| February 19, 2008

[NIST Tech Beat Search] [Credits] [NIST Tech Beat Archives] [Media Contacts] [Subscription Information]

NIST, NCI, SAIC Partner on New Method for Detecting HER2 Breast Cancer
|
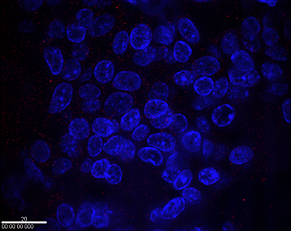
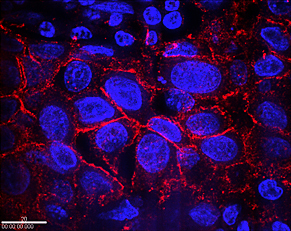
Immunohistochemical detection of HER2 cancer biomarker with IgY antibody and quantum dots is demonstrated in these samples of breast cancer cells from a patient with normal HER2 expression (top) and one with over production (bottom). Cell nuclei are stained blue, quantum dots responding to the HER2 protein light up in bright red at the cell membranes. Treatment with trastuzumab could only be effective for the latter patient.
Credit: NIST
View hi-resolution image of top image
View hi-resolution image of bottom image |
Generations of mothers have served up chicken soup to remedy the common cold, but now the therapeutic fowl may find use in diagnosis as well. Researchers at the National Institute of Standards and Technology (NIST), the National Cancer Institute (NCI) and the scientific research firm SAIC recently showed how chicken antibodies may one day improve the detection of an aggressive form of breast cancer.
HER2 is one of a family of genes that help regulate the growth and proliferation of human cells. Normal cells have two copies of HER2, but about 20 to 25 percent of breast cancers have multiple copies of the gene, resulting in the overproduction of a HER2-encoded protein (called HER2) that stimulates tumors to be particularly fast growing and difficult to treat in a subset of breast cancer patients.
Patients with that form of breast cancer—about 40,000 women in the United States annually—can be treated with a monoclonal antibody called trastuzumab that targets and inhibits the growth of tumor cells with higher-than-normal levels of the HER2 protein. But because the treatment can have adverse side effects, it’s important to screen for those patients who would benefit from it by testing them for one or both of the two relevant biomarkers: the amplified HER2 gene or its overexpressed HER2 protein. Unfortunately, the existing tests for these biomarkers can yield a significant number of false positives—as many as 23 percent of patients in one 2006 clinical study—resulting in some women getting a somewhat risky and expensive treatment that can’t help them.
In a paper in the International Journal of Cancer,* the NIST-NCI-SAIC research team found that chicken immunoglobulin Y (IgY) antibody created against the HER2 protein could be tagged with quantum dots (tiny, intense and tunable sources of colorful light) to more reliably detect the HER2 biomarker than the existing diagnostic tests using mammalian antibodies tagged with conventional fluorescent dyes. Overall, the improvement in sensitivity to the HER2 biomarker was about 40-50 percent.
The increased sensitivity of the HER2 quantum dot-based quantitative bioimaging system stems from the broad genetic differences between avian and human species. The chicken IgY antibody to HER2 reacts strongly with the target protein while ignoring other human proteins that can interfere with current diagnostic tests.
Other advantages of the novel NIST-NCI-SAIC system include faster and larger-scale production of the antibodies and a more reliable quantitative measure of HER2 biomarker level, in part because the quantum dot tags will stay bright and detectable while fluorescent dyes fade over time.
The research was funded under an interagency agreement between NIST and NCI. NIST also is funding the development of a standard reference material to support HER2 testing.
* Y. Xiao, X. Gao, G. Gannot, M.R. Emmert-Buck, S. Srivastava, P.D. Wagner, M.A.Amos and P.E. Barker. Quantitation of HER2 and telomerase biomarkers in solid tumors with IgY antibodies and nanocrystal detection. International Journal of Cancer, posted online Jan. 23, 2008.
Media Contact: Michael E. Newman, michael.newman@nist.gov, (301) 975-3025 

