Med. 47: 182-8.
LaMontagne, A.D., and K.T. Kelsey. (1989). OSHA's Renewed Mandate for Regulatory Flexibility Review: In Support of the 1984 Ethylene Oxide Standard. American Journal of Industrial Medicine. 34:95-104.
LaMontagne, A.D., and K.T. Kelsey. (2001). Evaluating OSHA's Ethylene Oxide Standard: Exposure Determinants in Massachusetts Hospitals. American Journal of Public Health. 91:412-417.
Lynch, D.W., Lewis, T.R., Moorman, W.J., et al. (1984). Carcinogenic and toxicological effects of inhaled EtO and Propylene Oxide in F344 rats. Toxicol. and Applied Pharmacol., 76: 69-84.
Mori, K., Kaido, M., Fujishiro, K., et al. (1991). Dose dependent effect of inhaled EtO on spermatogeneses in rats. Br. J. of Ind. Med. 48: 270-274.
National Toxicology Program. (1987). Toxicology and carcinogenesis studies of ethylene oxide in B6C3F1 mice (inhalation studies). Washington, DC: US GPO, 1987. (NIH publication no. 88-2582).
National Toxicology Program. (2000). 9th Report on Carcinogens. Carcinogen Profiles 2000. (Revised 2001). NIEHS, RTP, N.C.
NIOSH. (1983). Occupational Exposure to EtO. Testimony to Dept. of Labor on OSHA proposed rule. J. Donald Millar, M.D. 7/20/1983. Ex. 205-17-1.
NIOSH (1997). Letter from Paul A. Schulte. Ex. 2-10.
Office of Technology Assessment (1995). Gauging Control Technology and Regulatory Impacts in Occupational Safety and Health.
Olsen, G.W., Lacy, S.E., Bodner, K.M. (1997). Mortality from pancreatic and lymphopoietic cancer among workers in ethylene and propylene chlorohydrin production. JOEM; 54:592-598.
Rowland A.S., Baird D.D., Shore D.L. et al. (1996). EtO Exposure may increase the risk of spontaneous abortion, preterm birth, and postterm birth. Epidemiology 7:363-368.
Rutledge, J.C., Generoso, W.M. (1989). Fetal pathology produced by EtO treatment of the murine zygote. Teratology 39: 563-572.
Shore, R.E., Gardner, M.J., Pannett, B. (1993). Ethylene Oxide: an assessment of the epidemiological evidence on carcinogenicity. Br. J. Ind. Med., 50: 971-97.
Snellings, W.M., Weil, C.S., and Maronpot, R.R. (1984). A two-year inhalation study of the carcinogenic potential of ethylene oxide in Fischer 344 rats. Toxicol. and Applied Pharmacol., 76, 510-518.
Steenland K., Stayner, L., Griefe, A., et al. (1991). Mortality Among Workers Exposed to EtO. New England Journal of Medicine. 324:1402-71. Ex.2-10-R.
Steenland K., Whelan, E., Deddens, J., et al. (2002). Ethylene oxide and breast cancer incidence in a cohort study of 7576 women (United States). Cancer Causes and Control, 14:531-539.
Steenland, K., Stayner, L., and Deddens, J. (2004). Mortality analyses in a cohort of 18 235 ethylene oxide exposed workers: follow up extended from 1987 to 1998. Occup. Environ. Med. 2004; 61:2-7.
Teta, M.J., Benson, L.O., and Vitale, J.N. (1993). Mortality study of ethylene oxide workers in chemical manufacturing: a 10 year update. Br. J. Indust. Med.50(8):704-9.
Wong, W., Trent, L.S. (1993). An epidemiology study of workers potentially exposed to ethylene oxide. Br. J. Ind. Med. 50: 308-316.
CHAPTER IV
PUBLIC COMMENTS CONCERNING THE STANDARD
OVERVIEW
OSHA requested public comments on the Regulatory Flexibility Act and Executive Order 12866 review of the EtO Standard, Docket H-200C. In the Federal Register Notice announcing the opening of Docket H-200C, OSHA specifically asked commenters to comment on the rule, including the benefits and utility of the rule in its current form or in an amended form; any feasible alternatives to the rule; the continued need for the rule; the complexity of the rule; evidence of overlap, duplication, or conflict between the rule and other federal, state, and local rules; information on economic, technological, and other material changes since the promulgation of the rule; alternatives to the rule or portions of the rule that could minimize any significant impacts on small businesses; and the effectiveness of the rule as implemented by small entities [FR Vol. 62, No. 101, 5/27/97,
pp. 28649-28650]. Comments were accepted both at a public meeting on the Lookback review of the EtO standard and as written submissions to the docket.
The organizations that submitted comments to OSHA during this review included affected employers (BASF Corporation [Ex. 2-3], Clariant Corporation [Ex. 2-5], and Griffith Micro Science [Ex. 2-4]), a manufacturer of EtO-containing sterilant blends (Pennsylvania Engineering Company [Ex. 2-1]), industry associations (the Ethylene Oxide Industry Council (EOIC) [Ex. 3-2] and the American Hospital Association (AHA) [Ex. 2-2 ]), employee representatives and professional associations (the American Federation of Labor and Congress of Industrial Organizations (AFL-CIO) [Ex. 3-1], the American Federation of State, County, and Municipal Employees (AFSCME) [Ex. 3-4], the American Nurses Association (ANA) [Ex. 2-7], the American Association of Nurse Anesthetists (AANA) [Ex. 2-13], and the Massachusetts Nurses Association (MNA) [Ex. 2-12]), as well as several federal agencies (the Environmental Protection
Agency (EPA) [Ex. 2-8], the National Institute for Occupational Safety and Health (NIOSH) [Ex. 2-10], and the Office of Advocacy of the Small Business Administration (SBA) [Ex. 2-11]. Comments were also received from several individuals.
SUMMARY OF DOCKET COMMENTS
As required by Section 610 of the Regulatory Flexibility Act and Section 5 of Executive Order 12866, OSHA asked commenters to tell the Agency about their experience in complying with the EtO standard, any changes in technology or economic conditions that may have occurred since the standard was issued, and several other aspects of the rule, including alternatives to the rule that would minimize any significant impacts of the rule on small businesses. Comments were also solicited on the benefits and usefulness of the rule, the continued need for the rule, the rule's effectiveness in small businesses, and the rule's complexity and interaction with other Federal, state, and local rules. OSHA's responses to these comments are found in Chapter VII.
There was widespread support for the continuance of the standard across a broad spectrum of commenters, including employers and industry trade associations, employee representatives, professional organizations, and other government and public health agencies. However, the Small Business Administration noted that few small entities participated in the Lookback review process, though some did and the trade associations represent small as well as large employers. Most commenters supported continuance of the standard's current 1-ppm permissible exposure limit (PEL), even though some organizations, like the EOIC, believe that the evidence on EtO's health effects that has become available since issuance of the standard suggests that the risk of cancer is less than OSHA estimated in 1984. Some commenters presented evidence that they believed pointed to a more stringent PEL; (see the comments
of NIOSH [Ex. 2-10], the EPA [Ex. 2-8], the AFL-CIO [Ex. 3-1], AFSCME [Ex. 3-4], and ANA [Ex. 2-7].) Others urged OSHA to concentrate on issuing outreach materials instead of expending Agency resources on a reopening of the Standard; (see comments of Dr. LaMontagne and his co-authors [Ex. 2-9, Ex. 3-3]).
Several participants reported that the standard's exposure monitoring requirements are feasible for employers and have contributed to maintaining exposures at levels below the PELs. Some companies recommended relaxing the monitoring requirements to increase compliance flexibility; (see the comments of the BASF [Ex. 2-3] and Clariant Corporations [Ex. 2-5].) Other participants, including the AFL-CIO and Dr. LaMontagne and his co-authors, urged OSHA to add additional exposure monitoring requirements to the standard.
The standard's emergency situation requirements elicited comments from several participants. Dr. LaMontagne pointed out that many of the Massachusetts hospitals he studied were unclear about the meaning of these requirements and particularly about the most effective kinds of alarm mechanisms to use to warn employees to evacuate the area and the appropriate EtO level to set as the evacuation level. The AHA, the AFSCME, and Dr. LaMontagne all urged OSHA to provide additional guidance on alarm equipment, EtO trigger levels, and methods of detecting emergency EtO releases. AFSCME urged OSHA to continue to encourage or enforce compliance with the standard because doing so also has the effect of reducing accidental releases. The EOIC recommended against making any change to the standard's emergency situation requirements that would require the use of alarms (fixed-point monitors).
The standard's medical surveillance provisions were the subject of comments from many commenters, including the AFL-CIO, Dr. LaMontagne, the EPA, the EOIC, and the ANA. These commenters raised issues related to the utility of using the action level as a trigger for medical surveillance, the appropriateness of using the standard's excursion limit as a trigger, the need for inclusion of a complete blood count with leukocyte differential as a required screening test in the standard, and the need to enhance current compliance in the hospital sector with the standard's requirement to take an occupational history as part of the medical surveillance process. The EOIC cautioned against modifying the standard's medical surveillance provisions on the grounds that these requirements are "well-considered and consistent with other OSHA rules" [Ex. 2-6]. The American Nurses Association urged
OSHA to expand the list of those authorized by the standard to conduct medical surveillance to include "all appropriate health care professionals," as later OSHA health standards have done.
The AHA and others supported the standard's training requirements and stated that they were functioning as intended by OSHA. Dr. LaMontagne urged OSHA to enhance the effectiveness of EtO training by requiring more interactive training approaches and taking care that the trainees' literacy levels and language skills were taken into consideration. The AFL-CIO urged similar improvements to the training requirements. The AHA and the Clariant Corporation asked OSHA to make the training requirements more flexible by adopting a performance-based approach to refresher training and extending the interval between initial and refresher training to three years rather than the one year interval currently mandated.
Several participants in this Lookback review addressed OSHA's enforcement policy in their comments. For example, several groups urged OSHA to continue strong enforcement of the standard. One of these commenters, Dr. LaMontagne, pointed to evidence gathered in his study of Massachusetts hospitals, which showed that enforcement of the standard in that state had significantly enhanced employers' compliance. Dr. LaMontagne also suggested that OSHA rely more heavily that it does at present on EPA inventory release data for enforcement targeting purposes.
AFSCME recommended that OSHA's compliance directive for EtO be revised to include more information on ways to comply with the standard's emergency alert requirements. Several participants made recommendations about protections needed by workers who change EtO tanks, with some urging OSHA to require workers to wear respiratory protection during these operations and others stating that such protection is not necessary if engineering and administrative controls are effective in maintaining exposures during tank changing operations. (OSHA requires the use of respiratory protection during tank changing operations, on the grounds that engineering controls are not generally feasible.)
Many commenters urged OSHA to provide the regulated community with additional compliance assistance materials in the following areas:
- Emergency alert requirements
- Exposure monitoring
- Medical surveillance
- Training
- Respirator use during tank changing operations.
Some commenters, particularly Dr. LaMontagne and his co-authors, attribute at least some of the difficulty employers have had with the standard's medical surveillance and emergency alert requirements to the standard's complexity. Most commenters, however, found the standard relatively straightforward and consistent with other OSHA health standards (see the comments of the EOIC [Ex. 3-2] and the Pennsylvania Engineering Company [Ex. 2-1]).
OSHA received few comments on the issue of overlap, duplication, or conflict between the EtO standard and rules issued by other agencies or levels of government. The concern reflected in these comments was that compliance with the EPA NESHAP for EtO might require the use of pollution control technologies that increase employee exposures to EtO. This issue is discussed further in Chapter V of this review.
A few participants commented on changes in technology that have occurred since 1984, particularly those occurring in exposure monitoring and alarm technologies, sterilizer technology, EtO gas blend usage patterns, and substitution away from EtO use as a sterilant. These commenters emphasized that improvements in these areas had helped to decrease employee exposures to EtO and, in some cases, had reduced the costs of compliance for employers, particularly in the hospital and contract sterilizer industries. Economic and technological changes, and their significance for the regulated community, are discussed further in Chapter V of this review.
CHAPTER V
CHANGES IN TECHNOLOGICAL AND ECONOMIC CONDITIONS
OVERVIEW
The Ethylene Oxide standard was determined by OSHA to be technologically and economically feasible at the time of issuance. Commenters to the docket for this Lookback review (Docket H200-C) have reported that the standard has been and continues to be technologically and economically feasible in practice. In fact, in the years since promulgation of the standard, many technological changes, some of which have resulted in substantially lower costs for the regulated community, have taken place. These advances include improvements in ethylene oxide production processes and sterilizer technology, exposure monitoring technologies, and in substitutes for EtO sterilization. Although some of these changes have enhanced compliance for employers in all EtO-producing, using and handling industries -improvements in exposure monitoring technology is a case in point - the most important changes have
taken place in the industries in which EtO is used as a sterilant.
EXISTING TECHNOLOGY AND CHANGES IN TECHNOLOGICAL CONDITIONS
Chemical Manufacturing Industries
The controls described below in the EtO production and ethoxylation industries were known and in use at the time of the rulemaking, although it is likely that modernization efforts and plant consolidations have resulted in a larger proportion of the facilities in these industries having these advanced systems in place than was the case in 1984. There have been no technological changes in these industries that have significantly impacted the EtO production process or control procedures.
Ethylene Oxide Production Facilities
EtO production facilities and ethoxylators operate large facilities. In these facilities, EtO is typically contained in reaction vessels and piping systems. The tight control of ethylene oxide emissions normally found at chemical plants has typically been related to process stream/recycling improvements and flammability and explosion concerns. Nevertheless, the enclosed nature of many of the processes at these plants and the attention to safety found at many chemical production facilities have also resulted in lowered employee exposures. Other factors contributing to the generally low exposures at EtO production facilities are the high degree of automation and the out-of-doors location of the production units.
Conventional control technologies, many of which were already in use at the time of the 1984 rulemaking, include engineering efforts in production/conversion units that have been used to reduce exposure potential, such as double mechanical pump seals, double seated valves, check valves, plugs on vents and drains, flanged leak detectors, returns for process vents to combustion or recovery systems, remote isolation of EtO pumps, and exhaust hoods at sampling points [Ex. 2 6G]. The following controls are currently in place at some facilities: a centralized sampling system with a vented hood, nitrogen blanketing of railcar contents and nitrogen purging of loading lines, a fixed multi point leak detection system, manual leak detection, double mechanical seals (and associated buffer liquid pots) on pumps, and flushing connections on equipment to facilitate pre maintenance flushing [Ex. 2-6G].
Ethoxylators
Ethoxylation units are usually batch reaction units in which EtO is added to a reaction vessel that is filled with another reactant and one or more catalysts. Ethoxylation reactions are generally carried out in tightly closed and highly automated systems consisting of pipes, reaction vessels, pumps, and heat exchangers. These reaction facilities are generally located outdoors, and workers spend much of their work shift in the control room away from the process equipment.
In some facilities, isolated control rooms, closed sampling systems, vent collection systems, and nitrogen purges are used to control exposures. Other controls are closed loop bomb sampling systems, nitrogen blanketing of the EtO storage tank, an ethoxylation unit designed so that operators do not need to spend much time in the unit near the processing equipment, and vent collection systems.
INDUSTRIES THAT USE ETO AS A STERILANT OR FUMIGANT
Hospitals and commercial sterilizers have both been affected by improvements in the automation of sterilizer systems, improvements in control technology, and changes in the use pattern of EtO as a sterilant. The principal difference between the sterilizers used to sterilize medical products, e.g., baby diapers, and those used in hospitals, is their size. Industrial sterilizers are typically measured in terms of the number of pallets they can accommodate in one cycle.
According to one manufacturer, the industrial sterilizer market begins at sterilizers with a capacity of greater than 100 cubic feet. According to the Cosmed Web site, the world's largest sterilization chamber has a volume of 6,000 cubic feet.81 Contract sterilizers typically have sterilizers with capacities ranging from 1 to 24 pallets (24 pallets is a full 40 foot truck load). Most facilities have many sterilizers at a single site.
Commercial sterilizers often use gas diffusion systems to ensure that medical and other sensitive products are sterile. One such system is the Sterijet system, which combines a package sealer with an EtO injection machine. The sterilization process begins with placement of the finished product inside a bag. The mouth of the bag is placed inside the processor. The processor removes excess air from the bag, and then injects a preprogrammed amount of vaporized EtO into the bag. After the injection nozzle withdraws, the bag is heat sealed. After bags have been filled, evacuated, and injected with sterilant, the bags are transferred to a temperature controlled room with specially designed ventilation. The room is heated, which provides heat for both the diffusion and sterilization process. As aeration progresses, and EtO diffuses out of the package, the bag shrinks around the inner sterile
product. This vacuum packing protects the product from damage and provides an indication if the package seal is broken. Because of the design of the special packaging, this process uses less EtO, cuts the time and energy required to aerate products to FDA mandated residual levels, and reduces the demand on the facility's emission control system. See Cosmed brochure, from: http://www.cosmedgroup.com/homepage.htm. Devices sterilized with the system "use 30 to 80 % less EtO than industrial vacuum/pressure vessels achieving the same SAL [sterility assurance level]."82
Since 1985, the most dramatic changes in sterilization were stimulated by EPA's ban on the use of chlorofluorocarbons (CFCs). This has caused employers to shift away from the 88 percent CFC and 12 percent EtO mixture traditionally used for sterilization to the use of 100% EtO, other EtO blends, or non-EtO-using sterilants. Some of the newer EtO blends are described below.
Hydrochlorofluorocarbons (HCFCs) and carbon dioxide can both be used to form a sterilant mixture with EtO. Such mixtures are considerably more expensive than 100 percent EtO, especially for large sterilizers. In a mix with EtO, carbon dioxide provides stability only in high proportions. Use of a carbon dioxide and EtO blend also reduces the efficacy of EtO sterilization. Meanwhile, although HCFCs are being used in smaller proportions, they are ozone depletors. The Clean Air Act places HCFCs under Class II, which requires warnings regarding HCFC's ozone depleting potential, beginning in the year 2015.83
According to EPA, 100% cartridges are typically used in larger, dedicated sterilizing facilities, while blends are used in smaller laboratory and health care settings. According to EPA's Office of Pesticides, in 1997, there were seven companies holding FIFRA registrations for EtO products. The current blends include 100% EtO, 20% EtO/80% CO2, 8.5% EtO/91.5% CO2, and 30% EtO/70% CO2. With regard to HCFC blends, there are 12%EtO/80% dichlorodifluoromethane, 8.6% EtO/91.4% chlorotetrafluoroethane, 10% EtO/27% chlorodifluoromethane, 63% chlorotetrafluoroethane, and 10% EtO/90% undisclosed HCFCs.
Sterilization processes increasingly use pure EtO. This trend raises concerns because EtO is highly flammable and explosive. Sterilization equipment using EtO must meet fire hazard codes and requires more frequent replacement of smaller EtO storage tanks, which can lead, in turn, to a greater potential for worker exposure. When using 100 percent EtO, there must be additional safeguards in place. For example, all electrical components must be located in hazardous or special rooms; i.e., sterilizer, gas dispensing and scrubber rooms rated for explosive gases. These Damage Limiting Construction (DLC) rooms consist of a system of walls that are pressure retaining and venting and direct any explosions away from occupants.84 Use of pure EtO also requires compliance with 29 CFR 1910.106, OSHA's standard on the handling of flammable liquids.
There are some advantages associated with the use of 100 percent EtO, however. First, EtO sterilizers are relatively inexpensive, and operating costs are lower than when using the EtO/CFC mixture. Second, the risk associated with leakage of EtO/CFC cylinders is reduced with the use of pure EtO because the gas cartridges are placed entirely within the sterilizer and are only perforated at the beginning of the cycle. Negative pressure in the chamber ensures that gas does not escape to the outside.
The disadvantages of 100 percent EtO are that the sterilization cycle is long, up to 16 hours, because the sterilized products need to be aerated as well as sterilized. A recent study indicates that the ability of 100 percent EtO to kill micro organisms, and, therefore, the margin of safety for 100 percent EtO, vaporized hydrogen peroxide, and ion plasma sterilizers is less than that of a sterilizer using the 88 percent CFC and 12 percent EtO mixture in the presence of products containing serum or salt.85 Table V-1 shows the advantages and disadvantages of various sterilant mixtures and processes.
Table V-1
Advantages and Disadvantages of Different Methods of Sterilization
(As Alternatives to EtO Sterilization)
| Sterilization Method |
Advantages |
Disadvantages |
Ethylene Oxide
Larger sterilizer units or table-top sterilizers that use single-use EtO cartridges are used. Items are washed, packaged, and placed in baskets or racks before being loaded in the sterilizer. The sterilizer is sealed and the cycle started. EtO is highly toxic for all microbiological life forms
Exposure Limit : OSHA has a 1 ppm TWA PEL and NIOSH a 0.1 ppm TWA REL |
Ideal for heat and moisture sensitive equipment.
Temperatures used for EtO sterilization are much lower than those used in steam or dry heat sterilization.
EtO can permeate through wrapped or prepackaged materials |
Toxic to humans and the environment.
