PART D: SCIENCE BASE
Section 5: Carbohydrates
INTRODUCTION
Carbohydrates—the sugars, starches
and fibers found in fruits, vegetables,
grains and milk products—are an important
part of a healthy diet. Sugars and starches
supply energy to the body in the form
of glucose, which is the primary energy
source for the brain, central nervous
system and red blood cells. Fibers, unlike
sugars and starches, do not supply glucose
to the body. They promote healthy laxation
and decrease the risk of certain chronic
diseases such as coronary heart disease
and diabetes.
NOMENCLATURE FOR CARBOHYDRATES
The nomenclature for carbohydrates
is somewhat confusing. Sugars can be
one sugar unit (monosaccharides) such
as glucose, fructose, and galactose;
and they can be two sugar units linked
together (disaccharides) such as sucrose,
lactose, and maltose. A further distinction
is sometimes further made between intrinsic
and extrinsic sugars. The term intrinsic
sugar means those
sugars that are naturally occurring within
a food, whereas extrinsic sugars are those that are added to foods. The
U.S. Department of Agriculture (USDA)
has defined added sugars as sugars and
syrups that are added to foods during
processing or preparation, and also includes
sugars and syrups added at the table.
There is no difference in the molecular
structure of sugar molecules, whether
they are naturally occurring in the food
or added to the food.
Starches are many glucose units linked
together (polysaccharide). Although most
starch can be broken down by human enzymes
into glucose for absorption, some starch
does not undergo digestion in the small
intestine and is called resistant starch, which is found in plant foods
such as legumes, pasta, and refrigerated
cooked potatoes. Fibers, like starches,
are polysaccharides made up mostly of
glucose units (in the case of cellulose)
or other combinations of monosaccharides.
However, the monosaccharides in fibers
are bonded to each other differently
than they are in starches, and human
enzymes cannot break the bonds in the
fibers. Thus, fibers are not absorbed
from the small intestine and pass relatively
intact into the large intestine, as does
resistant starch.
Back to Top
RECOMMENDATIONS FOR THE INTAKE
OF SUGARS AND STARCHES
The Institute of Medicine report Dietary
Reference Intakes for Energy, Carbohydrate,
Fiber, Fat, Fatty Acids, Cholesterol,
Protein, and Amino Acids (IOM, 2002)
established a Recommended Dietary Allowance
(RDA) for carbohydrate of 130 g per day
for adults and children. This value is
based upon the amount of carbohydrate
(sugars and starches) required to provide
the brain with an adequate supply of
glucose. Glucose is the only energy source
for red blood cells and the preferred
energy source for the brain, central
nervous system, placenta, and fetus.
When muscle cells operate anaerobically
(without oxygen), they rely 100 percent
on glucose. If glucose is not provided
in the diet and the body's storage form
of glucose (glycogen) is depleted, the
body will break down protein in muscles
to maintain glucose blood levels and
supply glucose to the brain (IOM, 2002).
The Institute of Medicine (IOM) also set an Acceptable Macronutrient Distribution Range (AMDR) for carbohydrate of 45 to 65 percent of total calories. At the low end of this range it is very difficult to meet the recommendations for fiber intake, and at the high end of the range overconsumption of carbohydrates may result in high blood triglyceride values. A comparison of the RDA to the AMDR shows that the recommended range of carbohydrate intake is higher than the RDA. For example, if an individual with a caloric intake of 2,000 kcal per day were to consume 55 percent of calories as carbohydrate (the mid-range of the AMDR) that would mean that 1,100 kcal would be from carbohydrate. This equates to 275 g carbohydrate (1 g carbohydrate = 4 kcal), well above the RDA of 130 g per day. In summary, the primary beneficial physiological effect of sugars and starches, and the basis for setting an RDA for carbohydrate, is the contribution of glucose as an energy source for the brain. However, the amount of glucose needed by the brain is lower than the AMDR for carbohydrate (45 to 65 percent of total calories).
Back to Top
RECOMMENDATIONS FOR THE INTAKE
OF FIBER
Fibers are different from sugars and
starches in that they are not digested
and absorbed in the small intestine and
converted to glucose. Humans do not have
the necessary enzymes to break down fibers
into their constituent parts so that
they can be absorbed into the body. Therefore,
fibers pass from the small intestine
into the large intestine relatively intact.
There they can be fermented by the colonic
microflora to gases such as hydrogen
(H2) and carbon dioxide (CO2)
and to short chain fatty acids. Although
fibers are not converted to glucose as
are sugars and starches, some of these
short chain fatty acids are absorbed
and can be used for energy in the body.
However, determining the amount of calories
supplied by fiber is complex since it
depends on such factors as the fermentability
of the fiber, the individual's colonic microflora, how long fiber stays in the
colon, etc. The IOM has set an Adequate
Intake (AI) value for fiber of 14g of
fiber per 1,000 kcal. This AI is based
on the totality of the evidence for fiber
decreasing the risk of chronic disease
and other health-related conditions,
but the actual numbers for the AI were
derived from the data supporting a decreased
risk for the development of coronary
heart disease (CHD). The major food sources
of fiber are fruits, vegetables (particularly
legumes), and grains. Milk does not contain
fiber although certain milk-containing
products may.
Back to Top
MAJOR FOOD SOURCES OF CARBOHYDRATES
(FRUITS, VEGETABLES, GRAINS, AND MILK
PRODUCTS)
Since the RDA for carbohydrate is relatively
easy to meet, and carbohydrates (sugars
and starches) supply calories, it is
important to choose food sources of carbohydrates
carefully to maximize nutrient value
per calorie. Also, since fiber has known
health benefits (e.g., promoting a healthy
laxation and decreasing the risk of CHD
and diabetes) it is advisable to select
high-fiber foods where possible. For
example, fruits provide sugars, usually
at a relatively low calorie cost, and
they are important sources of fiber and
at least eight additional nutrients.
Some vegetables are high in starch and
some are very low in both starch and
sugar, but they all are important sources
of fiber. They also are important sources
of 19 or more nutrients, including vitamins
A, E, and folate and potassium, and,
in general, do not supply many calories.
Whole grains are high in fiber and starch,
but most of the fiber is removed when
grains are refined. Milk and milk products
contain the sugar lactose and generally
do not contain any fiber but may in certain
milk products.
Back to Top
OVERVIEW OF QUESTIONS ADDRESSED
Five of the questions about carbohydrates
and carbohydrate-rich foods are specific
to carbohydrates and are found in this
section. Other questions that also involve
carbohydrate-related issues are found
elsewhere in this report but are summarized
here to provide a better overview of
the role of carbohydrates in health.
The following questions specific to carbohydrates are found in this section
What is the relationship between intake of carbohydrates and dental caries? How important to human health is the glycemic response to carbohydrates? What is the utility of the glycemic index/glycemic load for providing dietary guidance for Americans? What is the significance of added sugars intake to human health? What are the major health benefits of fiber-containing foods?
In the interest of presenting a more comprehensive section on carbohydrates, below is a list of related questions, the section of the report in which they are found, and conclusions in brief.
- What are the major health benefits of carbohydrate-containing foods?
This information is found in Part D, Section 6, "Selected Food Groups (Fruits and Vegetables, Whole Grains, and Milk Products)." The conclusions are
Greater consumption of fruits and vegetables is associated with a reduced risk of stroke and perhaps other cardiovascular diseases, with a reduced risk of cancers in certain sites, and with a reduced risk of type 2 diabetes (vegetables more than fruit). Moreover, increased consumption of fruits and vegetables may be a useful component of programs designed to achieve and sustain weight loss. Consuming at least three servings of whole grains per day can reduce the risk of diabetes and coronary heart disease and may help with weight maintenance. Thus, daily intake of three or more servings of whole grains per day is recommended, preferably by substituting whole grains for refined grains. Consuming three servings per day of milk and milk products can reduce the risk of low bone mass and contribute important amounts of many nutrients. Furthermore, this amount of milk product consumption is not associated with increased body weight. Therefore, the intake of three servings of milk products per day is recommended. - What are the optimal proportions of dietary fat and carbohydrate to maintain body mass index and to achieve long-term weight loss?
This information is found in Section 2, " Energy," Question #3. The conclusion is weight maintenance depends on a balance of energy intake and energy expenditure, regardless of the proportions of fat, carbohydrate, and protein in the diet. For adults, well-planned weight loss diets that are consistent with the AMDRs for fat, carbohydrate, and protein can be safe and efficacious over the long term.
What is the evidence to support caloric compensation for liquid versus solid foods? This discussion and review of the literature is found in Section 2,
"Energy," Unresolved Issues. The conclusion is that the evidence is conflicting that liquid and solid foods differ in their effect on calorie compensation (the ability to regulate energy intake with minimal conscious effort, such as reducing the amount of food consumed on some occasions to compensate for increased consumption at other times).
Back to Top
QUESTION 1: WHAT IS THE RELATIONSHIP BETWEEN INTAKE OF CARBOHYDRATES AND DENTAL CARIES?
Conclusion
The intake of carbohydrates (including
sucrose, glucose, fructose, lactose and
starch) contributes to dental caries
by providing substrate for bacterial
fermentation in the mouth. Drinking fluoridated
water and/or using fluoride-containing
dental hygiene products help reduce the
risk of dental caries. A combined approach
of reducing the frequency and duration
of exposure to fermentable carbohydrate
intake and optimizing oral hygiene practices
is the most effective way to reduce caries
incidence.