‘NMR on a Chip’ Features NIST Magnetic Mini-Sensor
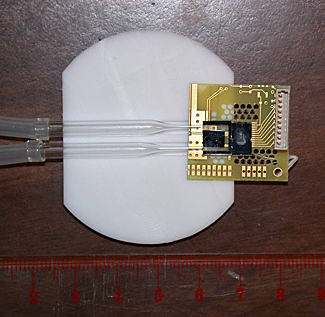 |
Prototype microchip device combining NIST’s miniature atomic magnetometer with a fluid channel for studies of tiny samples.
Credit: NIST
View hi-resolution image |
A super-sensitive mini-sensor developed at the National Institute of Standards and Technology (NIST) can detect nuclear magnetic resonance (NMR) in tiny samples of fluids flowing through a novel microchip. The prototype chip device, developed in a collaboration between NIST and the University of California, may have wide application as a sensitive chemical analyzer, for example in rapid screening to find new drugs.
As described in Proceedings of the National Academy of Sciences (PNAS),* the NMR chip detected magnetic signals from atomic nuclei in tap water flowing through a custom silicon chip that juxtaposes a tiny fluid channel and the NIST sensor. The Berkeley group recently co-developed this “remote NMR” technique for tracking small volumes of fluid or gas flow inside soft materials such as biological tissue or porous rock, for possible applications in industrial processes and oil exploration. The chip could be used in NMR spectroscopy, a widely used technique for determining physical, chemical, electronic and structural information about molecules. NMR signals are equivalent to those detected in MRI (magnetic resonance imaging) systems
Berkeley scientists selected the NIST sensor, a type of atomic magnetometer, for the chip device because of its small size and high sensitivity, which make it possible to detect weak magnetic resonance signals from a small sample of atoms in the adjacent microchannel. Detection is most efficient when the sensor and sample are about the same size and located close together, lead author Micah Ledbetter says. Thus, when samples are minute, as in economical screening of many chemicals, a small sensor is crucial, Ledbetter says.
Its small size and extreme sensitivity make the NIST sensor ideal for the microchip device, in contrast to SQUIDs (superconducting quantum interference devices) that require bulky equipment for cooling to cryogenic temperatures or conventional copper coils that need much higher magnetic fields (typically generated by large, superconducting magnets) like those in traditional MRI.
The results reported in the PNAS demonstrate another use for the NIST mini-sensor, a spin-off of NIST’s miniature atomic clocks. The sensor already has been shown to have biomedical imaging applications (see “New NIST Mini-Sensor May Have Biomedical and Security Applications”).
The principal investigator for the study described in PNAS is Alexander Pines, a leading authority on NMR. A joint university/NIST patent application is being filed for the microchip device. The research was supported by the Office of Naval Research, U.S. Department of Energy, a CalSpace Minigrant and the Defense Advanced Research Projects Agency.
* M.P. Ledbetter, I.M. Savukov, D. Budker, V. Shah, S. Knappe, J. Kitching, D. Michalak, S. Xu , and A. Pines. Zero-field remote detection of NMR with a microfabricated atomic magnetometer. Proceedings of the National Academy of Sciences, posted online Feb. 6, 2008.
Media Contact: Laura Ost, laura.ost@nist.gov, (303) 497-4880 

Optical ‘Frequency Comb’ Can Detect the Breath of Disease
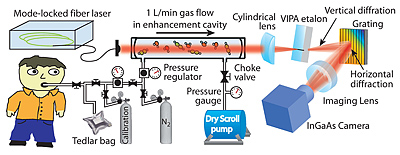 |
Schematic of a breath analyzer that uses “frequency combs,” an optical tool that led to a recent Nobel Prize in Physics for JILA fellow Jan Hall.
Credit: Univ. of Colorado/JILA
View hi-resolution image |
Exhale on a cold winter day and you will see the water vapor coming out of your mouth. Light up your breath with a Nobel-Prize-related tool, and you could potentially detect trace amounts of over 1,000 compounds, some of which provide early warning signs of disease. In a new paper,* a team led by Jun Ye, a physicist at JILA, a joint institute of the National Institute of Standards and Technology (NIST) and the University of Colorado at Boulder, has demonstrated an optical technique for simultaneously identifying tiny amounts of a broad range of molecules in the breath, potentially enabling a fast, low-cost screening tool for disease.
“It is exciting to imagine the potential of analyzing all major biomarkers in one’s breath at once,” says Ye. “For example, nitric oxide can indicate asthma, but it also appears in breath with many other lung diseases, including chronic obstructive pulmonary disease, cystic fibrosis and bronchiectasis. However, if we simultaneously monitor nitric oxide, carbon monoxide, hydro-peroxide, nitrites, nitrates, pentane, and ethane, all important biomarkers for asthma, we can be much more certain for a definitive diagnosis of this important disease.”
Existing methods for detecting trace amounts of molecules from the breath are either bulky, slow, limited to specific molecules, unable to distinguish very well between multiple compounds or inaccurate at measuring their concentrations. In this new approach, the researchers analyze human breath with “frequency combs,” an optical tool cited in the 2005 Nobel Prize in Physics shared by JILA fellow Jan Hall. Frequency combs are generated by a laser specially designed to produce a series of very short, equally spaced pulses of light. Each pulse may be only a few millionth billionths of a second long. The laser generates light as a series of very narrow frequency peaks equally spaced, like the teeth of a comb, across a broad spectrum.
In the experiment, student volunteers exhaled breath that entered an optical cavity where it was “combed” by the light pulses. By detecting which colors of light were absorbed and in what amounts—essentially looking for light absorbed near the “teeth” of the comb— the researchers could detect specific molecules and their concentrations. For example, a student smoker who participated in the experiment had a level of carbon monoxide that was five times greater than a nonsmoker in the experiment. The optical comb approach allows the researchers to simultaneously analyze a very broad spectrum, covering many possible molecular compounds, with high precision, frequency resolution and sensitivity. The technique is in early phases, and would require clinical trials before it could become available at a doctor’s office, but it could lead to one of the first widespread applications of frequency combs.
* M.J. Thorpe, D. Balslev-Clausen, M.S. Kirchner and J. Ye. Human breath analysis via cavity enhanced optical frequency comb spectroscopy. Optics Express, Vol. 16, No. 4, February 18, pp. 2387-2397.
Media Contact: Ben Stein, bstein@nist.gov, (301) 975-3097 