Sterilization takes approximately 10-24 hours
Certain plastics may deteriorate during EtO sterilization
Costs relatively more than other methods of sterilization like steam or radiation |
Gas Plasma
Radio frequencies or Microwave energy is used to generate an electrical field that changes hydrogen peroxide gas into plasma. As a result, reactive species including hydroxl and hydroperoxyl radicals are formed which are responsible for sterilization.
Exposure Limit : Hydrogen peroxide has an OSHA and NIOSH standard of 1 ppm TWA |
The system does not produce any toxic end products; the water and hydrogen produced are harmless.
Risk of exposure to hydrogen peroxide vapor is low since the sterilizer door cannot be opened when hydrogen peroxide is inside the chamber.
It is a lower temperature process
Short sterilization cycle time of approximately 1 hour
Can be used for most metals and plastics |
The sterilization will not occur if organic material is present. Contamination causes decomposition of sterilizing agent.
It cannot sterilize materials such as linen, paper, cotton, powders, and liquids since they tend to absorb hydrogen peroxide. Also, only wrapping materials made of low- absorbing non-woven proplene can be used.
The penetration of this system may not be as effective as with ethylene oxide.
The sterilizer cannot sterilize long or narrow lumens without the assistance of a "diffusion intensifier" |
Steam Autoclaving
Sterilization is achieved in a self-locking machine (autoclave), by steam under high pressure and temperature |
Non-toxic to humans and environment, simple, and efficacious
It is the cheapest method of sterilization
High pressure ensures saturation of wrapped surgical packs |
Cannot be used for heat-sensitive plastics, rubber, and delicate electronic devices that comprise a large fraction of materials used in modern medical care |
Radiation
High energy ionizing radiation (gamma rays or electron beams) destroys microorganisms and is used to sterilize prepackaged surgical equipment
Exposure Limit : Regulated by OSHA according to maximum REMS of radiation allowable for different body parts in each calendar quarter |
Useful for materials that cannot be sterilized by heat or chemicals
It is as effective as EtO and there is no holding time after sterilization
Is highly penetrating, leaves no chemical residues and requires no post-sterilization testing
Cheaper than EtO, especially at high annual outputs |
Some products are degraded by radiation. Pacemakers and certain plastics cannot withstand the radiation dose required for sterilization
The use of radiation for in-hospital applications is impractical and is viable for use only within industrial facilities. |
Vapor Phase Hydrogen Peroxide
This system uses a vacuum to pull a 30% vapor solution of hydrogen peroxide into a heated vaporizer
Exposure Limit : Hydrogen peroxide has an OSHA PEL and NIOSH REL of 1 ppm |
The liquid sterilant hydrogen peroxide is toxic neither to humans nor to the environment.
It is a low temperature process
No aeration of treated materials is required
This process requires only a 90 minute cycle |
It cannot be used for highly absorbent materials (such as cellulose) and is corrosive to materials such as nylon. It is also incompatible with iron and some plastics (which are susceptible to oxidation by hydrogen peroxide) |
Glutaraldehyde
This chemical is bactericidal, fungicidal, viricidal, and sporicidal. It is commonly used as a disinfectant, rather than a sterilant. Articles are totally immersed in a 2% to 3.2% solution of glutaraldehyde for 10 minutes for disinfection.
Exposure Limit : New ACGIH Ceiling Limit of 0.05 ppm TLV as of May 1997. |
Potential for use as alternative to EtO, within health care facilities since it is a high level disinfectant/steriliant (if left in solution for required time) |
Use of this chemical precludes the prepackaging of items
Handling operations subsequent to immersion may lead to recontamination
Instruments must be dry before immersion since wet items can lead to dilution of the solution
Up to 3 hours time needed to kill spores.
Repetitive use can reduce chemical efficacy of the solution, especially if the instruments are not clean
Less effective than use of EtO
Causes industrial asthma, severe allergic reactions, contact allergies; mucus membrane irritant |
Ozone
Oxygen is converted into ozone by an ozone generator inside a sterilizer, which is then humidified. Ozone's sterilization potential arises from the fact that is one of the most powerful oxidizing agents available
Exposure Limit : Both OSHA's PEL and NIOSH's REL for ozone are 0.1 ppm. |
Ozone can be quickly converted to non-toxic oxygen which is the end product of the process |
Ozone can be highly corrosive to many plastics, most metals, and rubber
Sterilization time data are highly variable; it can take from 1 to 3 hours for sterilization to be completed |
Peracetic Acid
The peracetic acid which is designed to be used only with the sterilizer is diluted with sterile water to 0.2% and circulated with the machine to sterilize all instruments it comes in contact with. Chemically, peracetic acid used for sterilization is a equilibrium mixture of hydrogen peroxide, peracetic acid, and acetic acid
Exposure Limit : OSHA's PEL for hydrogen peroxide is 1 ppm and 10 ppm for acetic acid |
It is a good method to sterilize heat sensitive items. It is compatible with plastics, rubber, and other heat sensitive items
Quick turnaround time for sterilization, complete cycle takes steam and EtO sterilization
Diluted peracetic acid solution is not hazardous for humans or the environment
Works even in the presence of organic material , though items must be thoroughly clean before being placed in the sterilizer |
Non-immersible scopes are not sterilizable
Maximum capacity of one flexible scope per sterilizer cycle
Biologic monitoring is not self-contained
In concentrated form, i.e., as greater than a 30 percent solution, it is corrosive to equipment and irritating to human tissue. |
Chlorine Dioxide
Chlorine dioxide has sporicidal properties and its gaseous sterilization process is very similar to that of EtO.
Exposure Limit : Both OSHA and NIOSH have a PEL and REL of 0.1 ppm and NIOSH also has a 0.3 ppm STEL. |
It is not ozone depleting and the nitrogen carrier gas is inert.
Relatively low in capital equipment and operating costs. |
Cannot be shipped and stored and must be generated on site, which would increase the complexity of the sterilizer design.
It is unstable and classified as a hazardous substance. |
Sources:
- Crow, Sue, "Sterilization Processes: Meeting the Demands of Today's Health Care Technology," Nursing Clinics of North America, September 1993, Vol. 28 No. 3, pp. 687-695.
- Crow, Sue, "Gas Plasma Sterilization - Application of Space Age Technology," Infection Control and Hospital Epidemiology," August 1995, Vol. 16 No. 8, pp. 483-487.
- Phillips, G. Briggs, "Critique of EPA's Preliminary Benefit Analysis of Ethylene Oxide," Health Industry Manufacturers Association (HIMA) Document No. 11 Vol. 1, December 1979.
- Shintani, Hideharu, "The Relative Safety of Gamma-Ray, Autoclave, and Ethylene oxide Gas Sterilization of Thermosetting Polyurethane," Biomedical Instrumentation and Technology, November/December 1995, pp. 513-519.
- American Hospital Association, appendix to comments on "Occupational exposure to Ethylene Oxide, 29 CFR 1910.1047," OSHA Docket H-200, July 30, 1997.
|
Hospitals
This Lookback review has shown that hospitals continue to be the major users of EtO sterilization processes and to employ the largest part of the EtO-exposed workforce, by far. Many changes in sterilizer technology occurred about the time the 1984 rule came out or shortly thereafter. In the years immediately preceding the rule, engineering controls were available that had the capability of reducing EtO worker exposures in hospital sterilization operations to below 1 ppm (TWA). These controls were generally retrofit controls, such as post cycle air purges or exhaust hoods installed over the sterilizer door. During 1979 to 1984, the three largest sterilizer manufacturers produced add ons: local exhaust ventilation (LEV) hoods, aerators, EtO supply tanks, and drain seals, and the addition of purge or air flush cycles.86 Beginning in 1982 or 1983,
companies began to include these controls as built ins. Examples included built-in local exhaust ventilation hoods; in chamber aeration; and reductions in the amount of EtO used during each sterilization cycle.
At the public meeting for this Lookback review, Mr. Pier George Zanoni of the MHA and AHA indicated that sterilizer technology featuring built in aerators as a control technology had helped in many smaller hospitals. Mr. Zanoni also commented that many smaller hospitals have switched to 100 percent EtO cartridges since EPA's ban on CFCs had forced them to shift from an 88 percent CFC/12 percent EtO mixture [Tr. Vol. 1, 6/30/97, p. 20].
Large hospitals have bought new sterilization equipment similar to that used by commercial sterilizers. (See descriptions below.)
Commercial Sterilizers
The process in commercial sterilization facilities involves materials that are palletized. Packaged products on pallets are placed in a holding area, and are then transferred to a preconditioning room. These rooms are temperature controlled (from 100 F to 120 F) and humidity controlled (from 45 % to 75 % relative humidity). The lots remain in the room for 24 to 96 hours. At this stage, the products are heated and moisture is introduced; in the next stage, the lots are placed into the sterilizer. Products are sterilized for lengths of time ranging from 5 to 12 hours, depending on material, packaging, and sterility assurance level. Products are then taken from the sterilizer to aeration rooms or cells, where they are typically aerated for 18 hours to ensure that EtO traces are minimal. The product is then quality checked and shipped back to the customer.
In the years since the standard was issued, the trends in commercial sterilization have been toward computerization, emissions control technology, and automation. In addition, some large sterilization facilities have installed new and improved sterilization systems that use 100 percent EtO, which are intrinsically safer than the older versions.
For example, Environmental Tectonics Corporation (ETC) is one of the companies producing an automated sterilization system that uses 100 percent ethylene oxide for sterilization.87 ETC offers ethylene oxide sterilization systems for the pharmaceutical, medical device, food and hospital industries. According to ETC, the 100 percent ethylene oxide sterilization system eliminates the use of ozone depleting CFCs and is, therefore, an environmentally conscious method of EtO sterilization that also increases productivity and minimizes costs while assuring worker safety.
The ETC product, and others like it on the market, has a very tight emissions control system that ensures worker safety while the in chamber circulation of the gas produces uniform sterilization of the products to be sterilized; the system is also fully automated. Safety features in these sterilizers include Door Logic Controls that prevent opening of the door during potentially hazardous conditions. The rear chamber port in the sterilizer is typically programmed to exhaust air from the chamber when the door is open, thereby forcing in outside clean facility air through the chamber.88 In some other sterilizers, the duct leading to the ventilation system at the rear of the sterilizer is blocked during the sterilization cycle by a butterfly valve, which is automatically activated when the door is opened.89
Emissions Control Technology
To develop control technology to comply with the EPA standard for EtO emissions, Griffith Micro Science reported to the docket that it is evaluating an alternative scrubber and absorber technology to control low EtO concentration streams. This commenter expressed concern that the new designs could have a negative effect on workers because they could increase the EtO concentration in some areas, such as the product aeration room.
There have also been further refinements of emission control systems. For example, ETC is also producing what it considers a final component of the ethylene oxide sterilization process, the EPAS Emission Control System.90 This patented process absorbs EtO from exhaust gas streams and converts it to ethylene glycol, a neutralized form of EtO. The company claims that this is achieved in a high efficiency, high volume, and low cost fashion. The system can be used with existing sterilizers or be included in new sterilization facilities; it can handle multiple sterilizer exhausts simultaneously. The system is also capable of handling all mixtures of EtO.
Sterijet and EO Gas Sterilizers
Gas diffusion technology is a fundamentally different approach to in house sterilization. According to the manufacturers, use of this technology allows for more economical compliance with EtO regulatory requirements.91 Use of this technology also controls gas emissions more effectively and reduces gas use compared to sterilization by large vacuum /pressure vessels, since there is no chamber dead air space to fill.
The two gas diffusion systems currently available are both produced by H.W. Anderson Products. The Sterijet system is designed for large scale production, while EOGas sterilizers are best suited for health care and small batch manufacturing. The EOGas sterilization system is considered a viable alternative to low temperature sterilization problems by the publisher of a journal focusing on sterilization techniques.92 The EOGas system delivers single load doses of EtO by means of EtO cartridges, and the 16 hour sterilization process includes aeration time. The technology allows several cycles to be initiated in the same cabinet. The unit cartridges can be handled and stored safely and contain only enough EtO to sterilize the contents of the bag for which they are designed. The EOGas sterilizer is safer than sterilizers that use EtO gas blends, since
there are no leaking tanks, according to one sterilization supervisor at a Bronx, N.Y. hospital.93
Gas diffusion sterilization can also easily be integrated into the medical device manufacturing environment. In addition, because the products can be continuously processed at the same conditions under which they were manufactured, climatic variations that cause condensation on product and packaging can be avoided.
Computerization of the Sterilizing Process
Many of the large contract sterilizing firms use computers to analyze and monitor the total sterilization process, and take appropriate corrective action if the computer indicates that such action is necessary; this aspect, of course, also makes the sterilization process more efficient. The technologically advanced hardware and software used in these systems provide timely warnings if conditions arise that might lead to an event compromising the integrity of the sterilization process or the safety of anyone in the facility.94 For example, the Digital Dynamics Sterilizer Control System used by Griffith Micro Science consists of a computer with all the necessary inputs and outputs to control the sterilization equipment as well as a manual override capability, whose use is documented by computer.95 Also, there
is no need for human intervention in the newer installations where automatic conveyer belts transport products through the various phases of the sterilization process.
One contract sterilizing company is reporting use of a new technology for controlling the release of EtO. According to the company, this new "parametric release" technology permits the optimization of sterilization cycles using EtO concentrations of 200 400 mg/L instead of the 800 1200 mg/L used by other systems and also eliminates the need for the biological indicators traditionally used to indicate successful sterilization; these features make the sterilization process more efficient. As a result, sterility testing costs are significantly lower and sterilized products may be shipped to the market immediately after sterilization.96
ETO PRODUCTION, USAGE, AND SUBSTITUTION TRENDS
Production Trends in the Chemical Manufacturing Industries
As reported in Chapter II, the U.S. ethylene oxide production industry has operated at annual capacity rates generally in the 84 89% range since 1985. Further, this industry has added over 2 billion pounds of capacity since 1988. This capacity increase resulted from the addition of two new plants as well as significant capacity improvements made to six other existing plants. Domestic production of EtO had risen from 5,957 million pounds in 1984 to 7,275 million pounds in 1996.97
Usage and Substitution Trends Within Industries Using EtO as a Sterilant Or Fumigant
There have been many changes since 1984 in the sterilizing medium used by industries for their sterilizing needs. Although EtO has long been a reliable method for sterilizing and fumigating different products, gamma and E beam sterilization have been successful in capturing a substantial share of the sterilizing market. Attitudes in various industries toward different sterilization technologies are based on cost factors, avoidance of regulatory issues, efficacy, and ease of use. There are an increasing number of substitutes for EtO sterilization processes, but not all of these can be used for all uses. Many organizations that use EtO for sterilizing a variety of medical devices and equipment maintain that it is irreplaceable as a sterilant. For many of its other uses as well, no comparable substitute has been identified. Medical devices sterilized with EtO include essential and lifesaving
tools used in every phase of health care delivery; they include heart pacemakers, dialyzers, heart valves, and respiratory care nebulizers. EtO sterilization capabilities possess the following advantages over other sterilizing methods:98
- EtO is highly diffusive. As a result, it can penetrate areas that are not ordinarily accessible to liquids or steam.
- Since EtO is very penetrating, products sealed in packages or cartons can be sterilized by it.
- EtO causes little damage to materials exposed to it.
- EtO sterilization requires lower temperatures than those required for dry heat or steam sterilization.
- EtO sterilization provides flexibility for sterilizing a variety of device designs or a large number of device combinations. EtO sterilization methods are preferred for custom kits that involve multiple devices of different materials.
Over the years, FDA inspections confirm that devices sterilized with EtO maintain an impressive sterile device compliance record. Alternate methods of sterilization (and an increasing number of them have become available over the years), may not have as proven a compliance record.
Among the many substitutes available for EtO sterilization, some may be good substitutes for the still widely used EtO sterilization methods. The worldwide sterilizer market consists of about 25,000 EtO systems, almost half of them in use in the United States, with approximately 10 percent replaced each year.99
Equipment using EtO substitutes for sterilization is increasingly available. An article published in Biomedical Market Newsletter in 1995 reported that a market research study conducted by the market research firm of Frost and Sullivan had concluded that between 1994 and 2001, alternatives to EtO sterilization would be one of the most promising market segments in the $679 million hospital infection control product market. The same study projected a strong compound annual growth rate of 8% in the hospital infection control product market over the same time period, reaching nearly $1.2 billion by 2001.100
Other materials and processes have been considered for use as EtO substitutes for sterilization, such as ozone, vapor phase hydrogen peroxide, gas plasma systems, methyl bromide, and radiation. Some of these other sterilization methods may not pose as great a health risk as ethylene oxide, when used according to proper instructions, but may present other disadvantages. Some of these and other technologies for sterilization, like the use of peracetic acid, vapor phase hydrogen peroxide, hydrogen peroxide plasma, and formaldehyde liquids currently are costly and difficult to use in a wide variety of settings; almost all of them also have exposure limits regulated by OSHA and recommended by other agencies (see Table V-1 for comparisons of EtO substitutes). Some of these and other alternatives to EtO in current use are discussed below.
Steam
Steam can be used to sterilize some medical instruments, but steam sterilization involves the use of extremely high temperatures and pressures. Not all devices and materials can withstand these conditions. Steam is one of the most cost effective methods of sterilization when it can be used, however. An EtO-resistant mold, Pyronema domesticum, was recently discovered in EtO processed cotton of Chinese origin. This led to a resurgence of steam sterilization for cases where products might be degraded or discolored by radiation doses that produce sterility.101
Gamma Radiation
Radiation is a fast and effective method of killing bacteria that leaves no toxic residue after sterilization is complete. Increased regulatory control of EtO and growth in radiation resistant plastics led to growth in the gamma sterilization market in the 1980s. Gamma sterilization use was primarily limited to simple products, because companies resisted the research and development investment that would have been necessary to convert more complex devices to gamma radiation sterilization. Nevertheless, between 1980 and 1990, gamma radiation gained 25 percent of the medical device sterilization market due to a growth in the construction and operation of gamma facilities.102 Gamma radiation can affect the molecular structure of substances like propylene and damage products made out of these substances. However, radiation stable grades of most medical
polymers are available from resin manufacturers. Contract sterilization facilities perform most of the gamma sterilization being done today because of the large expense involved in the construction of such facilities.
Electron Beam
Electron beam (E beam) sterilization is another method that has recently increased in use and represents approximately 10 percent of the disposable medical device market. This process uses electrons, can provide a small dose range, and is environmentally friendly. The cost of setting up an E-beam facility is high and, therefore, is limited primarily to contract sterilization facilities.
Gas Plasma
Gas plasma sterilization involves the use of non toxic gases. Gas plasma generates gaseous elements that surround items being sterilized with a rarified atmosphere that is effective at killing bacteria. Johnson and Johnson's low temperature gas plasma STERRAD Sterilization System is reputed to be a fast, safe, and effective sterilization process.103 According to an article in Hospital Materials Management, the list price of the unit in 1996 was $108,000. At that time, Johnson and Johnson reportedly had a greater than 90 percent share of the market.104 This system is available worldwide as a general purpose sterilizer and generates a low temperature gas plasma from hydrogen peroxide. There are no chemical emissions produced during the hour long sterilization cycle. Because
there are no residues, there is no need for an aeration phase.
The STERRAD System can sterilize most medical equipment. An article published in Hospital Materials Management reported three case studies in which hospitals reported significant cost savings from implementing the STERRAD system. A 144 bed hospital in Washington reported first year savings of $100,000 in instrument costs. A 267 bed hospital in Pennsylvania reported $130,000 in operating cost savings over a three year period of use, and a 604 bed hospital in Georgia reported an annual reduction of $24,400 in its instrument repair costs.105
Since 1994, a gas plasma sterilizer called the Plazlyte Sterilization System from AbToX has become available.106 According to AbToX, the company manufacturing this sterilizer, its previous sales were in Europe. The APlazlyte" uses a mixture of hydrogen, oxygen, and inert gases alternatively with peracetic acid vapor.107
Ozone
The Ster O3 Zone 100 is a new ozone sterilizer that, according to its manufacturer, is compact, easy to operate, and cost effective in both hospitals and manufacturing settings. The FDA is considering marketing clearance for the first such sterilizer. Ozone sterilization does not result in harmful emissions to the atmosphere and the environment because, after sterilization is complete, the ozone converts back to oxygen before being released into the environment. EtO sterilizers can also be converted to function as ozone sterilizers.108
EtO Usage and Substitution Trends in Hospitals
In a study of Massachusetts hospitals conducted in 1993, 92 of the 159 hospitals surveyed reported using EtO.109 Sixty two of the hospitals reported that EtO was not used on site and 21 of the Anon users@ reported that they sent out some of their materials for sterilization, mostly to neighboring hospitals. Some changes in hospital usage occurred as a result of new and pending EPA regulations. Twenty six percent of EtO using hospitals used 100 percent EtO at the time of a 1992 survey;110 63 percent used an 88/12 EtO/freon mixture, and 11 percent used both sterilization methods. According to the study, as of 1993, many hospitals were considering switching to 100 percent EtO, 90:10 carbon dioxide/EtO mixtures, or "drop in" replacement mixtures because of the phase out of CFCs. The study also indicated
that the average number of EtO loads processed in a typical week in EtO-using hospitals was 10.