Rationale
Overview. The
process of dental caries formation involves three steps:
the fermentation
of substrate by cariogenic bacteria in
the mouth to produce acid, demineralization
of the enamel surface by the acid, and
subsequent bacterial invasion. Factors
that affect this process include the
type and amount of substrate, the bacterial
population, the length of time the substrate
is available to the bacteria, and the
susceptibility of the tooth to acid demineralization.
Review of the Evidence
The Substrate for Bacterial
Fermenation. The major substrates for
bacterial fermentation are sugars, including sucrose, glucose,
fructose, and lactose) and starch (Bibby,
1975; Lingstrom et al., 2000; Moynihan
and Petersen, 2004; Walker and Cleaton-Jones,
1992;). In contrast, certain dietary
fibers (e.g., cellulose and pectin) (Touger-Decker
and van Loveren, 2002) and dietary sugar
alcohols and certain other sugar substitutes
(e.g., xylitol, sorbitol, mannitol, maltitol,
isomalt, lactitol, hydrogenated starch
hydrolysates, hydrogenated glucose syrups,
erythritol, or a combination of these)
are much less cariogenic than other carbohydrates
(FDA, 1997, 2002; Makinen et al., 1995).
However, evidence as to whether or not
substituting sucrose with sugar substitutes
to reduce caries occurrence is effective
remains inconclusive (Lingstrom et al.,
2003).
Other Factors That Affect
Caries Formation. Formation.
Despite the known ability of both sugar
and starch to be fermented to acids that
can induce demineralization of enamel,
their overall contribution to caries
formation is less clear and not a simple
cause-and-effect relationship (Walker
and Cleaton-Jones, 1992). Other important
considerations are the form of the food,
how long it remains in the mouth, and
the frequency of consumption. The longer
a cariogenic substance remains in the
oral cavity, the greater the probability
of extended acid production and demineralization
(Kashket et al., 1996). The duration
of acid production also is affected by
the frequency and amount of fermentable
carbohydrate consumed (Lingstrom et al.,
2000). Sugars that are slowly released
(e.g., from hard candies) or sweetened
beverages that are sipped over time or
held in the mouth provide greater access
to bacteria than the same amount of sugar
in foods or beverages that are swallowed
quickly. Other important factors for
clearing fermentable substrate from the
oral cavity include the activity of salivary
enzymes and saliva flow. Clearance of
high-starch foods such as cookies and
potato chips has been found by some to
be slower than clearance of high-sugar,
low-starch foods such as caramels and
jelly beans (Edgar et al., 1975; Kashket
et al., 1996; Luke et al., 1999).
Dental hygiene may have a greater role in the development of dental caries than do the type of carbohydrate and its retention time in the mouth. For example, in a systematic review of the literature from 1980 to 2000 addressing the question of whether individuals with a high level of sugar intake experience greater caries severity relative to those with a lower level of intake. The authors report that only 2 papers found a strong relationship between sugar consumption and caries development, 16 found a moderate relationship, and 18 found a weak-to-no relationship (Burt and Pai, 2001). A separate study investigated the relative importance of dietary sugars, toothbrushing frequency, and social class as predictors of caries among 1,450 British preschool children. The strength of the association between social class and caries was twice that between toothbrushing and caries, and approximately three times that between sugar confectionery and caries (no other diet component was statistically significant) (Gibson and Williams, 1999). The relationship between sugar confectionery and caries was significant only in those children who brushed less than twice a day (Gibson and Williams, 1999). The authors concluded that regular brushing with a fluoride toothpaste may have greater impact on caries in young children than does restricting sugary foods (Gibson and Williams, 1999).
Heller et al. (2001) came to the same conclusion in their report on the association between sugared soda consumption and caries. Using the dietary and dental examination data from the Third National Health and Nutrition Study (NHANES III), they found no relationship between soft drink consumption and caries in individuals under age 25 but did find a positive association in people over age 25 (Heller et al., 2001). They attributed the lack of association in the younger age group to increased use of fluorides since the 1960s. They attributed the significant association in the older group to the cumulative effects of long-term soft drink consumption. In the development of dental caries, the role of sugar and other carbohydrates is not independent of factors such as fluoridation and oral hygiene ( Holbrook et al., 1995; Mascarenhas, 1998; McDonagh et al., 2000; Navia, 1994; Shaw, 1987; Tougher-Decker and van Loveren, 2002). Caries have declined in areas where the water has been fluoridated (McDonagh et al., 2000).
The impact of sugar intake on dental caries was reviewed by the IOM (IOM) to determine whether or not the science supported an upper level (UL) of intake for sugar based on its contribution to tooth decay (IOM, 2002). The report concludes,
"Because of the various factors that can contribute to dental caries, it is not possible to determine an intake level of sugar at which increased risk of dental caries can occur." (IOM, 2002). Studies published since those cited in the IOM report and reviewed by the Dietary Guidelines Committee (the Committee), including a systematic review (Burt and Pai, 2001), support the IOM's conclusion (Anusavice, 2002; Campaign, 2003, Heller et al., 2001; Tougher-Decker and van Loveren, 2002).
Young Children
Most of the studies of preschool children
report a positive association between
sucrose consumption and dental caries
(Paunio, 1993; Wendt and Birkhed, 1995).
Here again, however, other factors (particularly
frequent brushing with fluoridated toothpaste)
are more predictive of caries outcome
than is sugar consumption (Gibson and
Williams, 1999; Grindefjord et al., 1996;
Paunio, 1993; Stecksen-Blicks and Holm,
1995; Wendt, 1996). Some studies report
increased risk with sugar-sweetened beverages
(Wendt et al., 1996), others with candy
consumption (Grindefjord et al., 1996),
others with a variety of sugar-containing
products (Paunio et al., 1993), and still
others report that intake of certain
sucrose-containing products may be predictive
of caries in children with poor dental
hygiene (e.g., brushing once a day or
less) but not in children with better
dental hygiene (Gibson and Williams,
1999). In a longitudinal study tracking
caries and diet in children just prior
to and after starting school, increased
consumption of sugar-containing foods
was associated with increased caries
in 5-year-olds (Holbrook et al., 1995).
In Finland, Ruottinen et al. (2004) followed
two groups of boys (one in the highest
5 percentiles and the other in the lowest
5 percentiles for sucrose intake; n=
> 66,000 boys) from infancy to age
10 years. They found that children with
the highest sucrose intake had a higher
score for dental caries than those in
the low-sucrose intake group (Routtinen
et al., 2004).
Possible Confounding Factors
Interpretation of the cross sectional
studies showing a relationship between
sugar intake and dental caries needs
to consider the possibility that frequent
use of candy, sodas, and other sugar-containing
foods may be a proxy for a less than
optimal healthy lifestyle, rather than
a direct effect of the sugar itself.
In addition, a large proportion of the
studies on children and dental caries
have been conducted outside of the United
States making extrapolation to the U.S,
population somewhat difficult. Not only
are eating habits different in other
countries, but the degree of fluoride
availability is different. Nevertheless,
there is little doubt that the primary
initiating event in caries formation
is the fermentation of carbohydrates,
particularly sugars and starch.
Back to Top
QUESTION 2: HOW IMPORTANT TO HUMAN HEALTH IS THE GLYCEMIC RESPONSE TO CARBOHYDRATES?
Conclusion
A potential health concern for foods
that raise blood glucose levels and initiate
an insulin response is that they may
eventually lead to diabetes. Current
evidence suggests that there is no relationship
between total carbohydrate intake (minus
fiber) and the incidence of either type
1 or type 2 diabetes. The intake of fiber-containing
foods is associated with a decreased
risk of type 2 diabetes in a number of
epidemiological studies.
Rationale
Overview. The glycemic
response is
defined as the effects that carbohydrate-containing
foods have on blood glucose concentration
during the time course of digestion.
A number of factors influence the glycemic
response to food, including the amount
of carbohydrate (Gannon et al., 1998),
the type of sugar (glucose, fructose,
sucrose, lactose) (Wolever et al., 1994),
the nature of the starch (amylose, amylopectin,
resistant starch) (O'Dea et al., 1981),
cooking and food processing (degree of
starch gelatinization, particle size,
cellular form) (Snow and O'Dea, 1981),
food structure (Jarvi et al., 1995),
and other food components (fat and natural
substances that slow digestion—lectins,
phytates, tannins, and starch-protein
and starch-lipid combinations) (Hughes
et al., 1989). Other factors affecting
the glycemic response to food include
fasting and preprandial glucose concentrations
(Nielsen et al., 1989, Rasmussen and
Hermansen, 1991, Fraser et al., 1990;
Schvarcz et al., 1993), the severity
of glucose intolerance (Parillo et al.,
1996), and the second meal or lente
effect (Jenkins et al., 1982). A major
concern with increasing postprandial
glucose and insulin levels is that there
may eventually be a diminished insulin
response that could lead to diabetes.
Type 1 diabetes is an immunological disease in which the beta-cells of the pancreas are destroyed by auto-immune processes. Although a number of food-based substances have been invoked as important in the process, there is no clear evidence for any of them. Type 2 diabetes is a disease that is manifested by insulin resistance and a gradual deterioration of B-cell function. Any dietary insult that abets either of these processes could play a role in its etiology. Does carbohydrate intake predispose to type 2 diabetes? Evidence from four prospective observational studies indicates that it does not (Lundgren et al., 1989; Marshall et al., 1991; Salmeron et al., 1997a, 1997b). In these four studies, there was no association between an increased amount of total carbohydrate in the diet and the development of diabetes mellitus in the cohorts studied over periods as long as 16 years. Also, in an analysis of cross-sectional data from NHANES III, Yang et al. (2003) found no association between carbohydrate intake and HgbA1c (the amount of glycosylated hemoglobin in blood that provides an estimate of how well diabetes is being managed over time), plasma glucose, or serum insulin concentrations. In fact, there was an association between lower total carbohydrate intake and an elevation of serum C-peptide concentration, suggesting a possible association between low-carbohydrate diets and increased basal insulin secretion.