In testimony presented at the public meeting for this Lookback review, Mr. Pier George Zanoni of the MHA and AHA indicated that many smaller hospitals have switched to 100 percent EtO cartridges since EPA's action on CFCs forced them to shift from the 88 percent CFC/12 percent EtO mixture they used before [Tr. Vol. 1, 6/30/97, p. 20].
Hospitals in some areas have switched to contracting out for their sterilization needs. For example, in California, which has particularly strict air pollution regulations for ethylene oxide emissions, many hospitals have switched to using contract sterilizers.111
Hospitals can save money by reducing the amount of EtO sterilization they conduct. For example, a case study of an industrial hygiene/engineering redesign of a hospital sterilization facility recently reported that an evaluation of the need to use EtO to sterilize equipment led to a reduction of 50 percent in the quantity of items requiring EtO sterilization. Using different sterilization methods (e.g., steam sterilization) and cleaning processes for these items did, however, involve an increase of approximately $50 in operating costs per day. However, these costs were more than offset by the savings of about $180 per day generated by the reduction in the amount of EtO purchased.112
Another way some hospitals have saved money on sterilization costs is to turn to single use instruments, which may be more cost effective than reusable instruments. For example, a study conducted by Deloitte & Touche for a disposable instrument producer found that the use of single use instruments was a more cost effective alternative than reusable instruments for laparoscopic cholecystectomy procedures.113 Nine hospitals were surveyed for this study. Each of the hospitals had customarily used a mixture of reusable and disposable instruments.114
The cost analysis performed for this study included the purchase price of instruments, plus labor and supply costs for cleaning and sterilization, and the repair and maintenance costs associated with reusable instruments. The study found a variety of hidden costs related to cleaning and sterilizing reusable instruments. The report found that the average total cost for a standard mix of disposable and reusable instruments on a per procedure basis was $740. The cost of using all disposable instruments was $725. The report concluded that hospitals could replace existing reusable instruments with disposables and simultaneously reduce the risk of nosocomial infections and improve instrument performance.
EtO Usage and Substitution Trends at Commercial Sterilizing Facilities
Although contracting out may not be an option for some hospitals, medical device and spice manufacturers are increasingly turning to contract sterilizers and fumigators to meet their needs in a more cost effective manner. Contract sterilizing facilities also provide their services to pharmaceutical, cosmetics, seed, and packaging industries. Turning to contractors is a logical response to the need to avoid worker exposure to EtO and to concerns about releases of ethylene oxide to the environment.
Medical Device Manufacturers
For medical device manufacturers, the use of EtO gas sterilization, which had been exhibiting an upward trend, began to stabilize in the late 1980s when sterilization with gamma radiation became a viable alternative. When regulatory issues began to be associated with EtO, between 1980 and 1990, gamma radiation captured 25 percent of the medical device sterilization market. Although some medical device manufacturers still use EtO for sterilization purposes, more than half are using contract sterilization services to bypass EtO exposure for their workers and to take advantage of the economies of scale that come with use of sterilization contract services.
The use of EtO by health care manufacturers seems to be declining. Although EtO substitutes are not always feasible in these applications, replacement is occurring. A survey of health care manufacturers also discovered that gamma sterilization is considered superior in terms of quality of sterilization and process reliability, although it ranks equal to e-beam sterilization in this regard. Both of these sterilization processes were also ranked significantly higher than EtO sterilization. Short-term predictions also included a move from in-house EtO use to contracting out.
A breakdown of the sterilization methods used for medical products in 1995 revealed use of EtO to be 52 percent, while radiation was used in 46 percent of the instances. Steam comprised less than 2 percent and other methods 0.1 percent of all methods used.115 Over that period, there has been a noticeable trend toward contracting out the sterilization of single use medical devices. According to one survey, a total of 87 percent of health care manufacturers are currently using contract sterilization services.116 In 1996, 51 percent of the manufacturers using contract services were very satisfied with the service received, based on factors like turnaround time, technical expertise, competitive rates, and quality of working relationship with individual contractor staff. Companies experienced an average turnaround
of 8 to 12 days for items sterilized with EtO and 5 to 6 days for those sterilized with gamma radiation.117
Spice Manufacturers
According to a 1995 1996 survey by the American Spice Trade Association (ASTA),118 approximately 15 percent of imported and domestic spices were treated with EtO and approximately 6 percent were treated with alternative methods. In 1989, ASTA reported that the percentage of spices treated by EtO and alternative methods was approximately 22 percent and 6.8 percent, respectively. Although this survey could be interpreted to suggest that the use of EtO sterilization has declined in the last six years, the 1995 1996 survey captured only approximately 70 percent of the total sales volume of spices. Since the manufacturers of EtO state that the use of EtO by the spice industry has remained constant, ASTA considers it reasonable to conclude that the apparent 6.8 percent decline in the use of EtO since 1989 is accounted for by those companies not responding
to the survey and that the use of EtO has remained fairly constant from 1989 to 1995 1996. ASTA therefore concludes that the use of EtO by the spice manufacturing industry has been fairly constant over the years.
One hundred percent propylene oxide is sometimes used for in house sterilization in spice manufacturing facilities; however, propylene oxide is not as effective a sterilant as EtO. The fact that propylene oxide is subject to fewer regulatory controls than EtO is one reason given for its use as a sterilizer.119 Microwave sterilization has also been attempted by spice manufacturers, but this method is not as effective as EtO or gamma radiation. The two preferred sterilization methods for most spice manufacturers are EtO and gamma radiation.120
ECONOMIC CHANGES
Costs Associated With the Use of EtO Sterilization
The U.S. market for all types of sterilizer equipment (e.g., steam, EtO, and other types of sterilizers) peaked in 1992 1993 at around $200 million for both manufactured and remanufactured sterilizers. In 1996, the manufactured and remanufactured sterilizer market was estimated to be around $150 million to $175 million in annual sales. A decline in medical capital equipment sales, driven in part by concerns about the passage of health care legislation, particularly affected U.S. sterilizer manufacturers, resulting in changes in ownership of the two dominant firms in the industry. According to an article in Biomedical Market Newsletter, uncertainty about health care reform efforts in 1992 and cost cutting efforts and consolidations occurring industry wide at that time discouraged hospital administrators from making planned investments in capital equipment.
However, the reform was defeated and after that this factor would not play a role. Demand for sterilizer equipment was also reduced due to the discovery that existing sterilizer equipment did not become obsolete and thus its service life was longer than expected.121 Two major companies, AMSCO and MDT/Castle, dominate the market. . According to sources cited in the article, the average cost of a hospital sterilizer dropped by about $10,000 from 1992 to 1996.
In 1995 and 1996, the current total U.S. market size for new technology sterilizers, such as gas plasma sterilizers and paracetic acid sterilizers, exceeded $50 million and was estimated to have a growth rate in excess of 20% annually.122 However, neither of these technologies is effective or approved for all types of medical devices.
REDUCED COSTS OF ETO STERILIZERS AND 100% ETO SYSTEMS
New EtO sterilizer models are now available for almost half the cost of the ones available in 1984, and the costs of compliance today are thus significantly less than they were estimated to be in 1984. To come into compliance with the OSHA EtO standard, many hospitals needed to use only general dilution ventilation and improved work practices and did not have to purchase and install any additional equipment.123
With advances in EtO sterilizer designs, such as local exhaust ventilation and purge controls incorporated directly into the sterilizers, new equipment does not need add-on engineering controls to achieve the levels mandated by the standard. For the sterilizers in use in 1982, a retrofit package consisting of a chamber purge system was required to reduce EtO exposure levels to the 1 ppm level. For instance, a retrofit package available for an Amsco sterilizer in 1982 was priced at approximately $3500.124 In 1996 prices, the retrofit would cost $5700.125
Ethylene oxide sterilizers by Amsco, with built in aerators, but otherwise parallel to the ones used in 1982, could be obtained in the mid-1990's for as little as $4500.126 Thus it is possible to purchase an improved EtO sterilizer with built in controls at a price that is actually less than the cost of a retrofit package in 1996.
The cost of the 100 percent EtO using sterilizers made by H.W. Anderson products range from approximately $30,000 for a 6 cubic feet chamber to $60,000 for a 33 cubic feet chamber for their EOGas sterilization system. A 100 percent EtO automated sterilization system, used by the industrial market, costs between $130,000 for a 100 cubic foot sterilizer and $1 million for high-end automated systems of 1,500 cubic feet or greater.127 The high end automated systems are primarily used by the large contract sterilizing companies. Smaller sterilizers like the EOGas and 3M models are primarily used by hospitals.
Operating costs for sterilizers are dependent on size as well as the components of the sterilizing medium. Using 100 percent EtO is cheaper than non-explosive blends with CFCs, carbon dioxide, or HCFCs. Since companion gases do not help in the actual process of sterilization and more of the sterilizing medium has to be used in large automated sterilizers, the mixtures are neither as effective nor as cost efficient as the use of 100 percent EtO. The cost of using these mixtures for high volume sterilization may be high.
The proposed EPA Clean Air Act NESHAP would have required that 100 percent of EtO emissions from sterilizer exhaust be captured which would have increased the cost of sterilizing with EtO.128 The prior requirement was for 99 percent of EtO emissions to be captured.129 The final amendments, however, only require the capture of 99% of emissions.
Costs Associated With Using Alternative Sterilization Systems
The cost of gamma processing is competitive with both EtO and E beam sterilization for some applications. Because of the large costs involved in constructing a gamma facility, contract sterilization facilities perform most gamma sterilization. If more substitutes for radiation sensitive plastics emerge, there may be an increase in the market for gamma sterilization.130 E beam sterilization is perhaps the cheapest method of sterilization where sufficient penetration of the delivered dose is attained.131 Steam technologies require a capital investment of approximately $1.5 million.
For spice manufacturers, the irradiation of spices is more expensive than EtO sterilization per pound of spices. The cost of establishing an irradiation facility is approximately $2 million more than establishing an in house EtO facility.132
According to AbTox Inc., the producer of the gas plasma sterilization system called the "Plazlyte Sterilization System," the use of its system would cut the sterilization expenses of an EtO-using hospital by as much as two thirds.133 Its sterilizer had a list price in 1995 of $115,000 per unit, and the average cost per cycle was $20. The STERRAD Sterilization System manufactured by Johnson and Johnson sold at $106,000 for a single unit in 1995, about $9000 less than Plazlyte. The entire sterilization cycle with the J + J system is completed in about 75 minutes. Johnson and Johnson states that its system will save a hospital user more than $30,000 per year over 100 percent EtO operations.134 Thus, to the extent that gas plasma can replace EtO, worker exposure to EtO is eliminated and sterilization
costs are reduced if the producers of the equipment's claims are accurate.
For the sterilization of specific products, EtO substitutes can be both effective and cost saving. For example, according to an infection control professional at Children's Hospital in Boston, treating semi critical equipment with pasteurization disinfection technology instead of EtO sterilization can reduce reprocessing costs without increasing risks to patients.135 According to this source, the pasteurization process is proven and effective in keeping microbial levels down and has saved the hospital at least $35,000 over a six month period. The pasteurization processing includes respiratory therapy and anesthesia equipment, which were formerly designated for EtO sterilization only. After using the process for almost one year, no increases in enteric or non enteric gram positive bacteria have been discovered.
Economics of Outsourcing EtO Sterilization To Contract Sterilizers
For non users of contract sterilization, in house sterilizing operations allow for cheaper processing costs if it can be done efficiently, faster turnaround times, greater control, and reduced shipping costs. Use of contract sterilization by a company allows for highly competitive processing costs for some applications, avoidance of regulatory constraints, and the opportunity to save or deploy investment funds. According to a 1996 survey, in house sterilization costs per cubic foot were around 30 to 40 percent less than the costs for contract sterilization and over 50 percent less than the costs for gamma sterilization.136 However, a 2004 article states that contract sterilizations lead to decreased costs, K. Hemmerich, et al., Sterilization Methods Stand the Test of Time, MDDI, Aug. 2004, devicelink.com. But the widespread use of contract sterilization
indicates that many users must find the overall benefits of contract sterilization higher.
Technological Changes in Exposure Monitoring Methods for Ethylene Oxide
Air sampling to evaluate employee exposures to ethylene oxide has historically been a challenge, primarily because of this chemical>s reactivity. Prior to the 1970s, air samples for ethylene oxide were collected in large Mylar bags for direct injection into a gas chromatograph. This method was unwieldy and could not be adapted to collect personal breathing zone samples.
In the late 1970s, two researchers working for Union Carbide Corporation (A. Qazi and N. Ketchum) developed a sampling and analytical method that used a personal sampling pump and a sampling train consisting of two large (8 inch) charcoal tubes arranged in series. The charcoal tubes were desorbed using carbon disulfide and analyzed using gas chromatography. The advantage of this method was that it could be used for personal breathing zone sampling. However, the sampling train was large (at least 16 inches long) and was difficult to position and maintain in the worker's breathing zone. The Qazi-Ketchum method had a limit of detection of 0.25 parts per million (ppm) and was validated in the range of 0.5 to 50 ppm.
In the same timeframe, OSHA developed Method 30, which used two smaller charcoal tubes in series. The limit of detection was 0.05 ppm but was limited in sample volume to 1 liter. At the recommended flow rate of .05 liters per minute, the method was limited to a sample duration of 20 minutes. Thus, this method was cumbersome for evaluating full shift exposures. Both the Qazi-Ketchum method and OSHA Method 30 needed the samples to be analyzed within 15 days.
Subsequently, OSHA developed Method 50, which involved collecting ethylene oxide on a single, small tube containing charcoal treated with hydrogen bromide (HBr); 2-bromoethanol was produced in the charcoal tube, which was then analyzed using a gas chromatograph equipped with an electron capture detector. Because this method uses a personal sampling pump and a single small charcoal tube, personal breathing zone samples can be collected conveniently. The recommended sample volume is 24 liters, allowing a 4-hour sample duration at the recommended flow rate of 0.1 liters per minute ( lpm). The limit of detection for this method is 3 parts per billion (ppb).
The development of passive dosimeters was a further improvement in the sampling and analytical method for evaluating ethylene oxide exposures. OSHA Method 49 is based on using 3M model 3551 passive dosimeters, which contain charcoal that has been treated with HBr. Analysis of the passive dosimeter is similar to the procedures contained in OSHA method 50. Personal breathing zone sampling using this method is very convenient because no personal sampling pump is needed. The limit of detection for this method is 0.7 ppb. Only one passive dosimeter is required to evaluate a full shift exposure. The only major disadvantage of the passive dosimeter is that it can only be used for area sampling in environments where ambient air velocity exceeds 15 feet per minute.
Some Organizational Changes in EtO Using Industries
Hospitals
EtO continues to be the chemical of choice for hospital sterilization, and this use of EtO continues to be the source of the greatest number of worker exposures. According to a 1996 study,137 EtO using hospitals tended to be larger than non users (an average of 250 patient beds versus 160 patient beds). Also, 83 percent of the EtO using hospitals were privately operated compared with 53 percent of the non EtO using hospitals. Ninety five percent of the EtO using hospitals were acute care facilities, while only 19 percent of the non-users were acute care facilities.
Community hospitals, as defined by the American Hospital Association (AHA), are non federal, acute care facilities. Although the number of for profit community hospitals increased from 730 to 750 between 1980 and 1995, the number of state and local and nonprofit community hospitals has decreased from 5,100 hospitals in 1980 to almost 4,400 hospitals in 1995 (see Table II-1). Although the number of AHA registered hospitals declined by almost 10 percent between 1980 and 1995 (see Table II-1), the number and percentage of smaller hospitals among AHA registered hospitals has increased (see Table II-2). None of these changes resulted from the EtO standard, but from other factors effecting hospitals.
Medical Device Manufacturers
Employment within the medical device manufacturing sectors rose by approximately 25 percent between 1984 and 1996. The number of employees within the manufacturers and sterilizers of medical products sector increased from approximately 74,000 in 1984 to 93,000 in 1996, indicating an increase in the production and number of companies within these sectors.138
The Spice Industry
The spice industry has experienced some downsizing as well as consolidations and increases in foreign ownership since 1989.139 This change was not the result of the EtO standard. However the change in industry structure has reduced the number of spice companies performing EtO sterilizations in-house. Many spice companies also use or have switched to contract sterilization.
This chapter presents the Lookback review's information on the impacts of the EtO standard on affected small entities. It first describes and quantifies these entities. It then reviews relevant public comments. Next, it identifies and characterizes the changes in impacts on small entities since the standard's enactment.140 Finally, the chapter concludes by summarizing why OSHA believes that the standard has not had a significant economic impact on a substantial number of small entities.
INDUSTRIAL SECTORS CONTAINING AFFECTED SMALL ENTITIES
Entities affected by the EtO standard include EtO producers, ethoxylators, and sterilizers including hospitals, medical and pharmaceutical product manufacturers, spice manufacturers, contract sterilizers, and other sterilization and fumigation operations. The following discussion characterizes (by standard industrial classification (SIC) code) and quantifies the small entities affected by the EtO standard within each of these groups.
Ethylene Oxide Producers
EtO producers manufacture ethylene oxide by the direct oxidation of ethylene. These firms are classified within SIC 2869.
Sic 2869, Industrial Organic Chemicals, Not Elsewhere Classified
There are 10 known producers of EtO in the U.S., including BASF Corporation, Dow Chemical, Eastman Chemical Company, Equistar Chemical, Formosa Plastics Corporation, Huntsman Corporation, Old World Industries, PD Glycol, Shell Chemical Company, and Sunoco, Inc.141 Most of these entities also operate ethoxylation facilities. Criteria established by the Small Business Administration (SBA) characterize firms in this industry as small if they have no more than 1,000 employees. None of these firms qualify as small entities under the SBA definition. Besides these 10 firms, one specialty chemical manufacturer produces glycol ethers at a single facility (but does not produce EtO).
Ethoxylators
Ethoxylators are chemical manufacturing firms that use EtO as a chemical feedstock to make chemical products such as ethylene glycol, glycol ethers, ethanolamines, surfactants, and other specialty chemicals.
Sic 2843, Surface Active Agents, Finishing Agents, Sulfonated Oils, and Assistants
EtO is used as a process input during the production of three different types of surfactants: alcohol ether sulfates, alcohol ethoxylates, and alkylphenol ethoxylates. These surfactant compounds are used to manufacture shampoos, light-duty liquid dish detergents, heavy-duty laundry detergent powders and liquids, and specialty cleansers.
An estimated 21 firms engage in the manufacture of surfactants through ethoxylation reactions and these 21 firms operate a total of 46 surfactant ethoxylation facilities. The SBA small firm definition for this industry is 500 employees or fewer.
Hospitals
An estimated 52 percent of U.S. hospitals have on-site EtO sterilization operations. These include hospitals that operate for profit, non-profit hospitals (including university hospitals), and hospitals owned by government entities. EtO-using hospitals are typically acute-care facilities. As a result of the EtO standard demand for modern EtO sterilizers increased and cost came down for sterilizers meeting OSHA requirements (see discussion below). Despite the reduced cost of EtO sterilizers, OSHA found strong evidence indicating that the number of hospitals using EtO sterilization systems has significantly declined since 1984. This decline is attributable to several factors, including the increased use of disposable instruments, the increased use of contract sterilizers, and the increased popularity of alternative sterilization methods, as well as a general consolidation of the hospital
industry. There is no evidence that the switch to EtO alternatives has caused any financial strain to hospitals. EtO sterilization came down in cost, and of course, sterilization is just a tiny part of a hospital budget.
SIC 8062, General Medical and Surgical Hospitals
SIC 8069, Specialty Hospitals
OSHA estimates the total number of hospitals with on-site EtO sterilizer units at 3,271. For the reasons discussed above, this figure shows a decline relative to the 6,237 EtO-using hospitals estimated in OSHA's 1988 Regulatory Impact Analysis (II-5). Most hospitals, however, are not small entities. The SBA small firm definition for profit-making enterprises in the hospital sector is $5 million in revenues.142 The proportion of hospitals that qualify as small using this criterion is estimated at between 10 percent and 20 percent.143 Therefore, as many as 654 hospitals affected by the standard may qualify as small entities. The actual number of small entities is likely to be less than 654, however, as studies have shown that EtO-using hospitals are somewhat larger on average than non-using hospitals (II-9).
This information suggests that smaller hospitals may be somewhat less likely to use EtO and thus to have fewer compliance responsibilities related to the EtO standard. In addition, EtO-using hospitals are more likely to be privately operated than are non-using hospitals (II-9).
Medical and Pharmaceutical Product Manufacturers
Only a small subset of medical and pharmaceutical products manufacturers appears to perform EtO sterilization in house, with most (over 80 percent) making significant use of contract sterilization services.
3841, Surgical and Medical Instruments and Apparatus
3842, Orthopedic, Prosthetic, and Surgical Appliances and Supplies
Medical products manufacturers use EtO to sterilize equipment, medical devices, and supplies that might be damaged by other sterilization methods. Most EtO-using medical products manufacturers are found in SIC 3841, Surgical and Medical Instruments and Apparatus, and SIC 3842, Orthopedic, Prosthetic, and Surgical Appliances and Supplies. The SBA small business size definition for both SICs 3841 and 3842 is 500 employees. As reported in Chapter II, six small entities are estimated to be affected by the EtO standard in these sectors. An additional five small entities affected by the standard are found in other health-related suppliers (SICs 3079, 3693, 5086, 2821, 2211, 2879, 3069, 3569, 3677, 3999), reflecting the diversity of products that can be sterilized using EtO.
2834, Pharmaceutical Preparations
A few pharmaceutical products manufacturers use EtO to sterilize products such as bulk powders, ingredients used in ophthalmic ointments and suspensions, and packaging materials, although some of these uses seem to be in decline. Most pharmaceutical manufacturers are typically found in SIC 2834, Pharmaceutical Preparations. The SBA small business size definition of this industry is 750 employees. As reported in Chapter II, two small entities are estimated to be affected by the EtO standard in these sectors.
Spice Manufacturers
2099, Food Preparations, NEC
Spice manufacturers are typically classified in SIC 2099, Food Preparations Not Elsewhere Classified. This SIC has an SBA size class definition of 500 employees. As reported in Chapter II, three small entities are estimated to be affected by the EtO standard in these sectors.
EtO is used to fumigate spices worth an estimated 15-22 percent of all spice sales. OSHA found evidence indicating that the sterilization of spices has shifted from spice manufacturers to contract sterilizers since 1984. The vast majority of EtO-treated spices now are believed to be fumigated by contract sterilizers.
Contract Sterilizers
7389, Business Services, NEC
Contract sterilizers typically are classified in SIC 7389, Business Services Not Elsewhere Classified (although some may also fall into SIC 7218, Industrial Launderers or SIC 8091, Health and Allied Services). This SIC currently has an SBA size class definition of $5.0 million dollars in annual sales. As reported in Chapter II, 12 small entities are estimated to be affected by the EtO standard in these sectors.
Other Sterilization and Fumigation Operations
8411, Museums and Art Galleries
8231, Libraries
For reasons discussed in Chapter II, OSHA assumes there are no longer any entities using in-house EtO sterilizers in these industry sectors.
PUBLIC COMMENTS ADDRESSING SMALL ENTITIES
OSHA did not receive any comments indicating that the EtO standard was imposing significant or unnecessary burdens on affected small entities. The American Hospital Association, which represents the hospital sector and most of the small entities subject to the standard, submitted comments indicating that the standard was feasible, "effective as written," and "requires no significant change in the context of this review [Exh. 2-2]." Similarly, the Ethylene Oxide Industry Council (EOIC), which represents individual companies in each of the sectors other than hospitals that are affected by the EtO standard, indicated that it "supports OSHA's EtO standard as technically and economically feasible [Exh. 2-6]."
One comment stated that small-scale users of EtO, such as hospitals, have carefully re-examined EtO use and found considerable opportunity for use reduction, resulting in hazard reduction as well as considerable cost savings. The comment noted a specific example in which one hospital had reduced its EtO usage by over 75 percent by switching to steam sterilization for items that could be safely sterilized using that method, which is both less expensive and safer. (Chapter IV)
In its comments, the Office of Advocacy of the SBA expressed concern about the lack of direct small business participation in the EtO standard lookback review process and indicated that OSHA should take this into consideration if changes to the standard are contemplated. Although OSHA did not receive any comments from individual small businesses or entities, the comments provided by industry associations representing the industries affected by the standard (discussed above), including small businesses in these sectors, suggest that there have been no significant impacts on a substantial number of small businesses or entities as a result of this standard.
Although one comment indicated that, in the hospital sector, compliance with the EtO standard is basically similar regardless of establishment size, some commenters recommended areas in which additional OSHA compliance assistance would be useful for the hospital sector and other small employers (e.g., the emergency alert, medical surveillance, exposure monitoring, and worker training requirements). OSHA has therefore decided to provide additional outreach and compliance assistance to small hospitals and other small entities (as discussed in ChapterVII).
IMPACTS ON AFFECTED SMALL ENTITIES
As part of this Lookback review, OSHA conducted independent research to identify trends since the promulgation of the standard that are relevant to the standard's costs and impacts on small entities. These trends suggest that the compliance costs and associated impacts attributable to the EtO standard have declined since its enactment:
- The major economic development that has occurred in the affected industries was a reduction in the cost of sterilizing equipment, despite concurrent improvements in sterilizer technology. New EtO sterilizer models are now available for almost half the cost of the ones available in 1984 (V-19). The average cost of a hospital sterilizer dropped by about $10,000 from 1992 to 1996. (V-19) Also, with advances in EtO sterilizer designs, new equipment no longer needs add-on engineering controls to achieve the levels mandated by the standard. For the sterilizers in use in 1982, a retrofit package was required to reduce EtO exposure levels to the 1 ppm level. Today it is possible to purchase an improved EtO sterilizer with built-in controls at a price that is less than the real cost of a retrofit package in 1996. (V-20)
- Additional sterilization methods are available and are used increasingly frequently when the unique advantages of EtO-based sterilization are not essential. In particular, the use of gamma radiation and E-beam sterilization are increasingly common. Gamma sterilization in particular is considered superior in terms of quality of sterilization and process reliability. Also, hospitals are making increased use of steam where feasible.
- Single-use instruments have become prevalent in many applications at hospitals (V-16). This reduces the need to use EtO units to re-sterilize the instruments.
- Medical device manufacturers and spice manufactures are increasingly turning to contract sterilizers both for reasons of cost-effectiveness (resulting from economies of scale) and to avoid the potential for health and environmental issues related to EtO (partly due to EPA regulations enacted since OSHA's standard took effect). Approximately half of all medical device manufacturers now use contract sterilizers. (V-17)
In addition, OSHA examined available data on small firms in seven relevant SIC codes. Figure VI-1 summarizes the relative changes in the size distribution of small establishments - those employing fewer than 500 employees144 in the affected SICs between 1983 (i.e., the year before EtO standard implementation) and 1997. The figure indicates that the percentage of establishments employing fewer than 500 employees did not significantly change in any of the eight SIC codes between 1983 and 1997, except in the hospital industry where there was about a 10 percentage-point drop in the percentage of hospitals employing 500 or fewer employees. This drop occurred largely in the 1987-1989 period and is most likely attributable to the general industry consolidation that occurred during that time. Though there was a percentage drop,
the actual number of small hospitals increased.
Figure VI-2 provides a closer look at hospital establishments that employ fewer than 500 employees. Hospitals are examined under SIC 806 (which encompasses general medical and surgical hospitals (SIC 8062) and specialty hospitals (SIC 8069)), which contains as many as 654 small entities.145 The figure shows (in the right-most pair of bars) that the number of hospital establishments employing fewer than 500 employees increased between 1983 and 1997. The figure also indicates growth in small hospital establishments employing 50-99, 100-249, and 250-499 people.
Appendix A presents similar information regarding the change in the number of establishments in all relevant SIC codes between 1983 - 1997. These data, although representing fewer affiliated small entities, also support the conclusion that the EtO standard did not have a negative economic impact on small businesses in the affected industries. Seven of the eight SIC codes had an increase in the number of establishments with one to 499 employees. In the eighth SIC code the number of establishments decreased by less than five percent.
The various trends described above provide a strong indication that the EtO standard is not imposing a significant economic impact on a substantial number of small entities. EtO systems cost less and are being used more selectively by affected entities. The public comments discussed earlier indicate that compliance with the standard is high and similar across the substantial range of workplace sizes. OSHA therefore concludes that neither small hospitals (which comprise the vast majority of small entities affected by the standard) nor other small entities have been significantly negatively impacted by the standard. Indeed the reduced costs of EtO sterilization and switching to less costly alternatives when effective have reduced sterilizer costs for small hospitals.
Figure VI-1
Percentage of Establishments with Fewer than 500 Employees, 1983-1997
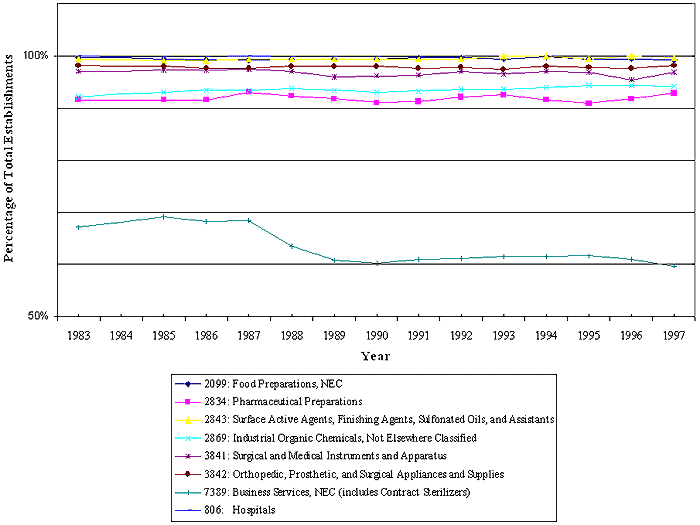 |
Text version of Chart
Chart Title: Figure VI-1 Percentage of Establishments with Fewer than 500 Employees, 1983-1997
Chart Type: Line graph
Chart Elements: 9 lines, one for each establishment with fewer than 500 employees in the affected SICs with points representing percentage for each year between 1983 and 1997.
Chart Data:
2099: Food Preparations, NEC
2834: Pharmaceutical Preparations
2843: Surface Active Agents, Finishing Agents Solfonated Oils, and Assistants
2869: Industrial Organic Chemicals, not Elsewhere Classified
3841: Surgical and Medical Instruments and Apparatus
3842: Orthopedic, Prosthetic, and Surgical Appliances and Supplies
7389: Business Services, NEC (includes Contract Sterilizers)
806: Hospitals
| |
2099 |
2834 |
2843 |
2869 |
3841 |
3842 |
7389 |
806 |
| 1983 |
99.56% |
91.61% |
99.51% |
92.19% |
97.01% |
98.19% |
99.89% |
67.05% |
| 1984 |
99.52 |
91.59 |
99.27 |
92.63 |
97.13 |
98.05 |
99.86 |
68.14 |
| 1985 |
99.49 |
91.57 |
99.04 |
93.07 |
97.25 |
97.91 |
99.87 |
69.22 |
| 1986 |
99.30 |
91.59 |
98.99 |
93.38 |
97.26 |
97.52 |
99.85 |
68.31 |
| 1987 |
99.21 |
93.00 |
99.50 |
93.35 |
97.48 |
97.64 |
99.88 |
68.39 |
| 1988 |
99.40 |
92.34 |
99.51 |
93.72 |
97.01 |
97.88 |
99.87 |
63.51 |
| 1989 |
99.37 |
91.70 |
99.49 |
93.39 |
95.98 |
97.97 |
99.86 |
60.71 |
| 1990 |
99.50 |
91.03 |
99.49 |
93.06 |
96.10 |
97.99 |
99.82 |
60.25 |
| 1991 |
99.59 |
91.18 |
99.50 |
93.19 |
96.28 |
97.55 |
99.83 |
60.95 |
| 1992 |
99.55 |
92.08 |
99.50 |
93.50 |
96.96 |
97.73 |
99.79 |
61.12 |
| 1993 |
99.41 |
92.55 |
100.00 |
93.67 |
96.59 |
97.45 |
-- |
61.53 |
| 1994 |
99.71 |
91.50 |
100.00 |
93.92 |
97.00 |
97.96 |
-- |
61.48 |
| 1995 |
99.48 |
90.86 |
99.50 |
94.27 |
96.80 |
97.84 |
-- |
61.65 |
| 1996 |
99.35 |
91.77 |
100.00 |
94.33 |
95.33 |
97.54 |
-- |
60.92 |
| 1997 |
99.15 |
92.87 |
99.52 |
94.08 |
96.94 |
98.07 |
-- |
59.61 |
CONCLUSION
The EtO standard has not had a negative economic impact on the industries affected by the standard, generally, or on small businesses in those industries. The major economic development that has occurred has been a reduction in the cost of sterilizing equipment, despite concurrent improvements in sterilizing technology. Substitutes for EtO and the use of EtO contractors are increasing for all sizes of companies. Much of the impetus for the increase in substitution and contracting out, however, has come from the need for companies to comply with EPA regulations, rather than with OSHA regulations.
Figure VI-2
Numbers of Small Hospital Establishments, 1983 to 1997
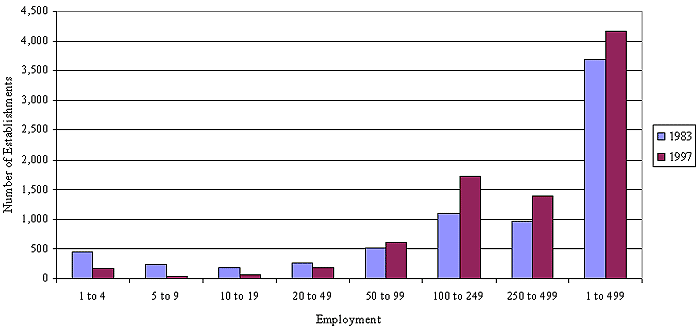 |
Text version of Chart
Chart Title: Figure VI-2 Numbers of Small Hospital Establishments, 1983 to 1997
Chart Type: Vertical bar graph
Chart Elements: 16 - Two bars, one for 1983 and one for 1997, for each employment range showing the number of small hospital establishments.
Chart Data:
| |
Employment |
| 1-4 |
5-9 |
10-19 |
20-49 |
50-99 |
100-249 |
250-499 |
1-499 |
| Number of Establishments |
1983 |
488 |
226 |
177 |
266 |
508 |
1,102 |
963 |
3,690 |
| 1997 |
160 |
38 |
61 |
181 |
616 |
1,721 |
1,387 |
4,164 |
This Lookback review also found no evidence of any technological or economic changes that have occurred since promulgation that have adversely affected the ability of EtO-using small entities to comply with the standard; instead, technological and economic changes have made compliance less burdensome for the regulated community, including small businesses.
Most of the small businesses affected by the EtO Standard are hospitals, medical device manufacturers, and spice manufacturers. There are no indications that the regulation of occupational exposure to EtO has impaired the economic well being of businesses in any of these sectors or has disproportionately affected small businesses. Public comments received by OSHA during this review indicated compliance was feasible. No public comments suggested that the standard has had a significant economic impact on a substantial number of small entities. This is consistent with the Agency's findings at the time the EtO standard was issued. OSHA concludes that its standard does not require modification to minimize significant impacts on a substantial number of small entities.
FINDINGS
OSHA has completed its review of the Ethylene Oxide standard (29 CFR 1910.1047) in accordance with the requirements of Section 610 of the Regulatory Flexibility Act and Section 5 of Executive Order 12866. The Agency concludes that the rule should be continued without change and does not need to be rescinded or amended to minimize significant impacts on a substantial number of small entities or for any other reason.
OSHA also finds that the Ethylene Oxide standard is necessary to protect employee safety and health, is compatible with other OSHA standards, is not duplicative or in conflict with Federal, State, local, or other government rules, is not inappropriately burdensome, and is consistent with the priorities and principles of EO 12866. In addition, information gathered during this Lookback review supports OSHA's earlier (1984) findings concerning the economic and technological feasibility of the Ethylene Oxide standard and demonstrates that the standard has been effective in reducing occupational exposure to EtO. Specifically, OSHA finds that: (1) there is a continued need for the standard; (2) OSHA can best address the specific complaints and comments about the standard by providing additional compliance assistance; (3) the rule is not particularly complex; (4) the standard does not overlap
other regulations and (5) no changes have occurred in technological, economic, or other factors that would warrant a reopening of the EtO rulemaking.
OSHA'S RESPONSES TO CONCERNS RAISED BY STAKEHOLDERS
Small Business Impacts
No comments received by OSHA during this review (Docket H200 C) suggest that the standard has had a significant economic impact on a substantial number of small entities. This is consistent with the Agency's findings at the time the EtO standard was issued. This Lookback review also found no evidence of any technological or economic changes that have occurred since promulgation that have adversely affected the ability of EtO using small entities to comply with the standard; in fact, the many technological and economic changes that have occurred since 1984 have made compliance easier for the regulated community, including small businesses. Therefore, OSHA concludes that the standard does not require modification to minimize significant impacts on a substantial number of small entities.
Most of the small entities affected by the EtO standard are employers operating EtO sterilization processes in the hospital sector, although affected small entities are also found in the contract sterilizer and spice manufacturing sectors. Articles and reports submitted to the Lookback Docket (H200-C) indicate a high degree of current compliance with many of the key requirements of the standard in the hospital sector and also demonstrate that the "implementation and effectiveness of the EtO standard is similar across the substantial range of workplace sizes examined" [Exs. 2 9; 2 9K]. The standard resulted in the development of sterilizers, which met the standard and cost less than earlier sterilizers that did not. Economic data do not show any negative impacts on small entities. OSHA therefore concludes that small hospitals and other small entities are not unduly burdened
by the standard.
Some commenters, however, recommended areas in which additional OSHA compliance assistance would be useful for hospital sector and other small employers (e.g., the emergency alert, medical surveillance, exposure monitoring, and worker training requirements). OSHA compliance officials and OSHA State plan state staff who were asked about their experience with the standard concurred that the exposure monitoring requirements and the emergency alert requirements of the EtO standard were areas in which some small businesses could use additional assistance in understanding the standard. OSHA has therefore determined that providing additional compliance assistance would be beneficial for small hospitals and other small entities. The compliance assistance OSHA intends to provide is described in the last section of this chapter.
FINDINGS CONCERNING SPECIFIC PUBLIC COMMENTS AND COMPLAINTS
The Current Permissible Exposure Limits
Many commenters submitted comments concerning the appropriateness of the current OSHA permissible exposure limits (PELs) for EtO [Exs. 2 1; 2 2; 2 4; 2 6; 2 7; 2 8; 2 10; Tr. 6/30/97]. Several of these commenters submitted epidemiological studies to the record in addition to offering their evaluations of the appropriateness of the current PELs [Exs. 2 1; 2 6; 2 10]. Comments requesting modification of the PEL were based on analyses of EtO's carcinogenic effects as well as other health effects.
The industry representatives who commented on the standard's current 8-hour TWA PEL generally concluded that it should be maintained at its current level [Exs. 2 1; 2 2; 2 6]. The American Hospital Association (AHA), the Ethylene Oxide Industry Council (EOIC), and the Pennsylvania Engineering Company all indicated that they did not recommend changing the current PEL. However, the EOIC and Pennsylvania Engineering Company stated that they believe that new health effect data indicate that the risk may be lower than originally estimated by OSHA in its risk assessment for the 1984 standard [Exs. 2 1; 2 6]. Griffith Micro Science commented that it believed that an "increased action limit and PEL are justified with the new health effect data" and also stated that OSHA should revise the standard to lessen the stringency of the PEL based on these new data [Ex. 2 4].
Other commenters, including the American Federation of Labor and Congress of Industrial Organizations (AFL CIO), the American Federation of State, County, and Municipal Employees (AFSCME), the American Nurses Association (ANA), the National Institute for Occupational Safety and Health (NIOSH), and the Environmental Protection Agency (EPA), also commented on the appropriateness of the current PEL. These commenters recommended making the PEL more stringent either on the basis of new health risk data or on the basis of the risk assessment performed by NIOSH to develop its current NIOSH REL for EtO of 0.1 ppm as an 8 hour TWA [Exs. 2 7; 2 10; 2 8; Tr. 6/30/97]. NIOSH supported lowering the OSHA PEL to the current NIOSH REL of 0.1 ppm as an 8 hour TWA and indicated that control technology is available to control exposures to that level [Ex. 2 10]. EPA also recommended lowering the OSHA PEL
for EtO to the NIOSH REL of 0.1 ppm on the basis of EPA's estimates of high cancer risks at the current PEL and gaps in the neurotoxicity data for EtO [Ex. 2 8]. In addition, AFSCME recommended lowering the current OSHA excursion limit (EL) of 5 ppm for 15 minutes to match the 1989 NIOSH STEL recommendation of 5 ppm for 10 minutes [Tr. 6/30/97, p. 31].
As part of this Lookback review and as described above, OSHA examined evidence submitted by the commenters and from the scientific literature as it pertained to the findings of the risk assessment used to support the 1984 EtO PEL of 1.0 ppm as an 8 hour TWA and the 1988 EL of 5 ppm over 15 minutes. Since the promulgation of the EtO standard in 1984, some new information on the adverse health effects of EtO has become available, including a number of new animal inhalation studies and human studies.
OSHA was presented with two differing risk assessments. The risk assessment submitted by EOIC suggests that the cancer risk is lower than OSHA's 1984 risk estimates. The risk assessment submitted by NIOSH and EPA suggests that the cancer risk is higher than OSHA's 1984 estimate. The variations in the risk assessments for the most part depend on the assumptions made in extrapolating animal data to humans.
OSHA does not believe it should use its limited regulatory resources to reconsider this issue, especially since all commenters indicate that there is a continued need for the standard and most exposures are less than 1 ppm and even 0.1 ppm.
OSHA further concludes that the new standard has successfully reduced exposure and risk for employees and has proved feasible for employers and small business with no significant negative impacts on small business. Finally, OSHA concludes that EtO is not a high priority for new complete rulemaking, which would take very substantial regulatory resources.
OSHA finds that new evidence of the health effects of ethylene oxide is not sufficient to require amendment of the PEL.