Furthermore, there is no evidence that total sugar intake is associated with the development of type 2 diabetes (Colditz et al., 1992; Janket et al., 2003). These two prospective longitudinal studies show no relationship between the total intake of sugar and the development of type 2 diabetes mellitus. One study actually shows a negative association between sugar intake and diabetes risk (Meyer et al., 2000). Clinical trials show that total dietary sugar does not increase plasma glucose concentrations to a greater extent than do isoenergetic amounts of dietary starch (Mann et al., 2002). However, further analysis within the Nurses' Health Study indicates that there may be an association between consumption of sugar-sweetened beverages, other than fruit juices, and an increased risk of type 2 diabetes in women, possibly by providing excessive calories and large amounts of rapidly absorbable sugars (Schulze, 2004).
In contrast, intake of fiber has been
inversely associated with type 2 diabetes
in a number of epidemiological studies
(Hu et al., 2001; Meyer et al., 2000;
Montonen et al., 2003; Salmeron et al.,
1997a, 1997b). For example, in the Nurses'
Health Study, Salmeron et al. (1997a)
reported on fiber intake and its relationship
to diabetes. There was a 28 percent risk
reduction from the highest to the lowest
quintile of fiber intake. However, the
source of fiber appears to be important,
as cereal fiber but not fruit or vegetable
fiber intake has been inversely associated
with risk for diabetes in several studies
(Salmeron et al., 1997b). In the Health
Professionals Follow-up Study (Hu
et al., 2001), the risk of developing
diabetes did not decrease with higher
total fiber intakes, but a risk reduction
of 30 percent was observed in the highest
quintile of cereal-fiber intake (median
10.2 g per day) compared to the lowest
quintile (median intake 1.14 g per day).
Again, as in the Nurses' cohort,
cereal fiber but not fruit or vegetable
fiber intake was associated with the
protective effect. Similarly, in the
Finnish Mobile Clinic Survey
(Montonen et al., 2003), cereal fiber
intake also was associated with a reduced
risk of type 2 diabetes. The relative
risk between the extreme quartiles of
cereal fiber intake was 0.39; P=0.01.
Back to Top
QUESTION 3: WHAT IS THE UTILITY OF THE GLYCEMIC INDEX/GLYCEMIC LOAD FOR PROVIDING DIETARY GUIDANCE FOR AMERICANS?
Conclusion
Current evidence suggests that the
glycemic index and/or glycemic load are
of little utility for providing dietary
guidance for Americans.
Rationale
Overview. The glycemic
index is a classification proposed to quantify the relative blood
glucose response to consuming carbohydrate-containing foods.
Operationally, it is the area under the curve for the increase in blood
glucose after
the ingestion of a set amount of carbohydrate in a food
(e.g., 50 g) during the 2-hour postprandial period, relative to the
same amount
of carbohydrate from a reference food (white bread or glucose)
tested in the same individual under the same conditions and using the
initial
blood glucose concentration as a baseline.
The glycemic load is an indicator of the glucose response or insulin demand that is induced by total carbohydrate intake. It is calculated by multiplying the weighted mean of the dietary glycemic index of the diet of an individual by the percentage of total energy from carbohydrate.
The glycemic response is defined as the effects that carbohydrate-containing foods have on blood glucose concentration during the time course of digestion.
Review of the Evidence Glycemic Index. Although the use of food with a low-glycemic index may reduce postprandial glucose, there is not sufficient evidence of long-term benefit to recommend general use of diets that have a low-glycemic index.
Glycemic Load. The glycemic load has been used primarily in observational epidemiological studies to examine the effect of diet on the risk of developing chronic diseases such as diabetes, heart disease, and cancer (IOM, 2002). The glycemic load has been reported to be positively associated with the risk of developing type 2 diabetes in men and women (Salmeron et al., 1997a, 1997b). In a cross-sectional study of healthy postmenopausal women, dietary glycemic load was inversely related to plasma high-density lipoprotein (HDL) cholesterol and positively related to fasting triglycerides (Liu et al., 2001). In the analysis of the NHANES III results, a high glycemic load was associated with a lower concentration of plasma HDL cholesterol (Ford and Liu, 2001).
The findings from epidemiological studies indicate a possible relationship between the propensity of diets with a high glycemic load to raise blood glucose levels and increase in the risk of type 2 diabetes. To determine the utility of glycemic load in predicting risk, long-term trials are needed in which diets with high glycemic load are compared to low glycemic load diets with regard to outcomes. Also, it is necessary to examine the effect of glycemic load of a mixed meal diet on postprandial glucose and insulin levels. A 4-week study by Wolever and Mehling (2003) comparing high-and low-glycemic index diets in impaired glucose tolerance subjects showed the high-glycemic index diet to have no significant change in glucose, but a lower insulin level than the low-glycemic diet.
Relationship of Glycemic Index and Load. The relationship of glycemic index and load has been examined in long-term prospective studies. These have shown inconsistent results. A followup study within the Nurses' Health Study confirmed the association between glycemic load and risk of type 2 diabetes. However, the Iowa Women's Health Study showed no significant relationship between glycemic index or load and the development of diabetes (Meyer et al., 2000).
The inconsistencies among studies are likely due to the poor tools available to measure these dietary components. Food frequency questionnaires can be extremely inaccurate, even in the best of hands. In addition, the food frequency questionnaires used in these studies were not designed to measure glycemic index or load. The validation data are weak. Prospective, randomized studies are needed to answer this question. The relationship between glycemic index and glycemic load and the development of type 2 diabetes is unclear at this time.
Back to Top
QUESTION 4: WHAT IS THE SIGNIFICANCE OF ADDED SUGARS INTAKE TO HUMAN HEALTH?
Conclusion
Compared with individuals who consume
small amounts of foods and beverages
that are high in added sugars, those
who consume large amounts tend to consume
more calories but smaller amounts of
micronutrients. Although more research
is needed, available prospective studies
suggest a positive association between
the consumption of sugar-sweetened beverages
and weight gain. A reduced intake of
added sugars (especially sugar-sweetened
beverages) may be helpful in achieving
recommended intakes of nutrients and
in weight control.
Rationale
Overview. Added sugars are sugars and syrups that are added to foods during processing or preparation or at the table. Major sources of added sugars include soft drinks, cakes, cookies, pies, fruitades, fruit punch, dairy desserts, and candy (USDA/DHHS, 2000). Specifically, added sugars include white sugar, brown sugar, raw sugar, corn syrup, corn-syrup solids, high-fructose corn syrup, malt syrup, maple syrup, pancake syrup, fructose sweetener, liquid fructose, fruit-juice concentrate, honey, molasses, anhydrous dextrose, and crystal dextrose. In 1994–1996 USDA food consumption survey data, nondiet soft drinks were the leading source of added sugars in Americans' diets, accounting for one-third of the intake of added sugars (Guthrie and Morton, 2000). Soft drinks were followed by sugars and sweets (16 percent), sweetened grains such as cakes and cookies (13 percent), fruitades/drinks (10 percent), breakfast cereals and other grains such as breakfast bars (10 percent), and sweetened dairy (9 percent). Together, these foods and beverages accounted for 90 percent of the Americans' intake of added sugars.
Solid foods with added sugars have a high energy density, while beverages that contain added sugars often are relatively low in energy density because of their high water content. The addition of sugar to a food (e.g., adding sugar to grapefruit or coffee) increases the energy density of the food or beverage as consumed. The issue with added sugars is not that sugars themselves are detrimental to health. Rather, as sugars are added to the diet they provide calories only. Thus, at some amount of additional added sugars, either one compensates by decreasing intake of more nutrient-dense foods, and/or one adds sugars on top of an existing diet and increases caloric intake. Increased calorie intake, in turn, may result in weight gain.
Does Intake of Added Sugars Contribute to Excess Intake of Energy?
The analysis of dietary data on added sugars may underestimate intake because of the underreporting of food intake, which is more pervasive among obese adolescents and adults than among their lean counterparts (Johnson, 2000). It appears that foods high in added sugars are selectively underreported (Krebs-Smith et al., 2000).
Cross Sectional Studies. Despite these research challenges, most cross-sectional studies have found that an increased intake of added sugars is associated with increased total energy intakes (Bowman, 1999; Gibson, 1996; Lewis et al., 1992; Overby et al., 2004; Storey et al., 2003). An analysis performed by the USDA Center for Nutrition Policy and Promotion (Britten et al., 2000), using the 1994–1996 Continuing Survey of Food Intakes by Individuals, divided the data set into four groups of equal number according to intake of added sugars expressed in teaspoons. About 59 percent of the group with the highest intake of added sugars consumed more than their 1989 Recommended Energy Allowance, in contrast with only 22 percent of all others (median added sugars consumption for the highest quartile was the equivalent of 36.7 teaspoons).