The Exposure Monitoring Requirements
A number of comments were received concerning the exposure monitoring provisions of the EtO standard (paragraph (d) of 1910.1047). The comments received on the monitoring requirements generally suggested that affected employers are capable of complying with the current exposure monitoring requirements and that it is technologically and economically feasible to do so [Exs. 2 2, 2 6, 2 9K].
Representatives of the BASF Corporation and the Clariant Corporation recommended that OSHA modify the exposure monitoring requirements of the standard in ways that would decrease monitoring frequencies under very specific conditions (e.g., creating an exemption from quarterly monitoring for employees engaged in well characterized tasks with exposures above the PEL that are performed in situations where engineering controls are infeasible) [Exs. 2 3, 2 14]. Several other commenters, including the AFL CIO, Dr. LaMontagne and Dr. Kelsey, and the EPA, recommended that OSHA modify the standard to add additional exposure monitoring requirements [Exs. 2 9, 2 8; Tr. 6/30/97]. These commenters suggested additions to the standard, such as requiring that periodic monitoring for EtO be performed without regard to existing exposure levels, adding a requirement for additional monitoring of workers
performing tasks where exposures in excess of the EL would be likely to occur, and adding a requirement for workplace re evaluation similar to a provision in OSHA's Cadmium standard 1910.1027 (l)(3)(ii)(A) [Exs. 2 9, 2 8; Tr. 6/30/97]. In contrast, the EOIC indicated that the exposure monitoring requirements of the EtO standard are "well considered and consistent with other OSHA rules" and generally supported retaining the current requirements of the standard unchanged [Ex. 2 6].
OSHA concludes as a result of this Lookback review that no changes are warranted in the existing exposure monitoring requirements of the standard at this time. In addition, OSHA notes that requiring periodic exposure monitoring without regard to previously monitored exposure levels would be inconsistent with the approach taken in other recent OSHA health standards. OSHA believes that compliance assistance activities will respond effectively to the concerns raised by commenters related to the exposure monitoring requirements of the standard.
The Emergency Situation Requirements
OSHA received comments on the standard's emergency situation requirements and emergency alert requirements. Compliance with these requirements plays an essential part in protecting workers from accidental overexposures to EtO. As indicated in Chapter II, non-compliance with the standard's emergency plan requirement was the most commonly cited violation of the standard across all size classes of employers from 1985 through 1997. Large employers (those employing more than 500 employees) were also frequently cited for deficiencies in compliance with the emergency alert requirement during this period.
Emergency Plan Requirement
Paragraph (h)(1) of the standard requires employers to have a written plan for addressing emergencies. The only comment received on this requirement concerned its implementation in the hospital sector. Dr. LaMontagne and Dr. Kelsey indicated that although the Massachusetts Hospital EtO Health and Safety Study found that 94 percent of EtO using hospitals (85 of 90) had written emergency response plans, only one third of the hospitals (31 of 90) had carried out EtO emergency drills in which workers actually evacuated the sterilization department [Ex. 2 9K]. (OSHA notes that the standard does not require employers to conduct such drills.) Dr. LaMontagne and Dr. Kelsey indicated that including evacuation drills as part of worker training would be one-way hospitals could improve their emergency preparedness. Dr. LaMontagne and Dr. Kelsey recommended that this primary preventive measure be
considered for incorporation as a requirement if the standard is reopened [Ex. 2 9]. As noted above, OSHA has decided that the rule should not be reopened at this time; however, the Agency is considering including a discussion of the value of worker evacuation drills in any compliance assistance materials OSHA develops for the EtO standard.
Emergency Alert Requirement
Paragraph (h)(2) of 1910.1047 is a performance requirement that requires employers to have a means of alerting potentially affected employees of emergencies in a prompt manner. The standard does not specify any particular means of warning employees but instead gives employers the flexibility to use any approach that will achieve the provision's goal. The use of continuous, real time EtO alarms is one possible method of complying with this requirement. The EtO standard also does not specify the EtO levels at which alarms must be set to sound in the event that employers choose to use them as a method of alerting employees.
Dr. LaMontagne and Dr. Kelsey, EPA, AHA, AFSCME, and the EOIC all commented on the emergency alert requirement of the standard [Exs. 2 9, 2 8, 2 2, 2 6;Tr. 6/30/97]. Dr. LaMontagne and Dr. Kelsey recommended that OSHA either modify the emergency alert requirement or provide compliance assistance concerning acceptable types of alarm equipment and appropriate settings for EtO alarms [Ex. 2 9]. EPA recommended modifying the standard to require EtO alarms [Ex. 2 8]. The AHA and AFSCME requested that OSHA provide non mandatory guidance [Ex. 2 2; Tr. 6/30/97, p. 32]. The AHA, as well as Dr. LaMontagne and Dr. Kelsey, indicated that if OSHA were to provide information concerning the appropriate alarm trigger levels, such guidance could help prevent hospitals from overspending on EtO alarms [Tr. 6/30/97, p. 22; 2 9]. The EOIC objected to comments received from other commenters urging OSHA to
revise the standard to include regulatory provisions related to alarm use [Ex. 2 6].
Dr. LaMontagne and Dr. Kelsey raised a number of concerns about the emergency alert requirement in their comments, including what they viewed as OSHA's somewhat inconsistent interpretation and enforcement of the requirement, the inability of voice alerts to protect workers from unrecognized leaks, employer confusion regarding the appropriate settings for EtO alarms, possible employer overspending caused by the lack of guidance on acceptable alarm types, and a lack of clear reference to NIOSH's alarm trigger level recommendations in OSHA compliance interpretations [Ex. 2 10F]. To resolve these issues, Dr. LaMontagne and Dr. Kelsey recommended that OSHA: (1) publicly clarify its interpretation of how to comply with the emergency alert requirement (with the strong implication that a clarified interpretation should indicate that EtO alarms must be used to comply with the emergency alert
requirement); (2) clarify that EtO alarms do not have to be set at levels at or below the permissible exposure limits to achieve the intent of this provision, and (3) recommend alarm trigger levels in the 20 to 50 ppm range [Ex. 2 9]. The EPA supported these recommendations but also recommended that the use of alarms capable of detecting releases of 20 ppm or above be required [Ex. 2 8].
In response to these comments, OSHA notes that its current interpretations (see the interpretations pertaining to EtO on OSHA's website) concerning the performance nature of the emergency alert requirement are consistent. Further, although OSHA agrees that EtO alarms may be more effective than voice communication as a means of alerting employees in large hospital settings, the EtO emergency alert provision was intentionally performance-based to be feasible for employers in all industries and to allow the use of any current or future technologies. As stated in the preamble to the 1984 Final Rule, OSHA determined that "The performance language of the emergency situation paragraph of the final standard will give employers the flexibility to choose any effective method of alerting employees, including communications systems, voice communication, or a bell or other alarm" [FR Vol.
49, No. 122, 6/22/84, p. 25783]. In 1987, OSHA also issued a compliance interpretation letter that stated that "a sophisticated alarm system might be unnecessary for some facilities such as small hospitals with only one EtO sterilizer." Thus, OSHA does not agree that the standard should be modified to specify that EtO alarms be used to satisfy the emergency alert requirement. OSHA continues to believe that employers are in the best position to identify an effective method of complying with this requirement for the circumstances of their particular workplaces. However, OSHA does agree that additional compliance assistance materials would aid employers in complying with this requirement and will consider including such information in any additional outreach or compliance assistance materials developed by the Agency for this standard.
Two other comments were made concerning the emergency situation requirements. The AHA requested that OSHA clarify the existing provisions to indicate that there is no need for continuous alarm monitoring after initial monitoring if exposures are below mandated levels [Ex. 2 2]. In addition, Dr. LaMontagne recommended modifying the standard to add a provision to trigger a mandatory reassessment of compliance procedures following emergency situations [Tr. 6/30/97, p. 74].
In response to the AHA, OSHA notes that the emergency situation requirements (both the written plan and emergency alert requirements) of the standard apply to employers whenever there is a possibility that an emergency (as defined in the standard) may occur - regardless of monitored airborne exposure levels. With regard to Dr. LaMontagne's recommendation, OSHA believes that the existing requirements of the standard already adequately address the issues that would be addressed by such a workplace reassessment provision. For example, the EtO standard currently requires employers to use feasible engineering controls and work practices to reduce and maintain employee exposure to or below the TWA PEL and EL, to have a compliance program that includes regular leak detection surveys, and to review the compliance program on an annual basis. OSHA therefore concludes that it is not necessary to
reopen the rulemaking to propose adding a new provision on workplace reassessment to the standard.
The Medical Surveillance Requirements
Dr. LaMontagne and Dr. Kelsey, NIOSH, the ANA, and the EOIC offered comments relating to modifications of the medical surveillance provisions of the standard [Exs. 2 9; 2 10; 2 7; 2 6]. The majority of the comments covered the following topics: (1) the utility of using the action level (AL) as a trigger for the provision of medical surveillance, (2) the possibility of using the excursion limit (EL) as a trigger for medical surveillance, (3) the utility of the requirement to perform a complete blood count with leukocyte differential as a mandatory part of an EtO medical examination, and (4) other recommendations for modification of the standard. On the other hand, the EOIC stated that the standard's medical surveillance requirements should not be modified because "the medical surveillance requirements of the EO standard are well considered and consistent with other OSHA rules"
[Ex. 2 6].
Action Level Exposure Trigger for Medical Surveillance
Paragraph (i)(2) of 1910.1047 requires employers to provide medical surveillance to employees who may potentially be exposed or who are in fact exposed to levels of EtO at or above the action level of 0.5 ppm for 30 or more working days per year. Paragraph (i)(2) also requires medical surveillance to be provided to employees exposed to EtO levels at or above the action level upon termination or reassignment. Thus, potential or actual exposure at or above the action level for more than 30 days per year is a "trigger" requiring compliance with the medical surveillance provisions of the EtO standard.
The findings of the Massachusetts Hospital EtO Health and Safety Study suggest that many hospital central sterile department managers are unfamiliar with the action level concept contained in §1910.1047 or are unaware that exposure above the action level for more than 30 days a year is a trigger for the provision of medical surveillance. The results of the Hospital Study indicated that while accidental worker exposure to EtO releases was strongly related to the provision of EtO medical surveillance in these settings, meeting or exceeding the AL trigger was not [Ex. 2 9K]. Dr. LaMontagne indicated that "simpler OSHA medical surveillance triggers or improved communication efforts appear warranted to address the poor understanding and implementation of the AL trigger" [Ex. 2 9K].
OSHA notes that relatively few of the participating Massachusetts hospitals actually reported exposure monitoring results in excess of the AL. Of 75 hospitals reporting their 1990 1992 exposure monitoring results to the Massachusetts Hospital EtO Health and Safety Study, only 17 hospitals (23%) reported exceeding the AL one or more times, and 11 of these 17 hospitals reported doing so on only one occasion [Ex. 2 10F]. Thus these hospitals would not be required by the standard to provide medical surveillance on the basis of these AL exceedances because the AL must be exceeded at least 30 days per year for medical exams to be required. The Massachusetts EtO Health and Safety Study also found generally good compliance with the standard's medical surveillance requirements. Thus the point about the AL trigger for medical surveillance raised by Drs. LaMontagne and Kelsey appears not in fact
to have represented non-compliance and thus not require the development of outreach materials.
Addition of the Excursion Limit as a Medical Surveillance Trigger
Dr. LaMontagne and Dr. Kelsey recommended that exceedance of the excursion limit (EL) should be added to the standard as a medical surveillance trigger if OSHA decided to reopen the record. These commenters based their recommendation on the fact that they view the EL as a health based exposure limit and their belief that making the EL a trigger for medical surveillance would clear up the difficulty some employers have in distinguishing between exceedances of the EL and accidental exposures resulting from emergency situations [Ex. 2 9; 2 9K]. The AFL CIO and EPA also supported this recommendation [Tr. 6/30/97, p. 14; Ex. 2 8]. In contrast, the EOIC opposed the recommendation, stating that "there is no known medical justification or benefit from medical surveillance after one short term exposure" [Ex. 2 6].
OSHA has decided not to reopen the record to address the issue raised by those commenters who favored making the excursion limit a trigger for medical surveillance, for a number of reasons. First, OSHA is unaware of any evidence that would suggest that the rare exceedances of the EL observed in the Hospital Study would pose a risk to workers warranting medical surveillance. Second, the principal purpose of the EL is to ensure that workplace conditions are sufficiently well controlled to ensure that the 8-hr TWA is maintained; thus, exceedances of the EL are almost always associated with exceedances of the action level and/or TWA PEL, and such exceedances would already trigger medical surveillance under the standard (providing that the 30-day trigger is also met). Third, a medical examination is required after emergency exposures when appropriate. Finally, it has not been OSHA's experience
that employers have difficulty distinguishing between emergency situations and brief excursions above 5 ppm. By definition, emergencies involve an "unexpected significant release" of a hazardous substance caused by ruptured containers, equipment failure, or some other unanticipated incident.
Utility of the Complete Blood Count with Leukocyte Differential
Paragraph (i) of 1910.1047 requires a medical examination for employees who meet one of the five criteria specified in §1910.1047 (i)(2)(A) (E). The specific components of the medical examination required by the standard include a requirement that a complete blood count (CBC) with differential leukocyte count be performed as part of the examination. Dr. LaMontagne and Dr. Kelsey commented that, based on the findings of their study, the "Utility of the Complete Blood Count in Routine Medical Surveillance for Ethylene Oxide Exposure," performing the CBC with leukocyte differential may not be useful in routine medical surveillance for EtO exposure [Exs. 2 9; 2 9C]. The results of their study indicated that alterations in the CBC seemed to occur only after EtO exposure at levels that also produce acutely toxic symptoms, thus rendering the test ineffective for the early detection
of chronic EtO health effects or in determining the adequacy of primary preventive measures [Ex. 2 9C]. These authors also state that the utility of the CBC as a screening test is questionable and that the comparison values needed to interpret the test results are not readily available [Ex. 2 9]. Drs. LaMontagne and Kelsey therefore recommended that, if the record is to be reopened, OSHA should consider leaving the administration of the CBC and leukocyte differential to the discretion of the attending health care professional [Ex. 2 9]. However, NIOSH cautioned against reliance on the LaMontagne and Kelsey study's findings concerning the CBC and leukocyte differential. NIOSH submitted a study performed by NIOSH researchers that characterized the data set evaluated by Dr. LaMontagne et al. as "small and inconclusive" and suggested that further investigations would be necessary
to resolve this debate [Ex. 2 100].
The Agency does not find the issue of including leukocyte differentials in the tests administered for medical surveillance sufficiently compelling to warrant such an undertaking at this time, particularly in light of NIOSH's concern.
Other Issues Related to the Standard's Medical Surveillance Provisions
Three other issues related to the medical surveillance portion of the standard were raised during this Lookback review: (1) The American Nurses Association asked that OSHA modify its medical surveillance requirements to include "all appropriate health care professionals" qualified to perform medical surveillance activities in the standard's medical surveillance provisions [Ex. 2 7]. (2) Dr. LaMontagne and Dr. Kelsey recommended adding a provision to the medical surveillance requirements of the EtO standard requiring workplace re evaluation similar to the exposure reassessment provision in OSHA's Cadmium Standard - 29 CFR 1910.1027(l)(3)(ii)(A). (3) Dr. LaMontagne and Dr. Kelsey also indicated that OSHA should consider conducting outreach activities to encourage better compliance with the occupational history requirement portion of the required medical examination [Ex. 2 9].
In response to the comments by the American Nurses Association, OSHA notes that its recent health standards, e.g., methylene chloride, use the term "physician or other health care professional" to permit qualified health care professionals to perform those medical surveillance activities for which they are licensed by the State in which they are practicing. However, this issue would require rulemaking to change and OSHA does not believe this is the highest priority use of its limited resources at this time.
With regard to Drs. LaMontagne and Kelsey's suggestion that certain medical surveillance results trigger a reevaluation of workplace conditions, OSHA believes that the EtO standard's current engineering control and compliance program requirements, working together, are sufficient to ensure that employers will take appropriate corrective actions if adverse health effects continue to occur among their workers as a result of overexposure to EtO.
As to Drs. LaMontagne and Kelsey's point about the failure of many Massachusetts hospitals to take adequate occupational histories, OSHA agrees that alerting employers to the value of such histories and encouraging them to ensure that the health care professionals they employ to perform medical surveillance include such histories in their medical examinations are appropriate issues for the Agency to pursue. The Agency will therefore emphasize the importance of complete occupational histories in any new compliance assistance or other compliance assistance materials OSHA issues for this standard in the future.
The Training Requirements
Several comments were received concerning implementation of the training requirements of the standard. Paragraph (j)(3) of 1910.1047 requires employers to provide training to employees who are potentially exposed to EtO at or above the action level or above the excursion limit and to do so at the time of initial assignment and at least annually thereafter. Both the AHA and Dr. LaMontagne provided evidence that these training requirements are functioning as intended by OSHA [Ex. 2 2; 2 9K]. Nevertheless, OSHA is concerned about employers' ability to comply with the training requirements, because, as indicated in Chapter II, non-compliance with the worker training requirements was the second most commonly cited violation of the standard across all size classes of employers from 1985 through 1997.
Dr. LaMontagne and Dr. Kelsey identified a number of ways in which the effectiveness of EtO training could be improved, including (1) the use of more interactive training methods; (2) providing instructional materials suitable for the needs of non native speakers of English and trainees with low literacy skills, (3) providing training to more potentially exposed workers (especially maintenance workers), and (4) providing more thorough coverage of medical surveillance topics during training [Exs. 2 9; 2 9K]. Dr. LaMontagne and Dr. Kelsey also recommended that OSHA consider proposing additional emergency training requirements if the Agency decides to reopen the record for the standard and suggested that OSHA also reconsider its action level trigger scheme because the Hospital Study had found no relationship between exceedances of the action level and the provision of worker training [Ex.
2 9].
The AFL CIO also commented on the need to improve the worker training requirements of the standard. The AFL CIO recommended that OSHA incorporate specific language into the standard that would require and encourage worker participation in the training process [Tr. 6/30/97, p. 13]. Two other commenters, the AHA and Clariant Corporation, recommended modifications to the standard's training requirements to give employers greater flexibility in scheduling refresher training. In its comments, the AHA recommended that OSHA permit a performance based approach to training in cases where staff turnover is rare [Ex. 2 2]. The Clariant Corporation commented that the annual refresher training requirement should be modified to match the frequency of a refresher training requirement (every three years) in the OSHA Process Safety Management Standard - §1910.119 (g)(2) [Ex. 2 5].
In response, OSHA notes that this Lookback review has generally shown that the worker training requirements of the standard have been effective, and thus the Agency believes it would be inappropriate to reopen the record primarily to revise these requirements. However, OSHA agrees with several of those participants who urged OSHA to make additional training materials available that are geared to the educational level of the workers being trained, are written in languages other than English, and that employ adult education principles, such as interactive approaches. The Agency will therefore consider making such changes to any compliance assistance or outreach materials it issues in the future for the EtO standard.
OSHA Enforcement Policy
Participants offered comments on four areas of concern regarding OSHA's enforcement/compliance policy. A number of commenters expressed strong support for vigorous enforcement of the standard [Exs. 2 7; 2 12; 2 9; Tr. 6/30/97, pp. 11 12, 33]. Other commenters raised specific issues related to enforcement policy, including (1) the use of EPA data to target OSHA enforcement efforts, (2) the enforcement of the emergency alert requirement, and (3) enforcement of requirements related to tank changing procedures.
Support for Enforcement Efforts
The findings of the Massachusetts Hospital EtO Health and Safety Study indicate that OSHA enforcement improved compliance with the implementation of the EtO standard's requirements [Ex. 2 9]. Many of the commenters, including AFSCME, ANA, the Massachusetts Nursing Association (MNA), the AFL CIO, and Dr. LaMontagne and Dr. Kelsey, indicated that they supported vigorous enforcement of the EtO standard because such enforcement, in their view, enhances employer compliance and thus worker protections [Exs. 2 7; 2 12; 2 9; Tr. 6/30/97, pp. 11 12, 33]. AFSCME also noted that enforcement encourages compliance, which, in turn, reduces the number of accidental releases of EtO occurring in the workplace.
As noted in Chapter II of this review, OSHA has continued to enforce the standard in the years since promulgation of the standard. Since the effective date of the EtO standard in February 1985, Federal OSHA has conducted well over 200 inspections where it monitored worker exposures to ethylene oxide. In addition, during this time period, several OSHA regions have conducted local emphasis programs targeting ethylene oxide hazards. OSHA personnel have also responded to reports of EtO releases in hospitals as well as to fires and explosions occurring at EtO using facilities in other industries. Fair and effective enforcement of OSHA standards is, and will continue to be, an essential part of the OSHA program.
Enforcement of the Emergency Alert Requirement
Both AFSCME and Drs. LaMontagne and Kelsey recommended that OSHA provide additional guidance to the public and the Agency's compliance staff concerning the standard's emergency alert requirement [Ex. 2 9; Tr. 6/30/97]. A review of the guidance available on OSHA's Website shows that OSHA has provided guidance on several aspects of the standard's emergency alert requirement. For example, OSHA guidance indicates that the emergency alert requirement is a performance requirement, that voice communication or other effective methods may be used to comply with the requirement, and that alarms do not have to be capable of detecting levels as low as the 1.0 ppm PEL. OSHA's guidance also indicates that alarms are not required to be set to sound at the EL, that the standard itself does not require the use of EtO alarms or specify alarm settings, and that with regard to specific compliance circumstances,
setting an EtO alarm to sound at 50 ppm could be an acceptable means of compliance with the emergency alert requirement. The Agency believes that the materials already available on its Website concerning the emergency alarm requirements are quite extensive, but will review them and consider expanding them in any additional compliance assistance materials developed for this standard.
Enforcement Requirements Related to Tank Changing Operations
Several commenters, including Dr. LaMontagne and Kelsey, the AFL CIO, and the AHA, indicated concern about OSHA compliance requirements for tank changing operations [Tr. 6/30/97, p. 16; Exs. 2 2; 2 9]. Comments submitted to Docket H 200C indicate that there is some confusion about whether or not OSHA requires that workers engaged in these operations wear respirators. The AHA indicated that "Agency clarifications have stated that because engineering controls are not feasible, respiratory protection (full face negative pressure respirators with canisters having visual end-of-service-life indicators) generally is required" [Ex. 2 2]. The AHA indicated that it disagrees with this position because the Association believes that administrative and engineering controls are "available and effective in controlling exposures to levels well below permissible limits during tank change
operations, even in the event of an accidental release" [Ex. 2 2]. The AHA recommended that OSHA amend its guidance to indicate that wearing respirators during tank changes would not be required if engineering controls and work practices are effective for achieving compliance with the TWA PEL and EL and if proper protective clothing is worn to protect against splashes of liquid EtO [Ex. 2 2]. In contrast, Dr. LaMontagne recommended that, as a good work practice, and for escape purposes only, employees should wear full face-piece cartridge respirators during tank changes [Tr. 6/30/97, p. 66].
In response to these comments, OSHA notes that its compliance interpretation on this issue is drawn directly from the requirements in the standard itself. In §1910.1047(f)(1)(iii), the EtO standard specifically identifies the changing of ethylene oxide tanks on sterilizers as one work operation for which engineering and work practice controls are "generally infeasible" and states that engineering controls would only be required where the Assistant Secretary demonstrates that such controls are feasible. In addition, §1910.1047(g)(1)(ii) indicates respirators must be used during work operations for which engineering and work-practice controls are not feasible. Under §1910.1047(g)(1)(iii), respirators are required to be used in work situations where engineering controls are not sufficient to reduce exposures to or below the TWA or excursion limit, and §1910.1047(g)(1)(iv) indicates
that respirators must be used in emergencies.
Three letters of interpretation provide OSHA's current guidance with respect to the requirements for respiratory protection during tank changing operations. First, in 1992, OSHA issued a compliance interpretation letter (available through the OSHA Website) stating that:
"The requirement for respiratory protection to be worn during tank changing operations is a precautionary measure which may provide adequate protection to employees from high or peak exposures that have the potential to occur quickly and without warning during this potentially hazardous procedure...the potential for worker exposure to a high level of ethylene oxide definitely exists during such operations. The requirement for employees who change these tanks to be provided with and to wear appropriate respiratory protection would protect them from a high, unexpected release, a reasonably predictable occurrence given the nature of this operation."
With regard to this interpretation, questions have arisen regarding the OSHA policy that lack of respiratory protection is not a citable violation unless or until the permissible exposure limit (PEL) is exceeded. Although this is OSHA's general policy for occupational overexposures to airborne concentrations of hazardous substances regulated under the §1910.1000 "Z" Table PELs, occupational exposure to ethylene oxide is regulated by the more specific §1910.1047 Ethylene Oxide standard. The EtO standard's substance-specific requirements take precedence over the Agency's more general policies applicable to occupational exposure to other airborne contaminants. The requirement for respiratory protection during tank changing operations is specifically addressed in the EtO rule, and exposure monitoring data demonstrating overexposure to the EtO PELs need not
be established before the requirement to wear respirators during these operations is effective.
The intent of the requirement at §1910.1047(g), to prevent exposure from a situation where potentially high, unexpected exposures could occur, could alternatively be met by providing real time, continuous exposure monitoring (instantaneous readout), of the area where the EtO tanks are maintained. Such monitors would, additionally, need to be set to act as an emergency alarm for employee evacuation of the area in the event of a leak or sudden release of EtO. In the event of such an emergency, employers must assure that the emergency procedures required under §1910.1047(h) are followed.
The implementation of these procedures could provide equivalent protection to employees and, although still a technical violation of the standard, their use would be considered "de minimis" if adequately demonstrated by the employer as providing equivalent employee protection.
In March of 1993, the above letter was supplemented by a clarification letter. This letter (also available through OSHA's Website) clarifies that while "use of real time continuous exposure monitoring coupled with emergency procedures" would be a way of providing additional protection to employees who operate or work in the general area of sterilizing equipment, employee use of a positive pressure full facepiece supplied air respirator or a positive pressure full facepiece self contained breathing apparatus (SCBA) would be required where the concentration of EtO is unknown and that "this would generally be the case if there was an unexpected release during tank changing operations."
Finally, in a June 1993 letter (one not available through the OSHA World Wide Web site), OSHA further clarified that workers must wear respiratory protection equipment during the changing of EtO cylinders on EtO sterilizers and stated that "air purifying full facepiece respirators can be used only to escape from a high, unexpected exposure during the changing of tanks on EtO sterilizers. If an employer does not have an alarm to warn the employee changing the tank of the occurrence of a high, unexpected release of EtO, then the employee must be provided and use a supplied air respirator acceptable for an unknown EtO concentration while changing the tank."
These letters comprise OSHA's current position on respiratory protection requirements for tank changing operations.
Compliance Assistiance
Several commenters requested that OSHA provide additional compliance assistance. These commenters included Dr. LaMontagne and Dr. Kelsey, the AHA, AFSCME, and the AFL CIO [Exs. 2 9; 2 2; Tr. 6/30/97]. Dr. LaMontagne and Dr. Kelsey suggested that, in general, OSHA could improve compliance with the standard by providing clarifications of interpretations of unclear requirements, by engaging in outreach efforts, and by participating in collaborative efforts with NIOSH, that might include providing information for a new NIOSH Current Intelligence Bulletin on EtO [Ex. 2 9]. Other specific recommendations made by Dr. LaMontagne and Dr. Kelsey, as well as by the AHA, AFSCME, and the AFL CIO are presented below, by topic.
Emergency Alert Requirement
A number of commenters indicated that OSHA should provide more guidance on the emergency alert requirement. Dr. LaMontagne and Dr. Kelsey recommended that OSHA publicly clarify its interpretation on how to comply with the emergency alert requirement (including whether or not EtO alarms are required), clarify that EtO alarms do not have to be set at levels at or below the permissible exposure limits in order to comply with the standard, and recommend alarm trigger levels in the 20 to 50 ppm range [Ex. 2 9]. The AHA asked OSHA to provide non mandatory guidance indicating acceptable types of alarm equipment and the level at which alarms should be set when in continuous use and indicated that this could be done by updating the non mandatory appendix material to §1910.1047 [Ex. 2 2; Tr. 6/30/97, p. 22]. The AHA further requested that OSHA clarify that there is no need for continuous alarm
monitoring after initial monitoring if exposures are below mandated levels [Ex. 2 2]. One other commenter, AFSCME, also recommended that OSHA provide additional guidance on methods of detecting emergency EtO releases. AFSCME suggested that such guidance could be provided through educational outreach efforts [Tr. 6/30/97, p. 32].
In response to the concerns expressed by these participants, OSHA notes that in the interval since the public meeting on this Lookback review was held in 1997, the Agency has made a host of EtO-related materials available on its Website, including materials developed by OSHA and various private-public sector coalitions. (See Chapter II for a discussion of these materials.)
Exposure Monitoring
Dr. LaMontagne and Dr. Kelsey, as well as the AFL CIO, recommended additional outreach efforts to educate employers about the importance of exposure monitoring [Ex. 2 9; Tr. 6/30/97, pp. 11 12]. Dr. LaMontagne and Dr. Kelsey indicated that OSHA could also use outreach efforts to encourage employers to perform additional EL monitoring of workers engaged in tasks such as tank changing that pose a risk of accidental exposure to EtO [Ex. 2 9]. OSHA believes that this issue warrants consideration for inclusion in any additional compliance assistance materials OSHA develops in the future for this standard.
Training
Dr. LaMontagne and Dr. Kelsey indicated that OSHA should encourage employers to provide emergency training to tank changing workers and other workers engaged in processes where accidental exposures could occur [Ex. 2 9]. The AFL CIO recommended that the Agency conduct a quality survey on how training is actually being conducted to comply with the standard [Tr. 6/30/97, p. 13].
OSHA notes that there is a wealth of material on its Website about tank changing; this material extensively covers all of the issues related to tank changing that participants raised.
FINDINGS CONCERNING COMPLEXITY OF THE STANDARD
A number of the comments presented at the public meeting and submitted to Docket H 200C requested clarification of the requirements of the standard. The provision for which commenters most frequently sought guidance was the emergency alert requirement [Exs. 2 2; 2 9; Tr. 6/30/97]. However, most commenters did not directly address the issue of whether the standard was considered to be complex.
The comments provided by representatives of large companies, such as the EOIC, BASF, and Clariant Corporation, suggested that these large companies understand the requirements of the standard and that they are not experiencing problems complying with the standard due to its complexity [Exs. 2 3; 2 5; 2 14]. More difficulty in understanding the requirements of the standard was reported in the hospital sector, which contains the majority of affected small entities. The findings of the Massachusetts Hospital EtO Health and Safety Study indicated that hospital sterilization department managers do experience some difficulty understanding and interpreting some of the requirements of the EtO standard. According to Dr. LaMontagne and Dr. Kelsey, hospital sterilizing department managers have reported difficulty in understanding "the AL trigger, the distinction between personal and area monitoring
and how they are complementary, how to comply with the alert requirement, and how long medical surveillance should continue to be provided once it has been triggered for an individual worker" [Ex. 2 9]. Dr. LaMontagne also concluded that medical surveillance providers may have an incomplete understanding of the medical surveillance requirements of the standard because of the observed deficits in collecting occupational histories and providing leukocyte differentials as part of medical surveillance [Ex. 2 9K]. These findings indicate that some employers may find some parts of the standard complex, but that, generally, compliance has not been unduly affected by the standard's complexity.
To improve employers' understanding of the standard, however, OSHA intends in the future to enhance compliance assistance materials that will aid employers in understanding some of the more complex portions of the standard. These include the medical surveillance, training, and emergency situation requirements.
FINDINGS CONCERNING DUPLICATION, CONFLICT OR OVERLAP
Major Federal Regulatory Entities with Responsibility for Regulating EtO
Ethylene oxide is currently regulated by four major federal regulatory entities in addition to OSHA - the Environmental Protection Agency, the Food and Drug Administration, the Department of Commerce, and the Department of Transportation. Each of these agencies regulates different aspects of EtO use and transport.
EPA regulates EtO as an air pollutant, as a pesticide, as a pesticidal residue in food, and as a hazardous waste [Ex. 2 9]. EPA regulates EtO under five of its major statutes; the Clean Air Act (CAA), the Federal Insecticide, Fungicide and Rodenticide Act (FIFRA), the Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA), the Superfund Amendments and Reauthorization Act (SARA), and the Toxic Substances Control Act (TSCA). EPA Clean Air Act regulations and FIFRA regulations are the regulations of most relevance to the current discussion. Under the Clean Air Act, EPA regulates airborne emissions of EtO from emissions sources such as chemical manufacturing plants and commercial sterilizing facilities by a national emissions standard for hazardous air pollutants (NESHAP). Under FIFRA, EPA is responsible for registering pesticides and regulating their use, application,
and labeling. EPA regulates EtO as a pesticide, primarily when it is used as a fumigant for medical items, spices, black walnuts, and other products. In 1996, EPA modified its FIFRA labels to include statements mandating that all users of EtO containing sterilants and fumigants observe the requirements of §1910.1047. This action had the beneficial effect of extending OSHA's worker protection requirements to public sector employers (primarily hospitals) that were not formerly under OSHA's jurisdiction.
The FDA regulates EtO sterilization of medical products and medical devices during manufacturing, regulates nonportable EtO gas sterilizers used to sterilize medical products, and sets acceptable levels for EtO residues in sterilized medical products and devices. The Food Quality Protection Act of 1996 (FQPA) recently transferred regulatory authority over liquid chemical sterilants and high level disinfectants used on critical and semi critical medical devices from EPA to FDA. The FDA also regulates EtO use in pharmaceutical manufacturing, food packaging materials, as a defoaming agent, as an industrial starch modifier, and in other miscellaneous uses.
The Department of Transportation regulates EtO under its Research and Special Program Administration (RSPA) regulations, which include general shipping and packaging requirements as well as packaging requirements for portable containers, tanks, and tank cars. The Coast Guard, both for port and shipping activities, classifies EtO as hazardous cargo and has specific requirements for its bulk shipment. The Department of Commerce regulates EtO as a commodity on the commodity control list for foreign trade.
The only regulatory conflict issue raised by commenters during this Lookback review involved an EPA air emissions standard for EtO using commercial sterilization and fumigation operations [Exs. 2 4; 2 9]. This EPA standard, the National Emission Standards for Hazardous Air Pollutants (NESHAP) for Ethylene Oxide Commercial Sterilization and Fumigation Operations, was promulgated in 1994 [FR Vol. 59, No. X, 12/6/1994, p. 62585]. It applies to commercial sterilization and fumigation operations using one ton or more of EtO per year. This standard does not apply to hospitals. Griffith Micro Science, a contract sterilizing company, as well as Drs. LaMontagne and Kelsey, made mention in their comments of the occurrence of several recent explosions at EtO using facilities. These explosions have been linked to the use of catalytic oxidizer emission control technology implemented to comply with
the EPA standard [Exs. 2 4; 2 9]. In response to this issue, EPA amended its rules by eliminating the maximum achievable control technology requirements for chamber exhaust vent emissions. According to Griffith Micro Science, the conflict that this standard raises with the OSHA standard is the possibility that in plant EtO concentrations (and thus, worker exposure levels) may increase due to the need to implement an alternative emission control technology to comply with this standard [Ex. 2 4]. Griffith Micro Science commented that such an increase in EtO concentrations would increase respirator usage and may even require use of pressurized air supply systems to achieve the PEL [Ex. 2 4]. EPA has not required alternative emission control technology. Employers can continue to ventilate the chamber before opening the sterilizer door. There should be no increase in worker exposures. Furthermore,
the two agencies' rules do not conflict in the sense that an employer complying with one agencies rules would in fact be in noncompliance with the other agency's rule. Finally, no other commenter raised this issue and no problems in practice seemed to have arisen.
FINDINGS CONCERNING CHANGES IN TECHNOLOGICAL AND ECONOMIC CONDITIONS
OSHA concludes that, at this time, there are no changes in technological or economic conditions that would warrant the reopening of the EtO standard. As part of this Lookback review, OSHA conducted independent research to identify significant technological and economic developments that occurred in the affected industries after the promulgation of the standard and also reviewed public comments on this subject. (These technological and economic developments are discussed in more detail in Chapter V.) Among the major technological developments that have occurred in the affected industries since the promulgation of the standard are: improvements in sterilizer technology for use in both hospital and commercial sterilization applications, the phasing out of CFCs in EtO gas blends, the increased use of 100% EtO following the phase out of CFC containing gas blends, the growth in use of a number
of alternative sterilants and sterilizing processes, and increasing use of contract sterilizers to perform EtO sterilization. Many of these technological developments have contributed to the observed reduction in occupational exposures to EtO. The major economic development that has occurred in the affected industries was a reduction in the cost of sterilizing equipment, despite concurrent improvements in sterilizer technology. However, none of these developments appears to have adversely affected the ability of small entities to comply with the EtO standard or to have increased the burden of the standard in a manner that would result in a significant economic impact on a substantial number of small entities.
Similarly, none of the comments received by OSHA indicated that there were any technological or economic feasibility problems preventing affected businesses or entities from complying with the EtO standard. Only a few of the commenters commented on the effects of new developments in technology or changes in economic conditions in the industry sectors affected by the EtO standard. The few comments related to technological changes that were submitted to Docket H 200C addressed changes in EtO gas blend usage patterns, improvements in monitoring technology, improvements in sterilizer technology, and substitution trends. Only two comments were received concerning economic changes. These comments addressed the cost of personal and area exposure monitoring and sterilization operation cost reductions driven by substitution and use reduction. Further, the American Hospital Association, which
represents the hospital sector, submitted comments to the record indicating that the standard was feasible, "effective as written," and "requires no significant change in the context of this review" and the EOIC, which represents individual companies in each of the sectors other than hospitals that are affected by the EtO standard, indicated that it "supports OSHA's EtO standard as technically and economically feasible" [Exs. 2 2; 2 6]. OSHA therefore finds that the standard should not be modified or rescinded due to changes in technological or economic conditions.
OUTCOME OF THE EXECUTIVE ORDER 12866 REVIEW
Under the review requirements of Section 5 of Executive Order 12866, OSHA is required to determine whether this rule should be eliminated or modified to make it more effective, less burdensome, or to bring it into greater alignment with the EO's priorities and principles. OSHA has concluded that there is a continued need for the standard. This finding is based on the following conclusions: (1) new epidemiological and health risk studies released since the promulgation of the standard in 1984 have supported findings of health risks associated with exposure to EtO; (2) exposure monitoring data from several sources indicate that occupational exposure to EtO has fallen markedly since the EtO standard went into effect; (3) OSHA enforcement program data indicate that overexposures and accidental releases of EtO continue to occur at workplaces that are not in compliance with the standard; and
(4) both employer and employee representatives indicate a continued need for the standard. OSHA has therefore concluded that the standard continues to be needed to protect workers' health and safety. This finding is discussed in more detail in Chapter III. In addition to the strong evidence supporting the continued need for the standard, no evidence was submitted to the docket or identified by OSHA in the course of this Lookback review to suggest that the rule was imposing either a significant impact on a substantial number of small entities or that it was causing an excessive compliance burden on any part of the regulated community. Accordingly, OSHA finds that there is no need at this time to modify the rule through a rulemaking process to make it more effective or less burdensome. However, as described below, OSHA has decided, in response to some of the concerns expressed by commenters,
to undertake a number of compliance assistance and outreach activities that will facilitate compliance with the standard.
COMPLIANCE ASSISTANCE ACTIVITIES OSHA IS CONSIDERING IN RESPONSE TO THIS LOOKBACK REVIEW
As a result of this Lookback review and the comments received from participants in the review process, OSHA is considering issuing the following outreach and compliance assistance materials and interpretations:
- Enhance compliance assistance materials clarifying and explaining the exposure monitoring requirements of the standard, particularly as these relate to the standard's emergency alert requirements and to operations potentially involving overexposures to the excursion limit. Examples of such operations include tank changing operations and tank car unloading operations.
- Enhance compliance assistance materials explaining the standard's medical surveillance requirements, particularly as these relate to the taking of occupational histories and the standard's triggers for medical surveillance.
- Review and enhance other training and compliance assistance materials to reflect comments and OSHA's experience.
- Review existing enforcement interpretations as these relate to tank changing operations and the use of respiratory protection in these operations.
APPENDIX A
Growth of Industries Affected by the EtO Standard, 1983-1997
This appendix presents added information related to the small entity analysis described in Chapter VI. Figures 1 to 8 show the growth or decline in the number of establishments of various sizes for the each of the eight SICs containing small entities affected by the EtO standard. For these same SICs, tables 9 to 16 show the number of establishments falling within various employment levels and the percentages of all establishments in the SIC that employ fewer than 500 people. All of these figures and tables were derived from data contained in the United States Department of Commerce's annual 1983 and 1985-1997 County Business Patterns reports.
Figure A-1: SIC 2099, Food Preparations, NEC
Text version of Chart
Chart Title: Figure A-1: SIC 2099, Food Preparations, NEC
Chart Type: Vertical bar graph
Chart Elements: 16 - Two bars, one for 1983 and one for 1997, for each employment range showing the number of establishments containing small entities affected by the EtO standard.
Chart Data:
| |
Employment |
| 1-4 |
5-9 |
10-19 |
20-49 |
50-99 |
100-249 |
250-499 |
1-499 |
| Number of Establishments |
1983 |
419 |
293 |
308 |
392 |
185 |
150 |
46 |
1,793 |
| 1997 |
522 |
266 |
283 |
331 |
181 |
131 |
42 |
1,756 |
Figure A-2: SIC 2834, Pharmaceutical Preparations
 |
Text version of Chart
Chart Title: Figure A-2: SIC 2834, Pharmaceutical Preparations
Chart Type: Vertical bar graph
Chart Elements: 16 - Two bars, one for 1983 and one for 1997, for each employment range showing the number of establishments containing small entities affected by the EtO standard.