Teens who reported consuming 26 or more ounces of soft drinks per day consumed a mean of 2,604 kcal per day in contrast to nonconsumers of soft drinks, who consumed 1,984 kcal per day (Harnack et al., 1999). Using NHANES III data, Troiano and colleagues (2000) found that soft drinks contributed a higher proportion of daily energy intake for overweight than for nonoverweight children and adolescents. However, a large number of cross-sectional studies show an inverse correlation between the consumption of added sugars and either body weight or BMI (Bolton-Smith and Woodward, 1994; Gibson, 1996; Lewis, 1992). Not all studies, however, adequately adjust for physical activity levels of the study subjects, suggesting that active people can consume added sugars without gaining weight.
Prospective Studies. The study by Newby et al (2004) compared changes in BMI in 1345 2-5 year-old children over 8 months with their sweetened drink intake. While no correlation was found, the very-low level of soda intake (~1 oz per day) weakens the generalizability of this finding. It should be pointed out that the study neither found correlations between BMI and drinks consumed in larger quantities, like milk (20 oz per day) or fruit juice (10 oz per day).
Other recent prospective studies, however, found a weak association between the consumption of sugar-sweetened beverages and weight gain. An observational study by Ludwig et al. (2001) reported a positive association between energy intake and the change in consumption of sugar-sweetened beverages in a group of 548 ethnically diverse schoolchildren followed for 19 months. After adjustments for physical activity and other variables, the increased consumption of sugar-sweetened beverages was a factor independently associated with a minor but statistically significant increase in absolute BMI values in children. Additionally, the change in consumption of diet soda intake was negatively associated with the incidence of obesity, which was defined on the basis of both BMI and triceps-skinfold thickness greater than or equal to the 85th percentile of age- and sex-specific reference data.
Berkey et al. (2004), in a large two-year prospective cohort study of boys and girls age 9 to 14, found that the consumption of sugar-added beverages was associated with small BMI gains. They concluded that the consumption of sugar-added beverages may contribute to weight gain among adolescents, probably because of their contribution to total energy intake.
In a short-term longitudinal study of 30 children, Mrdjenovic and Levitsky (2003) reported that excessive sweetened drink consumption (>12 oz per day) resulted in a reduction in milk intake and an increase in daily energy intakes. Also, these investigators found that the greater the sweetened-drink consumption, the greater the weight gain.
A study of women in the Nurses' Health Study examined the association between the consumption of sugar-sweetened beverages and weight change (Schulze et. al., 2004). Those with a stable consumption pattern of sugar-sweetened beverages (whether high or low) did not show a difference in weight gain. However, those who increased their sugar-sweetened soft drink consumption from low (<1 per week) to high (>1 per day) had the highest weight gain (4.69 kg in 4 years (1991–1995) and 4.20 kg for (1995–1999)). Those who decreased their intake of sugar-sweetened soft drinks had the smallest weight gain (1.34 kg for 1991–1995 and 0.15 kg for 1995–1999).
Another study, however, provided mixed results (Phillips et. al., 2004) followed 196 nonobese girls and found no relationship between total energy-dense food consumption and either BMI or the percentage of body fat. The energy-dense foods included baked goods, ice cream, chips, sugar-sweetened soft drinks, and candy. Sugar-sweetened soft drinks were the only food that was significantly related to BMI z-score1 over the study period, but it was not related to the percentage of body fat.
Intervention Studies. A
study by James et al. (2004) is one of the few intervention trials published
so far in which a decreased intake of carbonated beverages was a specific
target. The goal of the intervention was to prevent excessive weight gain.
The study, which included British schoolchildren age7 to 11, randomized
classrooms within each of six schools rather than randomizing individuals.
In this cluster scheme, the consumption of carbonated drinks over 12 months
decreased modestly by 0.6 glasses per day in the intervention group and
increased by 0.2 glasses in the control group but had no effect on BMI
or on Z score. The number of clusters with mean BMI above the 91st percentile
increased by one in controls (7.5 percent) and did not change in the intervention
Water intake increased in both groups, but there was no difference in water
intake between intervention and control clusters. The findings may have
been affected by the study design in that it allowed for intervention and
control classrooms to coexist in the same school, a likely source of contamination.
Mattes (1996) suggests that the form in which carbohydrates are consumed (solid or liquid) may be important since, at subsequent meals, people tend to compensate less for energy consumed in liquids than in solids; but this is controversial (see
"What Is the Evidence To Support Caloric Compensation for Liquids versus Solid Foods?" in Section 2). Rolls (2002) found, however, that meals including sugar-sweetened beverages are higher in energy content than meals without drinks by an amount roughly equal to the calorie content of the beverage.
In summary, although the evidence is not large and there are methodologic problems with this research, the preponderance of prospective data available suggest that added sugars (particularly in beverages) are associated with an increase in energy intake. As a result, decreasing the intake of added sugars (particularly in beverages) may help prevent weight gain and may aid in weight loss.
Does Intake of Added Sugars
Have a Negative Impact on Achieving Recommended
Nutrient Intakes?
The Committee reviewed 19 published
papers on the intake of added sugars
and corresponding micronutrient intake;
9 were used to develop this rationale.
Each of these papers (see Appendix G3
or Web site www.health.gov/dietaryguidelines)
shows a decreased intake of at least
one micronutrient with higher levels
of added sugar intake (Bolton-Smith and
Woodward, 1995; Bowman, 1999; Forshee
and Storey, 2001; Gibson, 1997; Lewis
et al., 1992; Nelson, 1991; Nicklas et
al., 2003; Rugg-Gunn et al., 1991). For
example, the Bowman study (Bowman, 1999)
used data from CSFII (1994-1996) (n=14,707)
and divided the intake data into three
groups: (1) Less than 10 percent of total
energy from added sugars (5,058); (2)
10 to 18 percent of total energy (n=4,488);
(3) greater than 19 percent of total
energy (n=5,158) (mean = 26.7 percent).
Group three had the lowest mean intakes
of all the micronutrients, especially
vitamin A, vitamin C, vitamin B12, folate,
calcium, phosphorus, magnesium and iron.
The individuals in Group 3 did not meet
the 1989 RDA for vitamin E, vitamin B6,
calcium, magnesium, and zinc. In terms
of food groups, Group 3 consumed more
soft drinks, fruit drinks, punches, ades,
cakes, cookies, grain-based pastries,
milk desserts, and candies. They had
lower intakes of grains, fruits, vegetables,
meat, poultry, and fish compared to those
in Groups 1 and 2.
In addition, an IOM panel developed tables to address the association between added sugars and specific micronutrient intakes at every fifth percentile of added sugar intake using data from NHANES III, 1988–1994 (IOM, 2002, Appendix J). Taken collectively, these data show a drop off in micronutrient intake at approximately 25 percent of calories coming from added sugars. The specific drop-off point depends upon the specific micronutrient and the age/sex of the group. Therefore, the IOM recommendation to keep added sugars intake below 25 percent of calories was based solely on the data of added sugar intake and micronutrient dilution—not on whether or not the people consuming added sugars were, for example, consuming more calories than required to meet their energy needs.
As noted previously, concern about added sugars arises when nutrient-poor foods are consumed at the expense of nutrient-dense foods. Not all foods that contain added sugars are poor sources of nutrients. Sugars can improve the palatability of foods and beverages that otherwise might not be consumed (FAO/WHO, 1998). Interestingly, a review of Appendix Table 1-9 in Appendix J of Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (IOM, 2002) shows that individuals consuming 5 to 10 percent of their calories as added sugars have higher micronutrient intakes than those consuming 0 to 5 percent of their calories as added sugars. Figure D5-1 illustrates this point.
A possible reason for this apparent beneficial effect of small amounts of added sugars is from a recent paper (Frary et al., 2004). These investigators conclude that, on average, the consumption of sweetened dairy foods and beverages and presweetened cereals had a positive impact on children and adolescents' diet quality, whereas sugar-sweetened beverages, sugars and sweets, and sweetened grains had a negative impact on their diet quality. The potential negative effects of added sugars appear to be excess calories or micronutrient dilution rather than a direct negative effect of sugar itself.
Added Sugar Intake and Discretionary Calories. Section 3 has a full discussion of the concept of discretionary calories. Added sugars fit into the category of discretionary calories because they are part of the difference between a person's energy requirement and his or her essential calories. As shown in Figures 3-1, 3-3a, and 3-3b in that section, individuals may have very few discretionary calories, particularly persons who are sedentary.
Back to Top
QUESTION 5: WHAT ARE THE MAJOR HEALTH BENEFITS OF FIBER-CONTAINING FOODS?
Conclusion
Diets rich in dietary fiber have a
number of important health benefits including
helping to promote healthy laxation,
reducing the risk of type 2 diabetes,
and decreasing the risk of coronary heart
disease (CHD). Prospective cohort studies
suggest that decreased risk of heart
disease is associated with the intake
of 14 g of dietary fiber per 1,000 calories.
Rationale
Overview. The
conclusion regarding the recommended
intake of dietary fiber is consistent
with the IOM's AI value of 14 g of fiber
per 1000 kcals (IOM, 2002). This AI for
fiber intake was based on the totality
of the evidence for certain health benefits
of dietary fiber, placing emphasis on
fiber's protective role against CHD but
also including its effect on laxation
(Burkitt 1972; Cummings, 1992; Kelsay
et al., 1978) and diabetes (Colditz et
al., 1992; Salmeron et al., 1997a). The
Committee evaluated the potential effects
of fiber on laxation and diabetes and
focused on the effects of fiber on CHD,
since that was the basis of setting a
value for fiber intake. Particular attention
was paid to studies published since the
IOM report. Studies on the association
between fiber and diabetes are discussed
under Question 2 in this section: How
important is the glycemic response to
carbohydrates to human health? Summaries
of the studies on the relationship of
fiber to healthy laxation and to risk
of CHD are shown below.