Chart Data:
| |
Employment |
| 1-4 |
5-9 |
10-19 |
20-49 |
50-99 |
100- |
250- |
1-499 |
| Number of Establishments |
1983 |
167 |
89 |
80 |
109 |
75 |
68 |
45 |
633 |
| 1997 |
203 |
74 |
110 |
129 |
79 |
94 |
53 |
742 |
Figure A-3: SIC 2843, Surface Active Agents, Finishing Agents, Sulfonated Oils, and Assistants
 |
Text version of Chart
Chart Title: Figure A-3: SIC 2843, Surface Active Agents, Finishing Agents, Sulfonated Oils, and Assistants
Chart Type: Vertical bar graph
Chart Elements: 16 - Two bars, one for 1983 and one for 1997, for each employment range showing the number of establishments containing small entities affected by the EtO standard.
Chart Data:
| |
Employment |
| 1-4 |
5-9 |
10-19 |
20-49 |
50-99 |
100- |
250- |
1-499 |
| Number of Establishments |
1983 |
45 |
32 |
36 |
54 |
22 |
12 |
1 |
202 |
| 1997 |
42 |
28 |
36 |
49 |
31 |
17 |
3 |
206 |
Figure A-4: SIC 2869, Industrial Organic Chemicals, Not Elsewhere Classified
 |
Text version of Chart
Chart Title: Figure A-4: SIC 2869, Industrial Organic Chemicals, Not Elsewhere Classified
Chart Type: Vertical bar graph
Chart Elements: 16 - Two bars, one for 1983 and one for 1997, for each employment range showing the number of establishments containing small entities affected by the EtO standard.
Chart Data:
| |
Employment |
| 1-4 |
5-9 |
10-19 |
20-49 |
50-99 |
100- |
250- |
1-499 |
| Number of Establishments |
1983 |
122 |
77 |
79 |
123 |
91 |
81 |
41 |
614 |
| 1997 |
127 |
63 |
78 |
145 |
109 |
133 |
44 |
699 |
Figure A-5: SIC 3841, Surgical and Medical Instruments and Apparatus
 |
Text version of Chart
Chart Title: Figure A-5: SIC 3841, Surgical and Medical Instruments and Apparatus
Chart Type: Vertical bar graph
Chart Elements: 16 - Two bars, one for 1983 and one for 1997, for each employment range showing the number of establishments containing small entities affected by the EtO standard.
Chart Data:
| |
Employment |
| 1-4 |
5-9 |
10-19 |
20-49 |
50-99 |
100- |
250- |
1-499 |
| Number of Establishments |
1983 |
296 |
112 |
130 |
144 |
69 |
82 |
42 |
875 |
| 1997 |
478 |
287 |
253 |
242 |
124 |
104 |
66 |
1,554 |
Figure A-6: SIC 3842, Orthopedic, Prosthetic, and Surgical Appliances and Supplies
 |
Text version of Chart
Chart Title: Figure A-6: SIC 3842, Orthopedic, Prosthetic, and Surgical Appliances and Supplies
Chart Type: Vertical bar graph
Chart Elements: 16 - Two bars, one for 1983 and one for 1997, for each employment range showing the number of establishments containing small entities affected by the EtO standard.
Chart Data:
| |
Employment |
| 1-4 |
5-9 |
10-19 |
20-49 |
50-99 |
100- |
250- |
1-499 |
| Number of Establishments |
1983 |
433 |
239 |
212 |
189 |
109 |
78 |
39 |
1,299 |
| 1997 |
560 |
268 |
245 |
292 |
168 |
141 |
56 |
1,730 |
Figure A-7: SIC 7389, Business Services, NEC (includes Contract Sterilizers)1
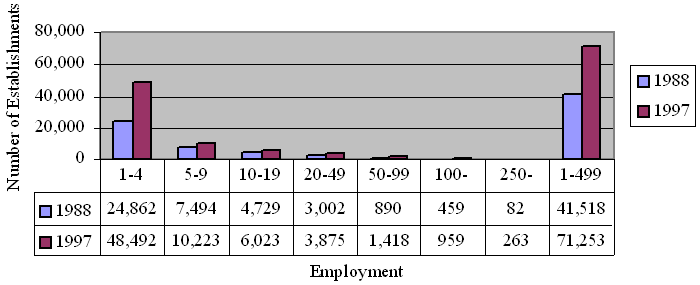 |
1Only 1988 - 1997 data were available for SIC 7389.
Text version of Chart
Chart Title: Figure A-7: SIC 7389, Business Services, NEC (includes Contract Sterilizers)
Chart Type: Vertical bar graph
Chart Elements: 16 - Two bars, one for 1988 and one for 1997, for each employment range showing the number of establishments containing small entities affected by the EtO standard.
Chart Data:
| |
Employment |
| 1-4 |
5-9 |
10-19 |
20-49 |
50-99 |
100- |
250- |
1-499 |
| Number of Establishments |
1988 |
24,862 |
7,494 |
4,729 |
3,002 |
890 |
459 |
82 |
41,518 |
| 1997 |
48,492 |
10,223 |
6,023 |
3,875 |
1,418 |
959 |
263 |
71,253 |
Figure A-8: SIC 806, Hospitals
 |
Text version of Chart
Chart Title: Figure A-8: SIC 806, Hospitals
Chart Type: Vertical bar graph
Chart Elements: 16 - Two bars, one for 1983 and one for 1997, for each employment range showing the number of establishments containing small entities affected by the EtO standard.
Chart Data:
| |
Employment |
| 1-4 |
5-9 |
10-19 |
20-49 |
50-99 |
100- |
250- |
1-499 |
| Number of Establishments |
1983 |
448 |
226 |
177 |
266 |
508 |
1,102 |
963 |
3,690 |
| 1997 |
160 |
38 |
61 |
181 |
616 |
1,721 |
1,387 |
4,164 |
Table A-9: Firms by Numbers of Employees
SIC 2099, Food Preparations, NEC |
| Year |
Number of Employees |
Number
<500 |
Percent
<500 |
| 1-4 |
5-9 |
10-19 |
20-49 |
50-99 |
100-249 |
250-499 |
500+ |
Total |
| 1983 |
419 |
293 |
308 |
392 |
185 |
150 |
46 |
8 |
1,801 |
1,793 |
99.6% |
| 1984 |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
| 1985 |
413 |
288 |
291 |
361 |
200 |
154 |
53 |
9 |
1,769 |
1,760 |
99.5% |
| 1986 |
413 |
284 |
256 |
354 |
198 |
155 |
49 |
12 |
1,721 |
1,709 |
99.3% |
| 1987 |
485 |
269 |
312 |
391 |
203 |
168 |
53 |
15 |
1,896 |
1,881 |
99.2% |
| 1988 |
355 |
253 |
265 |
314 |
163 |
116 |
26 |
9 |
1,501 |
1,492 |
99.4% |
| 1989 |
331 |
206 |
256 |
313 |
167 |
113 |
30 |
9 |
1,425 |
1,416 |
99.4% |
| 1990 |
328 |
215 |
241 |
309 |
164 |
116 |
30 |
7 |
1,410 |
1,403 |
99.5% |
| 1991 |
359 |
211 |
260 |
311 |
159 |
118 |
33 |
6 |
1,457 |
1,451 |
99.6% |
| 1992 |
414 |
248 |
254 |
336 |
162 |
109 |
34 |
7 |
1,564 |
1,557 |
99,6% |
| 1993 |
499 |
272 |
292 |
324 |
159 |
126 |
27 |
10 |
1,709 |
1,699 |
99.4% |
| 1994 |
519 |
277 |
287 |
319 |
158 |
124 |
36 |
5 |
1,725 |
1,720 |
99.7% |
| 1995 |
519 |
299 |
246 |
322 |
181 |
132 |
30 |
9 |
1,738 |
1,729 |
99.5% |
| 1996 |
497 |
261 |
262 |
318 |
180 |
145 |
29 |
11 |
1,703 |
1,692 |
99.4% |
| 1997 |
522 |
266 |
283 |
331 |
181 |
131 |
42 |
15 |
1,771 |
1,756 |
99.2% |
Table A-10: Firms by Numbers of Employees
SIC 2834, Pharmaceutical Preparations
| Year |
Number of Employees |
Number
<500 |
Percent
<500 |
| 1-4 |
5-9 |
10-19 |
20-49 |
50-99 |
100-249 |
250-499 |
500+ |
Total |
| 1983 |
167 |
89 |
80 |
109 |
75 |
68 |
45 |
58 |
691 |
633 |
91.6% |
| 1984 |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
| 1985 |
163 |
89 |
83 |
102 |
75 |
73 |
45 |
58 |
688 |
630 |
91.6% |
| 1986 |
152 |
97 |
75 |
103 |
65 |
77 |
52 |
57 |
678 |
621 |
91.6% |
| 1987 |
168 |
103 |
93 |
113 |
71 |
87 |
56 |
52 |
743 |
691 |
93.0% |
| 1988 |
146 |
99 |
97 |
125 |
62 |
81 |
53 |
55 |
718 |
663 |
92.3% |
| 1989 |
128 |
90 |
93 |
123 |
71 |
81 |
55 |
58 |
699 |
641 |
91.7% |
| 1990 |
125 |
67 |
96 |
133 |
69 |
78 |
51 |
61 |
680 |
619 |
91.0% |
| 1991 |
135 |
75 |
105 |
114 |
77 |
87 |
48 |
62 |
703 |
641 |
91.2% |
| 1992 |
153 |
85 |
120 |
132 |
75 |
89 |
52 |
61 |
770 |
709 |
92.1% |
| 1993 |
188 |
68 |
101 |
129 |
73 |
93 |
56 |
57 |
765 |
708 |
92.5% |
| 1994 |
159 |
66 |
83 |
122 |
71 |
88 |
57 |
60 |
706 |
646 |
91.5% |
| 1995 |
163 |
58 |
88 |
114 |
73 |
95 |
55 |
65 |
711 |
646 |
90.9% |
| 1996 |
190 |
80 |
95 |
126 |
86 |
96 |
52 |
65 |
790 |
725 |
91.8% |
| 1997 |
203 |
74 |
110 |
129 |
79 |
94 |
53 |
57 |
799 |
742 |
92.9% |
Table A-11: Firms by Numbers of Employees
SIC 2843, Surface Active Agents, Finishing Agents, Sulfonated Oils and Assistants
| Year |
Number of Employees |
Number
<500 |
Percent
<500 |
| 1-4 |
5-9 |
10-19 |
20-49 |
50-99 |
100-249 |
250-499 |
500+ |
Total |
| 1983 |
45 |
32 |
36 |
54 |
22 |
12 |
1 |
1 |
203 |
202 |
99.5% |
| 1984 |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
| 1985 |
47 |
28 |
38 |
49 |
29 |
14 |
1 |
2 |
208 |
206 |
99.0% |
| 1986 |
42 |
27 |
39 |
48 |
23 |
17 |
1 |
2 |
199 |
197 |
99.0% |
| 1987 |
45 |
31 |
27 |
51 |
24 |
19 |
2 |
1 |
200 |
199 |
99.5% |
| 1988 |
41 |
26 |
39 |
46 |
27 |
19 |
5 |
1 |
204 |
203 |
99.5% |
| 1989 |
35 |
28 |
39 |
43 |
25 |
20 |
5 |
1 |
196 |
195 |
99.5% |
| 1990 |
37 |
30 |
35 |
44 |
26 |
19 |
5 |
1 |
197 |
196 |
99.5% |
| 1991 |
45 |
32 |
31 |
44 |
21 |
21 |
5 |
1 |
200 |
199 |
99.5% |
| 1992 |
47 |
33 |
30 |
45 |
23 |
17 |
5 |
1 |
201 |
200 |
99.5% |
| 1993 |
45 |
29 |
33 |
49 |
22 |
15 |
6 |
0 |
199 |
199 |
100.0% |
| 1994 |
36 |
32 |
28 |
54 |
27 |
15 |
5 |
0 |
197 |
197 |
100.0% |
| 1995 |
33 |
26 |
32 |
55 |
30 |
19 |
3 |
1 |
199 |
198 |
99.5% |
| 1996 |
38 |
29 |
38 |
53 |
27 |
19 |
4 |
0 |
208 |
208 |
100.0% |
| 1997 |
42 |
28 |
36 |
49 |
31 |
17 |
3 |
1 |
207 |
206 |
99.5% |
Table A-12: Firms by Numbers of Employees
SIC 2869, Industrial Organic Chemicals, NEC
| Year |
Number of Employees |
Number
<500 |
Percent
<500 |
| 1-4 |
5-9 |
10-19 |
20-49 |
50-99 |
100-249 |
250-499 |
500+ |
Total |
| 1983 |
122 |
77 |
79 |
123 |
91 |
81 |
41 |
52 |
666 |
614 |
92.2% |
| 1984 |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
| 1985 |
111 |
60 |
80 |
117 |
97 |
84 |
42 |
44 |
635 |
591 |
93.1% |
| 1986 |
95 |
62 |
79 |
114 |
100 |
87 |
41 |
41 |
619 |
578 |
93.4% |
| 1987 |
95 |
72 |
82 |
115 |
96 |
91 |
39 |
42 |
632 |
590 |
93.4% |
| 1988 |
95 |
72 |
86 |
134 |
99 |
106 |
47 |
43 |
685 |
642 |
93.7% |
| 1989 |
86 |
71 |
75 |
131 |
89 |
111 |
45 |
43 |
651 |
608 |
93.4% |
| 1990 |
79 |
68 |
77 |
124 |
96 |
115 |
44 |
45 |
648 |
603 |
93.1% |
| 1991 |
99 |
62 |
79 |
120 |
98 |
110 |
48 |
45 |
661 |
616 |
93.2% |
| 1992 |
110 |
71 |
89 |
108 |
109 |
114 |
46 |
45 |
692 |
647 |
93.5% |
| 1993 |
99 |
81 |
86 |
125 |
106 |
109 |
45 |
44 |
695 |
651 |
93.7% |
| 1994 |
107 |
70 |
88 |
113 |
116 |
112 |
43 |
42 |
691 |
649 |
93.9% |
| 1995 |
120 |
68 |
80 |
124 |
100 |
125 |
41 |
40 |
698 |
658 |
94.3% |
| 1996 |
101 |
54 |
68 |
124 |
101 |
122 |
45 |
37 |
652 |
615 |
94.3% |
| 1997 |
127 |
63 |
78 |
145 |
109 |
133 |
44 |
44 |
743 |
699 |
94.1% |
Table A-13: Firms by Numbers of Employees
SIC 3841, Surgical and Medical Instruments and Apparatus
| Year |
Number of Employees |
Number
<500 |
Percent
<500 |
| 1-4 |
5-9 |
10-19 |
20-49 |
50-99 |
100-249 |
250-499 |
500+ |
Total |
| 1983 |
296 |
112 |
130 |
144 |
69 |
82 |
42 |
27 |
902 |
875 |
97.0% |
| 1984 |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
| 1985 |
278 |
120 |
125 |
160 |
87 |
801 |
35 |
25 |
910 |
885 |
97.3% |
| 1986 |
294 |
133 |
133 |
159 |
85 |
80 |
40 |
26 |
950 |
924 |
97.3% |
| 1987 |
350 |
190 |
164 |
170 |
84 |
90 |
37 |
28 |
1,113 |
1,085 |
97.5% |
| 1988 |
324 |
168 |
185 |
200 |
89 |
87 |
50 |
34 |
1,137 |
1,103 |
97.0% |
| 1989 |
309 |
191 |
160 |
203 |
104 |
90 |
41 |
46 |
1,144 |
1,098 |
96.0% |
| 1990 |
295 |
170 |
164 |
197 |
117 |
95 |
45 |
44 |
1,127 |
1,083 |
96.1% |
| 1991 |
315 |
160 |
201 |
190 |
116 |
106 |
52 |
44 |
1,184 |
1,140 |
96.3% |
| 1992 |
442 |
198 |
217 |
217 |
123 |
118 |
58 |
43 |
1,416 |
1,373 |
97.0% |
| 1993 |
417 |
207 |
201 |
209 |
117 |
110 |
71 |
47 |
1,379 |
1,332 |
96.6% |
| 1994 |
399 |
195 |
211 |
214 |
120 |
110 |
76 |
41 |
1,366 |
1,325 |
97.0% |
| 1995 |
370 |
223 |
193 |
215 |
126 |
108 |
97 |
43 |
1,345 |
1,302 |
97.0% |
| 1996 |
484 |
265 |
216 |
207 |
124 |
113 |
60 |
72 |
1,541 |
1,469 |
95.3% |
| 1997 |
478 |
287 |
253 |
242 |
124 |
104 |
66 |
49 |
1,603 |
1,554 |
96.9% |
Table A-14: Firms by Numbers of Employees
SIC 3842, Orthopedic, Prosthetic and Surgical Appliances and Supplies
| Year |
Number of Employees |
Number
<500 |
Percent
<500 |
| 1-4 |
5-9 |
10-19 |
20-49 |
50-99 |
100-249 |
250-499 |
500+ |
Total |
| 1983 |
433 |
239 |
212 |
189 |
109 |
78 |
39 |
24 |
1,323 |
1,299 |
98.2% |
| 1984 |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
| 1985 |
408 |
246 |
200 |
191 |
117 |
99 |
48 |
28 |
1,337 |
1,309 |
97.9% |
| 1986 |
425 |
248 |
197 |
194 |
114 |
97 |
45 |
33 |
1,333 |
1,300 |
97.5% |
| 1987 |
470 |
296 |
198 |
214 |
127 |
97 |
44 |
35 |
1,481 |
1,446 |
97.6% |
| 1988 |
408 |
274 |
218 |
224 |
147 |
110 |
52 |
31 |
1,464 |
1,433 |
97.9% |
| 1989 |
382 |
276 |
206 |
216 |
148 |
120 |
52 |
29 |
1,429 |
1,400 |
98.0% |
| 1990 |
401 |
267 |
218 |
226 |
129 |
118 |
58 |
29 |
1,446 |
1,417 |
98.0% |
| 1991 |
441 |
244 |
255 |
215 |
146 |
119 |
51 |
37 |
1,508 |
1,471 |
97.5% |
| 1992 |
566 |
293 |
244 |
255 |
144 |
126 |
51 |
39 |
1,718 |
1,679 |
97.7% |
| 1993 |
576 |
280 |
255 |
268 |
151 |
147 |
45 |
45 |
1,767 |
1,722 |
97.5% |
| 1994 |
546 |
308 |
252 |
276 |
156 |
135 |
58 |
36 |
1,767 |
1,731 |
98.0% |
| 1995 |
544 |
305 |
249 |
278 |
158 |
126 |
58 |
38 |
1,756 |
1,718 |
97.8% |
| 1996 |
500 |
264 |
227 |
258 |
166 |
123 |
49 |
40 |
1,627 |
1,587 |
97.5% |
| 1997 |
560 |
268 |
245 |
292 |
168 |
141 |
56 |
34 |
1,764 |
1,730 |
98.1% |
Table A-15: Firms by Numbers of Employees
SIC 7389, Business Services, NEC (includes Contract Sterilizers)
| Year |
Number of Employees |
Number
<500 |
Percent
<500 |
| 1-4 |
5-9 |
10-19 |
20-49 |
50-99 |
100-249 |
250-499 |
500+ |
Total |
| 1983 |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
| 1984 |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
| 1985 |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
| 1986 |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
| 1987 |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
| 1988 |
24,862 |
7,494 |
4,729 |
3,002 |
890 |
459 |
82 |
44 |
41,562 |
41,518 |
99.9% |
| 1989 |
21,547 |
6,917 |
4,682 |
3,144 |
860 |
439 |
114 |
52 |
37,755 |
37,703 |
99.9% |
| 1990 |
26,792 |
7,499 |
4,997 |
3,275 |
881 |
447 |
129 |
59 |
44,079 |
44,020 |
99.9% |
| 1991 |
31,582 |
8,158 |
5,251 |
3,575 |
958 |
521 |
130 |
77 |
50,252 |
50,175 |
99.8% |
| 1992 |
31,120 |
7,779 |
4,942 |
3,401 |
1,088 |
538 |
155 |
57 |
49,080 |
49,023 |
99.9% |
| 1993 |
37,408 |
8,467 |
5,299 |
3,655 |
1,122 |
651 |
151 |
76 |
56,829 |
56,753 |
99.9% |
| 1994 |
40,393 |
8,884 |
5,583 |
3,668 |
1,211 |
733 |
166 |
87 |
60,725 |
60,638 |
99.9% |
| 1995 |
35,444 |
7,695 |
4,944 |
3,338 |
1,231 |
670 |
180 |
94 |
53,596 |
53,502 |
99.8% |
| 1996 |
42,121 |
8,507 |
5,180 |
3,374 |
1,230 |
785 |
209 |
104 |
61,560 |
61,456 |
99.8% |
| 1997 |
48,492 |
10,223 |
60,023 |
3,875 |
1,418 |
959 |
263 |
149 |
71,402 |
71,253 |
99.8% |
Table A-16: Firms by Numbers of Employees
IC 806, Hospitals
| Year |
Number of Employees |
Number
<500 |
Percent
<500 |
| 1-4 |
5-9 |
10-19 |
20-49 |
50-99 |
100-249 |
250-499 |
500+ |
Total |
| 1983 |
448 |
226 |
177 |
266 |
508 |
1,102 |
963 |
1,813 |
5,503 |
3,690 |
67.1% |
| 1984 |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
| 1985 |
524 |
203 |
193 |
315 |
574 |
1,224 |
999 |
1,793 |
5,825 |
4,032 |
69.2% |
| 1986 |
498 |
161 |
142 |
289 |
566 |
1,253 |
991 |
1,809 |
5,709 |
3,900 |
68.3% |
| 1987 |
375 |
160 |
147 |
346 |
683 |
1,363 |
997 |
1,882 |
5,953 |
4,071 |
68.4% |
| 1988 |
168 |
38 |
45 |
218 |
540 |
1,288 |
1,019 |
1,905 |
5,221 |
3,316 |
63.5% |
| 1989 |
199 |
50 |
64 |
236 |
622 |
1,422 |
1,179 |
2,441 |
6,213 |
3,772 |
60.7% |
| 1990 |
247 |
70 |
73 |
230 |
593 |
1,455 |
1,144 |
2,515 |
6,327 |
3,812 |
60.2% |
| 1991 |
373 |
86 |
93 |
251 |
584 |
1,481 |
1,137 |
2,566 |
6,571 |
4,005 |
60.9% |
| 1992 |
427 |
143 |
131 |
293 |
586 |
1,477 |
1,148 |
2,675 |
6,880 |
4,205 |
61.1% |
| 1993 |
284 |
49 |
69 |
238 |
741 |
1,787 |
1,355 |
2,828 |
7,351 |
4,523 |
61.5% |
| 1994 |
278 |
78 |
83 |
238 |
728 |
1,836 |
1,331 |
2,864 |
7,436 |
4,572 |
61.5% |
| 1995 |
297 |
98 |
112 |
236 |
701 |
1,786 |
1,360 |
2,855 |
7,445 |
4,590 |
61.7% |
| 1996 |
276 |
66 |
92 |
234 |
639 |
1,741 |
1,388 |
2,846 |
7,282 |
4,436 |
60.9% |
| 1997 |
160 |
38 |
61 |
181 |
616 |
1,721 |
1,387 |
2,821 |
6,985 |
4,164 |
59.6% |
FOOTNOTES
163 FR 34139 (June 23, 1998).
2Stanford Research Institute, Chemical Economics Handbook (CEH), August 2000; 1996 data are from the Henry Chinn and Marjorie Petesch, Chemical Economics Handbook Marketing Research Report, "Ethylene Oxide," April 1997. OSHA initiated this Lookback review in 1997 and has continued to work on it as resources permitted through 2001. The data reported in this review derive from sources published in those years; OSHA has updated these data where more current information was available.