Review of the Evidence
Fiber and Laxation. Chronic
constipation is one of the most common disorders in Western countries (Roma
et al., 1999). Although there is no one accepted definition of what constitutes
normal laxation, constipation has been defined as difficulty in passing
stools or an incomplete or infrequent passage of hard stools (Anderson,
2003). Epidemiological studies have reported a negative correlation between
per capita fiber consumption and the incidence of chronic constipation
(Graham et al., 1982). Dietary fibers from whole grains, fruits, and vegetables
(including legumes) increase stool weight, which promotes normal laxation
in children and adults. In general, the greater the weight of the stool,
the more rapid the rate of passage through the colon (Birkett et al., 1997)
the better the laxative effect. The water holding capacity and bulking
ability that result in increased laxation are thought to reduce intracolonic
pressure and lower the risk for diverticular disease as well (Bodribb et
al., 1976).
Several factors affect stool weight, including the fermentability of the fiber (the less fermentable, the greater the fecal bulk) the water holding capacity of the fiber, and the contribution of the fiber to microbial mass, which also contributes to fecal bulk (Bach Knudsen et al., 1997; Blackwood et al., 2000 Chen et al., 1998). In addition, certain fibers may contain unfermented gel, which acts as an emollient and a lubricant (Marlett et al., 2000). Cummings reviewed over 100 studies of the effect of fiber intake on stool weight and calculated the increase in weight of the stool as a function of fiber intake (Cummings et al., 1992). There was a wide range of the contribution of dietary fiber to fecal weight (e.g. an increase of 5.7 g fecal bulk per gram of wheat bran fed compared to an increase of 1.3 g per gram of pectin in the diet). A meta-analysis of 11 studies in which daily fecal weight was measured accurately in 26 groups of people (n = 206) on controlled diets of known non-starch polysaccharide content shows a significant correlation between fiber intake and mean daily stool weight (r = 0.84) (Cummings et al., 1992). Although stool weight continues to increase as fiber intake increases (Burkitt et al., 1972; Wrick et al., 1983), there is a plateau effect for both intestinal transit time and fecal frequency. In general, most studies show that once intestinal transit time was less than 1 day and fecal frequency reached two to three per day the only effect of extra fiber in the diet was increased stool weight (Haack et al., 1998). The fecal weight required to achieve normalcy is variable, but the effect on decreasing transit time appears to plateau at fecal outputs >160 to 180 g per day (Burkitt et al., 1972). Many fiber experts have interpreted this as fiber having a
"normalizing" effect on laxation: once normal laxation has been achieved, additional fiber may contribute to other health benefits but not to laxation.
A number of recent feeding studies of healthy individuals provide further evidence to support the role of a fiber-rich diet in normal laxation and other purported benefits to colonic health ( Bach Knudsen et al., 1997; Blackwood et al., 2000; Chen et al., 1998 Haack et al., 1998). There are a large number of recent publications on the use of pre- and pro-biotics to alter the colonic microflora. Although a change in the microflora has been documented in several studies, functional endpoints are lacking at this time. (Cummings et al., 2002). Certain clinical studies have reported successful management of chronic constipation with fiber supplementation (Cummings, 1984; Hein et al., 1978; Loening-Baucke, 1994; Shafik et al., 1993).
Children. Consumption of adequate dietary fiber is associated with important health benefits throughout the lifecycle, but certain populations may require specific comment. For example, since the new AI for fiber is based on a decreased risk for CHD, some may assume that meeting the AI for fiber is less important for children than for adults. However, chronic constipation is one of the most common causes of morbidity in childhood (Bakwin, 1971; Leung, 1996; Loening-Baucke, 1995). Some studies have shown that up to 10 percent of children have chronic constipation (Bakwin, 1971; Leung, 1996; Loening-Baucke, 1995), which accounts for 25 percent of visits to pediatric gastroenterology clinics (Loening-Baucke, 1994). Several cross-sectional surveys on U.S. children and adolescents found inadequate dietary fiber intakes (Champagne et al., 2004; Cavadini et al., 2000). A randomized study of Greek children (291 with constipation and 1,602 controls) age 2 to 14 found that constipated children had lower caloric and nutrient intakes (P < 0.001), lower body weight/height (P < 0.001), and reported a higher prevalence of anorexia (P < 0.001). Despite the age of onset of constipation, dietary fiber alone was inversely correlated with chronic constipation (P < 0.001) (Roma et al., 1999). Another study found that children with constipation consumed approximately half as much fiber as a control group that was not constipated (McClung, et al., 1995). Similarly, Morais et al. (1999) reported that children with chronic constipation ate less fiber than their age-matched counterparts.
Elderly. There are a number of issues regarding healthy laxation and the elderly, some of which are summarized in Imershein et al. (2000). Some of these issues involve varied definitions of what constitutes normal laxation. However, there are also very real issues of drug interactions with laxation and lack of appropriate hydration due to concerns about urinary incontinence. Diuretics (often prescribed for hypertension) may result in hard stools, which are difficult to pass. Limitations on mobility also contribute to constipation. Not only is the prevalence of constipation higher in the elderly than in the general population (Grant, 1999), but the impact on quality of life appears to be greater (Pettigrew et al., 1997). Constipation may affect up to 20 percent of people over 65 years of age (Rouse et al., 1991).
Fiber and CHD. Evidence from a large number of epidemiological studies supports a protective role for dietary fiber against CHD (Fraser et al., 1992; Humble et al., 1993; Kromhout et al., 1982; Kushi et al., 1985; Khaw and Barrett-Connor et al., 1987; Morris,1977; Liu et al., 1999; Pietinen et al., 1996; Rimm et al., 1996; Wolk et al., 1999). The actual numbers used to set the AI were from three well-designed, adequately powered prospective epidemiological studies that measured the intake of fiber in healthy people and related the intake to later development of CHD (Pietinen et al., 1996; Rimm et al., 1996; Wolk et al., 1999) (see Table D5-1). Specifically, in the Health Professionals Follow-up Study, in which 43,757 men were followed, the relative risk for CHD for men in the highest quintile of fiber intake was 0.59 compared to 1.0 for the men in the lowest quintile of fiber intake (p<0.001). In the Nurses' Health Study, involving 68,782 U.S. women, the relative risk for the highest quintile of fiber intake was 0.77 compared to 1.0 for the lowest (Wolk et al., 1999). In the Finnish Men's Study, involving 21,930 men, the relative risk for CHD for men in the highest quintile of fiber intake was 0.45 compared to the lowest quintile (P <0.001) (Pietinen et al., 1996). As can be determined from data rows one through three of Table 5-1, the AI of 14 g of fiber per1,000 kcal was calculated using the average intake of fiber in the
"protected group" in each of the studies (i.e., the highest quintile of fiber intake) and dividing that intake by the average energy intake for that quintile to obtain grams of fiber per calorie. Fiber intake then was expressed as grams per 1,000 calories to provide a more useable number. In brief, to be in the group with the lowest risk for CHD, an average intake of 14 g of fiber per 1,000 kcal would need to be consumed.
Two more recent prospective cohort studies (Bazzano et al., 2003; Liu et al., 2002) provide further evidence that supports the AI of 14 g of fiber per 1000 kcal (Table D5-1). Liu et al. used prospective data from the Women's Health Study over a 6-year period to assess the relationship between total dietary fiber, soluble and insoluble fiber, and fiber sources on the risk of cardiovascular disease or myocardial infarction. A significantly smaller number of cardiovascular disease cases occurred in the highest quintile of intake than in the lowest quintile of intake (99 cases vs. 140 cases). The age and randomized treatment-adjusted relative risk (RR) of cardiovascular disease was 0.65 (P for the linear trend = 0.001) comparing the highest and lowest quintiles (Liu et al., 2002). Liu et al. also reported a pooled analysis of nine published dietary fiber and CHD epidemiological studies. This pooled analysis shows an RR of 0.83 associated with 10 g increases in dietary fiber intake (Liu et al., 2002). Bazzano et al. (2003) examined the relationship between total and soluble dietary fiber intake and the risk of CHD and cardiovascular disease in 9,776 adults who participated in the National Health and Nutrition Examination Survey 1 Epidemiologic Follow-up Study. They report that individuals in the highest quartile for dietary fiber intake (20.7 g per day) had an RR of 0.88 for CHD events compared to those in the lowest quartile (5.9 g per day).
A meta-analysis by Pereira et al. (2004) compared intakes of dietary fiber and its subtypes (cereal, fruit, and vegetable fibers) and risk of CHD. This pooled analysis of 10 large prospective studies reported that each 10 g per day increment of dietary fiber was associated with a 14 percent decreased risk of coronary death. Fiber from cereals and fruits also had a strong, inverse association with CHD risk (RR 0.75 and 0.70, respectively). This association was not found for vegetable fiber (RR 1.00).
When setting the AI for fiber, the IOM also took into consideration small-scale
clinical intervention trials and potential mechanisms for this observed
protective effect against CHD. As reviewed by Fernandez (2001), a large
number of relatively small-scale clinical intervention trials have shown
that viscous fibers can lower serum cholesterol. It is generally accepted
that a decrease in serum cholesterol is protective against CHD (see Part
D, Section 4, for further information). Notably, in the studies in the
Types and Sources of Dietary Fiber Summary Tables, Appendix G-3, total dietary
fiber from foods was shown to be protective against CHD, not
just those fibers that lower cholesterol. Whole grains, fruits, and vegetables
are
the food sources of fiber. Other possible mechanisms for the
protective effect of high-fiber diets include the delayed absorption of
macronutrients;
a decrease in serum triglyceride levels; and a lowering of
blood pressure. Also, whole grains, fruits and vegetables contain substances,
such as phytochemicals,
that may contribute to their beneficial effect in protecting
against CHD.