3CEH Marketing Research Report, "Ethylene Oxide," April 1997; CEH, August 2000.
4Ibid.
5Heiden Associates, Inc., "An Estimate of Industry Costs for Compliance with Two Ethylene Oxide Workplace STEL Scenarios: Ethylene Oxide Production and Ethoxylation Plants," February 5, 1988 [Docket H-200B, Ex. 205-6B].
6Ten of the 11 EtO producer firms produce ethylene glycol at 11 facilities, 5 EtO producer firms produce glycol ethers at 8 facilities, and 4 EtO producer firms produce ethanolamines at 4 facilities. Eliminating double-counting of firms and facilities, OSHA estimates that 10 EtO producer firms operate 11 ethoxylation facilities.
7Although EtO is also used as a process input in the production of polyether polyols, tetraethylene glycol, hydroxyethyl cellulose, and other miscellaneous chemicals, insufficient information was available on these uses to permit estimation of the number of firms involved in producing these products. These uses account for approximately 361 million pounds of EtO per year (approximately 5% of total consumption).
8Robert F. Modler, Rene Willhalm, and Yuka Yoshida, Stanford Research Institute CEH Marketing Research Report, "Surfactants, Household Detergents and Their Raw Materials," December, 1994.
9Ibid.
10Robert F. Modler, Eric Anderson, and Yuka Yoshida, CEH Marketing Research Report, "Detergent Alcohols," December, 1996.
11Forty-six surfactant production facilities operated by stand-alone ethoxylators + (9 surfactant production facilities operated by EtO producers - 3 facilities already included in the 12 facilities producing other EtO derivatives) = 52 facilities.
12The regulatory impact analysis for the 1988 rulemaking included both medical products manufacturers and contract sterilizers of medical products in the category of medical products sterilizers. It is unclear whether pharmaceutical manufacturers were included in this category [53 FR 11421].
13"Gas-Plasma Sterilization In, EtO Out With New AbTox Sterilizer System," Health Industry Today, April 1, 1995.
14Glaser, Z. R. "Special Occupational Hazards with Control Recommendations: Use of Ethylene Oxide as a Sterilant in Medical Facilities," Publication No. 77-200. Rockville, MD: National Institute for Occupational Safety and Health, U.S. Department of Health, Education, and Welfare, 1977.
15Dr. Anthony LaMontagne, "The Massachusetts Hospital EtO Health & Safety Study: A Summary Report for Study Participants & Supporters," 1996.
16Patricia B. McClearn, "Survey of Chemical Use in Colorado Hospitals," Colorado Hospitals for a Healthy Environment, Denver, Colorado, June, 1996, Docket H-200.
17Twenty of the responding hospitals had under 50 licensed beds; 10 had 50 to 100 licensed beds; 7 had 150 to 300 licensed beds; and 11 had over 300 licensed beds. (Four respondents chose not to reveal bed information in order to preserve their anonymity.)
18This 1984 estimate included state and municipal employees both in State-plan and non-State-plan States; only state and municipal employees in OSHA State-plan States are affected by OSHA rules.
19As with the 1984 estimate, this estimate includes state and municipal workers in Federal OSHA states. However, since current EPA labeling requirements for EtO sterilants require all users to comply with the requirements of §1910.1047, these workers are protected by the OSHA EtO standard to some extent.
20Environmental Protection Agency, OAR/OAQPS, "Ethylene Oxide Emissions from Commercial Sterilization/Fumigation Operations - Background Information for Proposed Standards" (Pub No. EPA-435/D-93-016), March 1993, pp. 8-1/8-2.
21Ibid.
22EPA's 1993 background information document drew upon the following sources of information on EtO-using commercial sterilizers: (1) a 1985 Health Industry Manufacturers Association survey of medical equipment suppliers, (2) a 1986 EPA information collection request, and (3) EPA follow-up letters submitted in July 1988 and July 1989, respectively, to subsets of the firms responding to the 1986 information collection request.
23In its 1993 report, EPA used annual sales of less than $3.5 million as the standard for defining a small entity.
24Karen G. Beagley, AEtO and Gamma Sterilization: Room for Both," Pharmaceutical and Medical Packaging News, May 1996, pp. 29-32.
25Food and Drug Administration, "Guide to Inspections of Sterile Drug Substance Manufacturers," available at http://www.fda.gov/ora/inspect_ref/igs/subst.html
26Office of Advocacy, Small Business Administration data provided by the Bureau of Census at: http://www.sba.gov/ADVO/stats/int_data.html>.
27The North American Survey developed by CMR and MDS Nordion presents a profile of 100 manufacturing sites and 16 contract sterilization sites. The survey was conducted between August and September 1996.
28Heiden Associates, Inc., "A Medical Products Industry Profile for Evaluating Compliance with Two Ethylene Oxide Workplace STEL Scenarios: 10 ppm STEL and 5 ppm STEL," 1988 [Docket H-200B, Ex. 205-6].
29The American Spice Trade Association, Inc., "Final Report to EPA: 1995-1996 Trade Practices Survey," July 31, 1996.
30Ibid.
31"Venture-backed IPOs: Gamma Irradiation Service Provider Plans IPO Launch," Going Public: The IPO Reporter, July 21, 1997.
32The North American Survey developed by CMR and MDS Nordion presents a profile of 100 manufacturing sites and 16 contract sterilization sites. The survey was conducted between August and September 1996.
33Roger Renstrom, "Sterilizing Methods Duke It Out In Market," Plastics News, March 3, 1997.
34Ibid.
35"California Has Twice as Many Medical Mfgrs As Any Other State, Official FDA Data Says", August 1, 1996.
36Roger Renstrom, "Sterilizing Methods Duke It Out In Market," Plastic News, March 3, 1997.
37<http://www.cosmedgroup.com/location.html>
38Massachusetts hospitals, which have a relatively high concentration of certified providers and a history of hospital EtO health and safety training efforts, may represent a best case scenario of compliance with the requirements of the EtO standard.
39La Montagne, A.D. and Kelsey, K.T., "Evaluating OSHA's Ethylene Oxide Standard: Employer Exposure-Monitoring Activities in Massachusetts Hospitals from 1985 through 1993," American Journal of Public Health, July 1997, Vol. 87, No. 7, pp. 1119-1125.
40Ibid.
41Ibid.
42Ibid.
43For comparison purposes, in 1997, Mr. Pier-George Zanoni, an industrial hygienist who testified at the public meeting on behalf of the American Hospital Association, estimated that 50% of EtO-using hospitals in the State of Michigan had installed continuous alarms [Letter from Pier-George Zanoni of the Michigan Health and Hospital Association to Margaret J. Hardy of the American Hospital Association, dated 7/31/97].
44"Evaluating OSHA's Ethylene Oxide Standard," July 1997.
45Ibid.
46Ibid.
47Anthony D. LaMontagne, Thomas W. Mangione, David C. Christiani, and Karl T. Kelsey, "Medical Surveillance for Ethylene Oxide Exposure: Practices and Clinical Findings in Massachusetts Hospitals," Journal of Occupational and Environmental Medicine, Vol. 38, No. 2, February 1996.
48Anthony D. LaMontagne, Rima E. Rudd, Thomas W. Mangione, and Karl T. Kelsey, "Determinants of the Provision of Ethylene Oxide Medical Surveillance in Massachusetts Hospitals," Journal of Occupational and Environmental Medicine, Vol. 38, No. 2, February 1996.
49Ibid.
50Ibid.
51Ibid.
52Anthony D. La Montagne, T.W. Mangione, D.C. Christiani, and K.T. Kelsey, "Medical Surveillance for Ethylene Oxide Exposure: Practices and Clinical Findings in Massachusetts Hospitals," Journal of Occupational and Environmental Medicine, Vol. 38, No. 2, February 1996.
53Meridian Research, Inc., "Ethylene Oxide: A Case Study in Hazard Identification, OSHA Regulation, and Market Response," final report submitted to OSHA's Office of Program Evaluation, July 21, 1992.
54U.S. Congress, Office of Technology Assessment, "Gauging Control Technology and Regulatory Impacts in Occupational Safety and Health - An Appraisal of OSHA's Analytic Approach," OTA-ENV-635, Washington, DC: U.S. Government Printing Office, September 1995.
55All of the exposure monitoring sample results used in this analysis were 8-hour TWA personal samples. The IMIS data used in this analysis included exposure data collected during both OSHA enforcement and consultation program activities. OSHA compliance activities are representative of worker exposures because OSHA tends to inspect establishments at higher risk or in response to employee complaints. Measurements of EtO exposures made during OSHA consultations may not be representative because consultations occur in establishments where employers request consultation services; nonetheless, these measurements can provide insight into the general patterns of exposures.
56Establishment size information in the IMIS database was used as a proxy for firm size.
57Glaser, Z. R. "Special Occupational Hazards with Control Recommendations: Use of Ethylene Oxide as a Sterilant in Medical Facilities," Publication No. 77-200, U.S. Department of Health, Education, and Welfare, National Institute for Occupational Safety and Health, 1977.
58Docket H-200, Exs. 99; 4-2; 4-8; 4-13; 4-14; 4-18; 4-53; 11-5; 11-20; 11-35; 11-37; 11-38; 11-40; 11-60; 11-77; 11-85; 11-87; 11-97; 11-100; 11-122; 11-132; and 11-156.
59Gschwandtner, G., Kruger, D., and Harman, P., "Compliance with the EtO Standard in the United States," Journal of Healthcare Material Management, November/December, 1986, pp. 38-41.
60Ringenburg, V.L., Elliott, L.J, Morelli-Schroth, P. and Molina, D., "Industrial Hygiene Characteristics of Ethylene Oxide Exposures of Hospital and Nursing Home Workers," U.S. Department of Health and Human Services, National Institute for Occupational Safety and Health, December, 1986.
61Mortimer, V.D. and Kercher, S.L., "Control Technology for Ethylene Oxide Sterilization in Hospitals," U.S. Department of Health and Human Services, National Institute for Occupational Safety and Health, September, 1989.
62Meridian Research, Inc., "Final Report--Assessment of Short-Term Exposures to Ethylene Oxide," prepared for the Department of Labor under Contract J-9-F-5-0060, Task Order 2, Option Year 1, May 8, 1987.
63Meridian Research, Inc., "Ethylene Oxide: A Case Study in Hazard Identification, OSHA Regulation, and Market Response," final report submitted to OSHA's Office of Program Evaluation, July 21, 1992.
64Op. cit., p. 3-1
65Ibid.
66Ibid.
67Daley, W.J., Morse, W.A., and Ridgeway, M.A., "Ethylene Oxide Control in Hospitals," prepared for the American Hospital Association and submitted as an attachment to the American Hospital Association's comments to OSHA Docket H-200, March 22, 1982.
68Gschwandtner, G., Kruger, D., and Harman, P., "Compliance with the EtO Standard in the United States," November/December, 1986, pp. 38-41.
69LaMontagne, A.D. and Kelsey, K.T., "Evaluating OSHA's Ethylene Oxide Standard: Employer Exposure-Monitoring Activities in Massachusetts Hospitals from 1985 through 1993," American Journal of Public Health, July 1997, Vol. 87, No. 7, pp. 1119-1125.
70LaMontagne, A.D. and Kelsey, K.T., Evaluating OSHA's Ethylene Oxide Standard: Exposure Determinants in Massachusetts Hospitals," American Journal of Public Health, March 2001, Vol. 91, No. 3, pp. 412-417.
71The percentage results associated with the IMIS data for the post-promulgation period are very close to the results of the 1985-1992 OCIS data analysis documented in the 1992 Meridian Research report.
72LaMontagne and Kelsey, 1997, op. cit.
73LaMontagne and Kelsey, 2001, op. cit.
74LaMontagne, A.D. and Kelsey, K.T., "OSHA's Renewed Mandate for Regulatory Flexibility Review: In Support of the 1984 Ethylene Oxide Standard," American Journal of Industrial Medicine, March, 1998, Vol. 34, pp. 95-104 (1989).
75R.G. Vanderwater, "Case History of an Ethylene Tank Car Explosion," Chemical Engineering Progress, December 1989, pp. 16-20.
76Rex Springston, "EPA Warns of Blasts/Pollution-Control Device Suspected in Explosions at Four Plants," Richmond Times-Dispatch, 8/2/97, p. A1.
77Gerald Ondrey, "Ethylene Oxide in the Safety Spotlight," Chemical Engineering, Vol. 12, December 1992, pp. 37-38.
78"OSHA Proposes $2,817,500 in Penalties Against Union Carbide for Alleged Safety/Health Violations," OSHA, Office of Information, U.S. Department of Labor News Release, September 12, 1991.
79"Union Carbide Complex Closed After Explosion," Platt's Oilgram News, March 14, 1991, Vol. 61, No. 51.
80Warrick, Joby, "EPA Delays Air Pollution Rules Following 4 Factory Explosions," The Washington Post, 8/2/97, p. A2.
81Cosmed brochure, from: http://www.cosmedgroup.com/homepage.htm
82Andersen, Lauren, Delvers, Marcus, and Hu. "An Introduction to Gas-Diffusion Sterilization," in Medical Device and Diagnostic Industry, May 1997.
83Personal communication, L. Nisenson, EPA, to K. Condit, OSHA, Aug. 6, 1997.
84Personal communication, L. Nisenson, EPA, to K. Condit, OSHA, Aug. 6, 1997.
85Alfa, M. et al., "Comparison of Ion Plasma, Vaporized Hydrogen Peroxide, and 100% Ethylene Oxide Sterilizers to the 12/88 Ethylene Oxide Gas Sterilizer," Infection Control and Hospital Epidemiology 17(2):92-100, 1996.
86Meridian Research, Inc., "Ethylene Oxide: A Case Study in Hazard Identification, OSHA Regulation, and Market Response," 1992, p. 3-10.
87Environmental Tectonics Corporation brochure, 1993.
88Personal communication, Brian O'Neill, Environmental Tectonics Corp., with K. Condit, OSHA, July, 1997.
89Griffith Micro Science, company brochure, 1997.
90Environmental Tectonics Corp. EPAS Emission Control System, company brochure, 1997.
91Ibid.
92Mayworm, D. "100% EtO Processing: Another Alternative," Infection Control and Sterilization Technology, 2(8), Aug., 1996.
93Ibid.
94Ibid.
95Ibid.
96Cosmed Group, Inc., "Cosmed Introduces Parametric Release to the Contract Sterilization Market," at the Web address cited earlier.
97Stanford Research Institute, Chemical Economics Handbook, Marketing Research Report, "Ethylene Oxide," April 1997.
98Booth, 1995.
99Ross Caputo, Pres. of AbTox, Inc., quoted in Health Industry Today, April 1995.
100"Sterilization Systems Lead Infection Control Market," Biomedical Market Newsletter, 5(7), July 1995.
101Booth, 1995.
102Ibid.
103Johnson and Johnson, Annual Report, 1995.
104"Amerinet Signs $57 Million Deal with J&J for Sterilization Systems," Hospital Materials Management, 20(7), July 1996.
105"Three Hospitals Save Following Conversion to H2O2 Gas Plasma Sterilization System," Hospital Materials Management, 20(6), June 1997.
106Health Industry Today, 1995.
107Caregiver Publications, at www.hrt.org/ozone008, July 1997.
108Enviro$en$e Factsheet, at www.es.inel.gov/techinfo/facts/ca-htm/htmfact4, June 1997.
109LaMontagne, A.D., "The Massachusetts Hospital EtO Health and Safety Study: A Summary Report for Study Participants and Supporters," 1996, pp. 1-44.
110Ibid.
111Cone, M., AEPA Halts Use of Certain Pollution Control Equipment," Los Angeles Times, Aug. 1, 1997, p. A25.
112Elias, J.D., Wylie, D.N., Yassi, A., and Tran, N. "Eliminating Worker Exposure to Ethylene Oxide from Hospital Sterilizers," J. Appl. Occ. Environ. Hyg. 8(8), Aug. 1993.
113"Study Deems Single-Use Instruments More Cost-Effective Than Reusable Lap Choly Procedures," Hospital Materials Management, 20(7), July 1995.
114Ibid.
115Scholla, M.H., and Wells, M.E., "Tracking Trends in Industrial Sterilization," in Medical Device and Industry Magazine, September, 1997.
116K.G. Beagley, "EtO and Gamma Sterilization: Room for Both," Pharmaceutical and Medical Packaging News, May 1996.
117Ibid.
118The American Spice Trade Association, Inc. (ASTA), "1995-1996 Trade Practices Survey," Final Report to EPA, July 31, 1996.
119Personal communication, R. Mann and H. Glombeck, of Cal Compack Foods, Inc., and Morris J. Glombeck, Inc., respectively, and K. Condit, OSHA, New York, July 22, 1997.
120Ibid.
121Caster, G., "U.S. Market for Sterilizer Equipment," Biomedical Market Newsletter, 6(8), Aug., 1996.
122Ibid.
123Meridian Research, Inc., 1992, op. cit.
124JRB Associates, Inc. Regulatory Impact Assessment of a Standard for Ethylene Oxide, 1983.
125Bureau of Labor Statistics Data, "Consumer Price Index - All Urban Consumers," August 1997.
126Production Engineering-Medical Equipment Division (PEMED), Autoclaves/Sterilizers, SPD, at PEMED's Homepage.
127Personal communication, Brian O'Neill, Environmental Tectonics Corp, with K. Condit, OSHA, July 10, 1997.
128Beagley, op. cit.
129Personal communication, K. Thomas, Griffith Micro Science, with K. Condit, OSHA, July 22, 1997.
130Booth, p. 69
131Op. cit., p. 65.
132ASTA, op. cit.
133Product Strategies, "Gas-Plasma Sterilization In, EtO Out With New AbTox Sterilizer System," Health Industry Today, April 1995, p. 4.
134Ibid.
135"Sterilization Changes Net $35,000 in Savings," Hospital Infection Control, Oct. 1996.
136Ibid.
137LaMontagne, 1996. op. cit.
138Bureau of Labor Statistics, Economist, Sept. 2, 1997.
139ASTA, op. cit.
140This chapter includes some information from other chapters.
141See Chapter II for further discussion.
142For other types of hospitals, special criteria apply. The Regulatory Flexibility Act defines a "small organization" as an organization that is independently owned and operated and is not dominant in its field. Use of this definition means that most private not-for-profit hospitals would be considered small entities. For cases where a not-for-profit hospital is owned by a local government, the applicable criteria for "small governmental jurisdiction" is an establishment that serves a population of less than 50,000. The above analysis assumes that the $5 million threshold provides a reasonable and consistent basis for analyzing the hospital sector overall.
143This estimate is based on analysis of 1997 U.S. Census data obtained from SBA (http://www.sba.gov/advo/stats/data.html#us).
144The Small Business Administration typically defines small businesses as those firms employing 500 or fewer employees.
145 The industry with the next most small entities is "Business Services Not-Elsewhere-Classified" (SIC 7389), which contains an estimated 12 small contract sterilizers. No data were available for SIC 7389.
|