Back to Top
SUMMARY
Carbohydrates—the sugars, starches,
and fibers found in grains, fruits, vegetables,
and milk products—are an important part
of a nutritious, healthy diet. The intake
of carbohydrates (including sucrose,
glucose, fructose, lactose and starch)
contributes to dental caries by providing
substrate for bacterial fermentation
in the mouth. Drinking fluoridated water
and/or using fluoride-containing dental
hygiene products help reduce the risk
of dental caries.
Compared with individuals who consume small amounts of foods and beverages that are high in added sugars, those who consume large amounts tend to consume more calories but smaller amounts of micronutrients. Although more research is needed, available prospective studies suggest a positive association between the consumption of sweetened beverages and weight gain. A reduced intake of added sugars (especially sugar-sweetened beverages) may be helpful in weight control and in achieving recommended intakes of nutrients.
To reduce the risk of CHD disease and promote healthful laxation, the Committee recommends the intake of 14 g of dietary fiber per 1,000 calories.
Back to Top
REFERENCES
Anderson KN. (ed). Mosby's Medical, Nursing, & Allied Health Dictionary. 6th Edition. Mosby Publishing Company, Inc., 2003.
Anusavice KJ. Dental caries: risk assessment and treatment solutions for an elderly population. Compendium of Continuing Education in Dentistry 23:12-20, 2002.
Bach Knudsen KE, Johansen HN, Glitsø V. Rye dietary fiber and fermentation in the colon. Cereal Foods World 72:690-694, 1997.
Bakwin H, Davison M. Constipation in twins. American Journal of Diseases of Children 121:179-181, 1971.
Bazzano, LA, He J, Ogden LG, Loria CM and Whelton PK. Dietary fiber intake and reduced risk of coronary heart disease in U.S. men and women. The National Health and Nutrition Examination Survey I Epidemiologic Follow-up Study. Archives of Internal Medicine 163:1897-1904, 2003.
Berkey, CS, et al. Sugar-added beverages and adolescent weight change. Obesity Research 12(5):778-788, 2004.
Bibby BG. The cariogenicity of snack foods and confections. Journal of the American Dental Association 90:121-32, 1975.
Birkett AM, Jones GP, de Silva AM, Young GP, Muir JG. Dietary intake and faecal excretion of carbohydrate by Australians: importance of achieving stool weights greater than 150 g to improve faecal markers relevant to colon cancer risk. European Journal of Clinical Nutrition 51:625-632, 1997.
Blackwood AD., Salter J, Dettmar PW, Chaplin MF. Dietary fibre, physicochemical properties and their relationship to health. Journal Review of Sociological Health 120(4):242-7, 2000.
Brodribb, AJM, Humphreys DM. Diverticular disease: Three studies. Part II - Treatment with bran. British Medical Journal 1:425-428,1976.
Bolton-Smith C and Woodward M. Dietary composition and fat to sugar ratios in relation to obesity. International Journal of Obesity Related Metabolic Disorders 18:820-828, 1994.
Bolton-Smith C and Woodward M. Antioxidant vitamin adequacy in relation to consumption of sugars. European Journal of Clinical Nutrition 49:124-33, 1995.
Bowman SA. Diets of individuals based on energy intakes from
added sugars Family and Economic Nutrition Reviews 12:31-8,
1999.
Britten P, Basiotis PP, Davis CA, Anand R. Is intake of added
sugars associated with diet quality? USDA Center for Nutrition
Policy and Promotion Nutrition Insights Insight 21, 2000.
(Online) http://www.usda.gov/cnpp/insights.html.
Burkitt, DP, Walker ARP, Painter NS. Effect of dietary fibre
on stools and transit-times, and its role in the causation of
disease. Lancet 1408-1412, 1972.
Burt BA, Pai S. Sugar consumption and caries risk: a systematic
review. Journal of Dental Education 65:1017-1023, 2001.
Campaign AC, Morgan MV, Evans RW, Ugoni A, Adams GG, Conn JA,
Watson M.J. Sugar-starch combinations in food and the relationship
to dental caries in low-risk adolescents. European Journal
of Oral Science 111:316-25, 2003.
Cavadini C, Siega-Riz AM, Popkin BM.. U.S. adolescent food intake
trends from 1965 to 1996. Archives of Diseases of Children
83(1):18-24, 2000.
Champagne C M, Bogle ML, McGee BB, Yadrick K, Allen HR, Kramer
TR, Simpson P, Gossett J, Weber J. Dietary intake in the lower
Mississippi delta region: results from the Foods of our Delta
Study. Journal of the American Dietetic Association;
104(2):199-207, 2004.
Chen HL, Haack VS, Janecky CW, Vollendorf NW, Marlett JA. Mechanisms
by which wheat bran and oat bran increase stool weight in humans.
American Journal of Clinical Nutrition Sep;68(3):711-719,
1998.
Colditz GA, Manson JE, Stampfer MJ, Rosner B, Willett WC, Speizer
FE. Diet and risk of clinical diabetes in women. American
Journal of Clinical Nutrition 55:1018-1023, 1992.
Cummings JH, Bingham SA, Heaton KW, Eastwood MA. Fecal weight,
colon cancer risk and dietary intake of non-starch polysaccharides
(dietary fiber). Gastroenterology 103:1783-1789, 1992.
Cummings JH. The effect of dietary fiber on fecal weight and
composition. In: Spiller, G.A. (ed.), CRC Handbook of Dietary
Fibre in Human Nutrition. Ann Arbor: CRC Press pp. 263-349,
1992.
Cummings JH. Constipation, dietary fiber and the control of large
bowel function. Postgraduate Medical Journal 60:811-819,
1984.
Edgar WM, Bibby BG, Mundorff S, Rowley J. Acid production in
plaques after eating snacks: modifying factors in foods Journal
of the American Dietetic Association 90:418-25, 1975.
FAO/WHO (Food and Agriculture Organization/World Health Organization).
Carbohydrates in Human Nutrition. Rome: FAO, 1998.
Food and Drug Administration (FDA). Administration. Rules and
Regulations -- Food Labeling. Health Claims, dietary sugar alcohols
and dental caries. Federal Register December 2, 63653-5,
1997.
FDA. Administration. Food Labeling: Health claims. D-tagatose
and dental caries. Federal Register 21 CFR (101):71461-70,
2002.
Fernandez ML. Soluble fiber and nondigestible carbohydrate effects
on plasma lipids and cardiovascular risk. Current Opinion
in Lipidology 12:35-40, 2001.
Ford ES, Liu S. Glycemic index as a determinant of serum HDL
cholesterol concentration among US adults. Archives of Internal
Medicine 161:572-576, 2001.
Forshee R and Storey M. The role of added sugars in the diet
quality of children and adolescents. Journal of the American
College of Nutrition 20, 32-43, 2001.
Fraser RJ, Horowitz M, Maddox AF, Horowitz M, Maddox AF, Harding
PE, Chatterton BE. Hyperglycemia slows gastric emptying in type
1 (insulin-dependent) diabetes mellitus. Diabetologia
Nov:33(11):675-80, 1990.
Fraser, GE, Sabate J, Beeson WL and Strahan TM. A possible protective
effect of nut consumption on risk of coronary heart disease: the
Adventist Health Study. Archives of Internal Medicine
152:1416-1424, 1992.
Frary DC, Johnson RK, Wang MQ. Children and adolescents' choices
of foods and beverages high in added sugars are associated with
intakes of key nutrients and food groups. Journal of Adolescent
Health 34, 56-63, 2004.
Gannon MC, Nuttall FQ, Westphal SA, Fang S, Ercan-Fang N. Acute
metabolic response to high-carbohydrate, high-starch meals compared
with moderate-carbohydrate, low-starch meals in subjects with
type 2 diabetes. Diabetes Care 21:1619-1626, 1998.
Gibson SA. Are diets high in non-milk extrinsic sugars conducive
to obesity? An analysis from the Dietary and Nutritional Survey
of British Adults. Journal of Human Nutrition and Dietetics
9:283-292, 1996.
Gibson SA. Non-milk extrinsic sugars in the diets of pre-school
children: association with intakes of micronutrients, energy,
fat and non-starch polysaccharides. British Journal of Nutrition
78:367-78, 1997.
Gibson SA, Williams S. Dental caries in pre-school children:
associations with social class, toothbrushing habit, and consumption
of sugars and sugar-containing foods. Further analysis of data
from the National Diet and Nutrition Survey of children aged 1.5-4.5
years. Caries Research 33:101-113, 1999.
Graham YD, Moser ES, Estes KM. The effect of bran on bowel function
in constipation. American Journal of Gastroenterology
77:599-603, 1982.
Grant E. An audit of laxative use and fibre/fluid intakes in
a care of ther elderly rehabilitation ward. Journal of Human
Nutrition and Dietetics 12:409-413, 1999.
Grindefjord M, Dahllof G, Nilsson B, Modeer T. Stepwise prediction
of dental caries in children up to 3.5 years of age. Caries
Research 30:256-66, 1996.
Guthrie JF, Morton JF. Food sources of added sweeteners in the
diets of Americans. Journal of the American Dietetic Association
100:43-8, 2000.
Haack V S, Chesters JG, Vollendorf NW, Story JA, Marlett JA.
Increasing amounts of dietary fiber provided by foods normalizes
physiologic response of the large bowel without altering calcium
balance or fecal steroid excretion. American Journal of Clinical
Nutrition 68(3):615-22, 1998.
Harnack L, Stang J, Story M. Soft drink consumption among U.S.
children and adolescents: nutritional consequences. Journal
of the American Dietetic Association 99:436-441, 1999.
Hein, AB, Beerends JJ. A successful pediatric program based on
dietary control bowel training and family counseling. Clinics
in Pediatrics 17:67-70, 1978.
Heller KE, Burt BA, Eklund SA. Sugared soda consumption and dental
caries in the United States. Journal of Dental Research
80:1949-53, 2001.
Holbrook WP, Arnadottir IB, Takazoe E, Birkhed D, Frostell G.
Longitudinal study of caries, cariogenic bacteria and diet in
children just before and after starting school. European Journal
of Oral Science 103:42-5, 1995.
Hu FB, Manson JE, Stampfer MJ, Colditz G, Liu S, Solomon CG,
Willett WC. Diet, lifestyle, and the risk of type 2 diabetes mellitus
in women. New England Journal of Medicine 345:790-797,
2001.
Hughes TA, Atchison J, Hazelrig JB, Boshell BR. Glycemic responses
in insulin-dependent patients with diabetes: effect of food composition.
American Journal of Clinical Nutrition 49: 658-666, 1989.
Humble CG, Malarcher AM, Tyroler HA. Dietary fiber and coronary
heart disease in middle-aged hypercholesterolemic men. Am
J Prev Med 9:197-202, 1993.
Institute of Medicine (IOM). Dietary carbohydrates: sugars
and starches. Dietary Reference Intakes for Energy, Carbohydrate,
Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids.
Washington, DC: National Academies Press, 2002.
Imershein N, Linnehan E. Constipation: a common problem of the
elderly. Journal of Nutrition for the Elderly 19:49-54,
2000.
James J, Thomas P, Cavan D, Kerr D. Preventing childhood obesity
by reducing consumption of carbonated drinks: cluster randomised
controlled trial. British Medical Journal 328(7450):1237,
2004.
Janket SJ, Manson JE, Sesson H, Buring JE, Liu S. A prospective
study of sugar intake and risk of type 2 diabetes in women. Diabetes
Care 26:1008-1015, 2003.
Jarvi A, Karlstrom B, and Granfeldt Y. The influence of food
structure on postprandial metabolism in patients with NIDDM. American
Journal of Clinical Nutrition 61:837-842, 1995.
Jenkins DHA, Wolever TM, Taylor RH, Griffiths C, Krzeminska K,
Lawrie JA, Bennett CM, Goff DV, Sarson DL, and Bloom SR. Slow
release dietary carbohydrate improves second meal tolerance. American
Journal of Clinical Nutrition 35:1339-1346, 1982.
Johnson RK. What are people really eating and why does it matter?
Nutrition Today 35:40-46, 2000.
Kashket S, Zhang J, van Houte J. Accumulation of fermentable
sugars and metabolic acids in food particles that become entrapped
on the dentition. Journal of Dental Research 75:1885-1891,
1996.
Kelsay, JL, Behall KM, Prather ES. Effect of fiber from fruits
and vegetables on metabolic responses of human subjects. American
Journal of Clinical Nutrition 31:1149-1153, 1978.
Khaw K, Barrett-Connor E. Dietary fiber and reduced ischemic
heart disease mortality rates in men and women: a 12-year prospective
study. American Journal of Epidemiology 126:1093-1102,
1987.
Krebs-Smith SM, Graubard BI, Kahle LL, Subar AF, Cleveland LE,
Ballard-Barbash R. Low energy reporters vs. others: a comparison
of reported food intakes European Journal of Clinical Nutrition
54:281-287, 2000.
Kromhout D, Bosschieter EB, de Lezenne Coulander C. Dietary fibre
and 10-year mortality from coronary heart disease, cancer and
all causes. The Zutphen Study. Lancet 2:518-522, 1982.
Kushi LH, Lew RA, Stare FJ. Diet and 20-year mortality from coronary
heart disease: the Ireland-Boston Diet-Heat Study. New England
Journal of Medicine 312:811-818, 1985.
Leung AK, Chan PY, Cho HY. Constipation in children. American
Family Physician 54:611-627, 1996.
Lewis CJ, Park YK, Dexter PB, Yetley EA. Nutrient intakes and
body weights of persons consuming high and moderate levels of
added sugars. Journal of the American Dietetic Association
92:708-713, 1992.
Lingstrom P, Holm AK, Mejare I, Twetman S, Soder B, Norlund A,
Axelsson S, Lagerlof F, Nordenram G, Petersson LG, Dahlgren H,
Kallestal C. Dietary factors in the prevention of dental caries:
a systematic review. Acta Odontologica Scandia 6:331-40,
2003.
Lingstrom P, van Houte J, Kashket S. Food starches and dental
caries. Critical Reviews in Oral Biological Medicine
11:366-380, 2000.
Liu S, Buring JE, Sesso HD, Rimm EB, Willett WC, Manson JE. A
prospective study of dietary fiber intake and risk of cardiovascular
disease among women. Journal of the American College of Cardiology
39:49-56, 2002.
Liu S, Manson JE, Stampfer MJ, Holmes MD, Hu FB, Hankinson SE,
Willett WC. Dietary glycemic load assessed by food-frequency questionnaire
in relation to plasma high-density-lipoprotein cholesterol and
fasting plasma triacylglycerols in postmenopausal women. American
Journal of Clinical Nutrition 73:560-566, 2001.
Liu SM, Stampfer MJ, Hu FB, Giovannucci E, Rimm E, Manson JE,
Hennekens CH, Willett WC. Whole-grain consumption and risk of
coronary heart disease: results from the Nurses' Health Study.
American Journal of Clinical Nutrition 70:412-419, 1999.
Loening-Baucke V. Functional constipation. Seminars in Pediatric
Surgery 4:26-34,1995.
Loening-Baucke V. Assessment, diagnosis and treatment of constipation
in childhood. Journal of Wound, Ostomy and Continence Nursing
21:49-58, 1994.
Ludwig DS, Peterson KE, Gortmaker SL. Relation between consumption
of sugar-sweetened drinks and childhood obesity: A prospective,
observational analysis Lancet 357, 505-508, 2001.
Luke GA, Hough H, Beeley JA, Geddes DA. Human salivary sugar
clearance after sugar rinses and intake of food stuffs. Caries
Research 33:123-129, 1999.
Lundgren H, Bengtsson C, Blohme G, Isaakson B, Lapidus L, Lenner
RA, Saaek A, Winther E. Dietary habits and incidence of noninsulin-dependent
diabetes melitus in a population study of women in Gothenburg,
Sweden. American Journal of Clinical Nutrition 49:708-712,
1989.
Makinen K, Bennett C, Hujoel P, Isokangas P, Isotupa K, Pape
H. Xylitol chewing gums and caries rates: a 40-month cohort study.
Journal of Dental Research 74:1904-1913, 1995.
Mann J, Hermansen K, Vessby B, Toller M. Evidence-based nutrition
principles and recommendations for the treatment and prevention
of diabetes and related complications. Diabetes Care
25:1256-1258, 2002.
Marlett JA, Kajs TM, Fischer MH. An unfermented gel component
of psyllium seed husk promotes laxation as a lubricant in humans.
American Journal of Clinical Nutrition 72:784-789, 2000.
Marshall JA, Hamman RF, Baxter J. High-fat, low-carbohydrate
diet and the etiology of non-insulin-dependent diabetes mellitus:
the San Luis Valley Diabetes Study. American Journal of Epidemiology
134:590-603, 1991.
Mascarenhas A. Oral hygiene as a risk indicator of enamel and
dentin cares. Community Dentistry and Oral Epidemiology
26:331-339, 1998.
Mattes RD. Dietary compensation by humans for supplemental energy
provided as ethanol or carbohydrate in fluids. Physiology
and Behavior 59:179-187, 1996.
McClung HJ, Boyne L, Heittlinger L. Constipation and dietary
fiber intake in children. Pediatrics 96:999-1001, 1995.
McDonagh MS, Whiting PF, Wilson PM, Sutton AJ, Chestnutt I, Cooper
J, Misso K, Bradley M, Treasure E, Kleijnen J. Systematic review
of water fluoridation. British Medical Journal 321:855-859,
2000.
Meyer KA, Kushi LH, Jacobs DR Jr, Slavin J, Sellers TA, Folsom
AR. Carbohydrates, dietary fiber, and incident type 2 diabetes
in older women. American Journal of Clinical Nutrition
71: 921-930, 2000.
Montonen J, Knekt P, Jarvinen R, Aromaa A, Reunanen A. Whole-grain
and fiber intake and the incidence of type 2 diabetes. Journal
of the American College of Nutrition 77:622-629, 2003.
Morais MB, Vitolo MR, Aguirre ANC, Fagundes-Neto UF. Measurement
of low dietary fiber intake as a risk factor for chronic constipation
in children. Journal of Pediatric Gastroenterology and Nutrition
29:132-135, 1999.
Morris JN, Marr JW, Clayton DG. Diet and heart: a postscript.
British Medical Journal 2:1307-1314, 1977.
Moynihan P, Petersen PE. Diet, nutrition and the prevention of
dental diseases. Public Health Nutrition 7:201-226, 2004.
Mrdjenovic G, Levitsky DA. Nutritional and energetic consequences
of sweetened drink consumption in 6- to 13-year-old children.
Journal of Pediatrics 142(6):604-610, 2003.
Navia J. Dietary carbohydrates and dental health. American
Journal of Clinical Nutrition 59 (Suppl):719S-27S, 1994.
Nelson M. Food, vitamins and IQ. Proceedings of the Nutrition
Society 50:29-35, 1991.
Newby PK, Peterson KE, Berkey CS, Leppert J, Willett WC, Colditz
GA. Beverage consumption is not associated with changes in weight
and body mass index among low-income preschool children in North
Dakota. Journal of the American Dietetic Association
104(7):1086-1094, 2004.
Nielsen PH, Nielsen GL. Preprandial blood glucose values: influence
on glycemic response studies. American Journal of Clinical
Nutrition 53:1242-1246, 1989.
Nicklas TA, Yang SJ, Baranowski T, Zakeri I, Berenson G. Eating
patterns and obesity in children. The Bogalusa Heart Study. American
Journal of Preventive Medicine Jul;25(1):9-16, 2003.
O'Dea K, Snow P, Nestel P. Rate of starch hydrolysis in vitro
as a predictor of metabolic responses to complex carbohydrates
in vivo. American Journal of Clinical Nutrition 34:1991-1993,
1981.
Overby NC, Lillegaard IT, Johansson L, Andersen LF. High intake
of added sugar among Norwegian children and adolescents. Public
Health Nutrition Apr;7(2):285-293, 2004.
Parillo M, Giacco R, Ciardullo AV, Rivellese AA, Riccardi G.
Does a high-carbohydrate diet have different effects in NIDDM
patients treated with diet alone or hypoglycemic drugs? Diabetes
Care 19:498-500, 1996.
Paunio P, Rautava P, Helenius H, Alanen P, Sillanpaa M. The Finnish
family competence study: the relationship between caries, dental
health habits and general health in 3-year-old Finnish children.
Caries Research 27:154-60, 1993.
Pettigrew M, Watt I, Sheldon T. Systematic review of the effectiveness
of laxatives in the elderly. Health Technology Assessment
1:13, 1997.
Pereira MA, O'Reilly E, Augustsson K, Fraser GE, Goldbourt U,
Heitmann BL, Hallmans G, Knekt P, Liu S, Pietinen P, Spiegelman
D, Stevens J, Virtamo J, Willett WC, and Ascherio A. Dietary fiber
and risk of coronary heart disease: a pooled analysis of cohort
studies. Archives of Internal Medicine 164(4):370-376,
2004.
Phillips SM, Bandini LG, Naumova EN, Cyr H, Colclough S, Dietz
WH, Must A. Energy-dense snack food intake in adolescence: longitudinal
relationship to weight and fatness. Obesity Research
12(3): 461-472, 2004.
Pietinen, P, Rimm EB, and Korhonen P. Intake of dietary fiber
and risk of coronary heart disease in a cohort of Finnish men:
the alpha-tocopherol, beta-carotene cancer prevention study. Circulation
94:2720-2727, 1996.
Rasmussen I, Hermansen K. Preprandial blood glucose values and
glycemic responses in insulin-dependent diabetes mellitus at constant
insulinemia. American Journal of Clinical Nutrition 53:
520-523, 1991.
Rimm, EB, Ascherio A, Giovannucci E, Spiegelman D, Stampfer MJ,
Willett WC. Vegetable, fruit, and cereal fiber intake and risk
of coronary heart disease among men. Journal of the American
Medical Association 275:447-451, 1996.
Rolls BJ, Morris EL, Roe LS. Portion size of food affects energy
intake in normal-weight and overweight men and women. American
Journal of Clinical Nutrition Dec;76(6):1207-13, 2002.
Roma E, Adamidis D, Nikolara R, Constantopoulos A, Messaritakis
J. Diet and chronic constipation in children: the role of fiber.
Journal of Pediatric Gastroenterology and Nutrition 28(2):169-174,
1999.
Rouse M, Mahapatra M. An open randomised parallel group study
of lactulose versus ispaghula ni the treatment of chronic constipation
in adults. British Journal of Clinical Practice 45:28,
1991.
Rugg-Gunn AJ, Hackett AF, Jenkins GN, Appleton DR. Empty calories?
Nutrient intake in relation to sugar intake in English adolescents
Journal of Human Nutrition and Dietetics 4:101-111, 1991.
Ruottinen S, Karjalainen S, Pienihakkinen K, Lagstrom H, Niinikoski
H, Salminen M, Ronnemaa T, Simell O. Sucrose intake since infancy
and dental health in 10-year-old children. Caries Research
38:142-148, 2004.
Salmeron J, Ascherio A, Rimm EB, Colditz GA, Spiegelman D, Jenkins
DJ, Stampfer MJ, Wing AL, Willett WC. Dietary fiber, glycemic
load, and risk of NIDDM in men. Diabetes Care 20:545-550,
1997.
Salmeron J, Manson JE, Stampfer MJ, Colditz GA, Wing AL, Willett
WC. Dietary fiber, glycemic load, and risk of non-insulin-dependent
diabetes mellitus in women. Journal of the American Medical
Association 277:472-477, 1997.
Schulze MB, Manson JE, Ludwig DS, Colditz GA, Stampfer MJ, Willett
WC, Hu FB. Sugar-sweetened beverages, weight gain, and incidence
of type 2 diabetes in young and middle-aged women. JAMA,
in press 2004.
Schvarcz E, Palmer M, Aman J. Hypoglycaemia increases gastric
emptying rate in patients with Type 1 diabetes mellitus. Diabetes
Medicine 10:660-663, 1993.
Shafik A. Constipation. Pathogenesis and management. Drugs
45:528-540, 1993.
Shaw JH. Causes and control of dental caries. New England
Journal of Medicine 317:996-1004, 1987.
Snow P, O'Dea K. Factors affecting the rate of hydrolysis of
starch in food. American Journal of Clinical Nutrition
34:2721-2727, 1981.
Stecksen-Blicks C, Holm AK. Between meal eating, toothbrushing
frequency, and dental caries in 4-year-old children in the north
of Sweden. International Journal of Paediatric Dentistry
5:67-72, 1995.
Storey ML, Forshee RA, Weaver AR, Sansalone WR. Demographic and
lifestyle factors associated with BMI among children and adolescents.
International Journal of Food Science and Nutrition Nov;54(6):491-503,
2003.
Touger-Decker R, van Loveren C. Sugars and oral health. ILSI
Conference: Sugars and Health. American Journal of Clinical
Nutrition 78 (suppl):881S-92S, 2002.
Troiano RP, Briefel RR, Carroll MD, Bialostosky K. Energy and
fat intakes of children and adolescents in the United States:
data from the National Health and Nutrition Examination Surveys.
American Journal of Clinical Nutrition 72:1343S-53A,
2000.
USDA/DHHS. Nutrition and your health: dietary guidelines for
Americans. Home and Garden Bulletin No. 252. Washington,
DC, 2000.
Walker ARP, Cleaton-Jones PE. Sugar intake and dental caries.
British Dental Joural 172:7, 1992.
Wendt LK, Birkhed D. Dietary habits related to caries development
and immigrant status in infants and toddlers living in Sweden.
Acta Odontologica Scandia 53:339-344, 1995.
Wendt LK, Hallonsten AL, Koch G, Birkhed D. Analysis of caries-related
factors in infants and toddlers living in Sweden. Acta Odontologica
Scandia 54:131-137, 1996.
Wolever TM, Mehling C. High-carbohydrate-low-glycaemic index
dietary advice improves glucose disposition index in subjects
with impaired glucose tolerance. British Journal of Nutrition
May; 87(5):477-487, 2003.
Wolever TM, Nguyen PM, Chiasson JL. Determinants of diet glycemic
index calculated retrospectively from diet records of 342 individuals
with non-insulin-dependent diabetes mellitus. American Journal
of Clinical Nutrition 59:1265-1269, 1994.
Wolk, A, Manson JE, Stampfer MJ, Colditz GA, Hu FB, Speizer FE,
Hennekens CH, Willett WC. Long-term intake of dietary fiber and
decreased risk of coronary heart disease among women. JAMA
281:1998-2004, 1999.
Wrick, KL, Robertson JB, Van Soest PJ, Lewis BA, Rivers JM, Roe
DA, Hackler LR. The influence of dietary fiber source on human
intestinal transit and stool output. Journal of Nutrition
113(8):1464-1479, 1983.
Yang EU, Kerver JM, Park YK, Kayitsinga J, Allison DA, Song WO.
Carbohydrate intake and biomarkers of glycemic control among U.S.
adults: the Third National Health and Nutrition Examination Survey
(NHANES III). American Journal of Clinical Nutrition
77:1426-1433, 2003.
Back to Top
1 The z-score, which represents the number of standard deviations away from the population mean in a normal distribution, indicates the degree to which an individual's measurement deviates from what is expected for that individual.
Back to Top
Table D5-1. Dietary Fiber Intake and Coronary Heart Disease (CHD): Prospective Cohort Studies
 Select icon to view table
Select icon to view table
Back to Top
Figure D5-1. Calcium Intake in 4- to 8-year-old Children as a function of added sugar intake
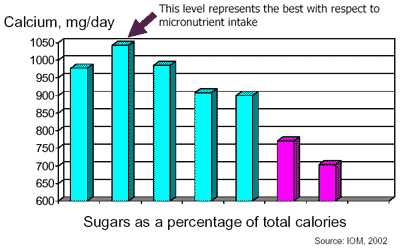 d d
Back to Top
|