Directed Self-Ordering of Organic Molecules for Electronic Devices
A simple surface treatment technique demonstrated by a collaboration between researchers at the National Institute of Standards and Technology (NIST), Penn State and the University of Kentucky potentially offers a low-cost way to mass produce large arrays of organic electronic transistors on polymer sheets for a wide range of applications including flexible displays, “intelligent paper” and flexible sheets of biosensor arrays for field diagnostics.
In a paper posted this week,* the team describes how a chemical pretreatment of electrical contacts can induce self-assembly of molecular crystals to both improve the performance of organic semiconductor devices and provide electrical isolation between devices.
Organic electronic devices are inching towards the market. Compounds with tongue-twisting names like “5,11-bis(triethylsilylethynyl) anthradithiophene” can be designed with many of the electrical properties of more conventional semiconductors. But unlike traditional semiconductors that require high-temperature processing steps, organic semiconductor devices can be manufactured at room temperature. They could be built on flexible polymers instead of rigid silicon wafers. Magazine-size displays that could be rolled up or folded to pocket size and plastic sheets that incorporate large arrays of detectors for medical monitoring or diagnostics in the field are just a couple of the tantalizing possibilities.
One unsolved problem is how to manufacture them efficiently and at low cost. Large areas can be coated rapidly with a thin film of the organic compound in solution, which dries to a semiconductor layer. But for big arrays like displays, that layer must be patterned into electrically isolated devices. Doing that requires one or more additional steps that are costly, time-consuming and/or difficult to do accurately.
The NIST team and their partners studied the organic version of a workhorse device—the field effect transistor (FET)—that commonly is used as a switch to, for example, turn pixels on and off in computer displays. The essential structure consists of two electrical contacts with a channel of semiconductor between them. The researchers found that by applying a specially tailored pretreatment compound to the contacts before applying the organic semiconductor solution, they could induce the molecules in solution to self-assemble into well-ordered crystals at the contact sites. These structures grow outwards to join across the FET channel in a way that provides good electrical properties at the FET site, but further away from the treated contacts the molecules dry in a more random, helter-skelter arrangement that has dramatically poorer properties—effectively providing the needed electrical isolation for each device without any additional processing steps. The work is an example of the merging of device structure and function that may enable low cost manufacturing, and an area where organic materials have important advantages.
In addition to its potential as a commercially important manufacturing process, the authors note, this chemically engineered self-ordering of organic semiconductor molecules can be used to create test structures for fundamental studies of charge transport and other important properties of a range of organic electronic systems.
* D.J. Gundlach, J.E. Royer, S.K. Park, S. Subramanian, O.D. Jurchescu, B.H. Hamadani, A.J. Moad, R.J. Kline, L.C. Teague, O. Kirillov, C.A. Richter, J.G. Kushmerick, L.J. Richter, S.R. Parkin, T.N. Jackson and J.E. Anthony. Contact-induced crystallinity for high-performance soluble acene-based transistors and circuits. Nature Materials Advanced Online Publication, 17 February 2008.
Media Contact: Michael Baum, michael.baum@nist.gov, (301) 975-2763 

Turtle Studies Suggest Health Risks from Environmental Contaminants
 |
NIST research biologist Jennifer M. Keller taking a blood sample from a loggerhead turtle as part of her study looking at the health impacts of perfluorinated compounds (PFCs) on the endangered marine reptile.
Credit: NIST
View hi-resolution image |
The same chemicals that keep food from sticking to our frying pans and stains from setting in our carpets are damaging the livers and impairing the immune systems of loggerhead turtles—an environmental health impact that also may signal a danger for humans.
Jennifer Keller, a researcher at the National Institute of Standards and Technology (NIST) Hollings Marine Laboratory in Charleston, S.C., reported on Feb. 16 at the annual meeting of the American Association for the Advancement of Science (AAAS) that a scientific team monitoring the blood plasma of loggerhead turtles along the U.S. East Coast consistently found significant levels of perfluorinated compounds (PFCs). PFCs are used as nonstick coatings and additives in a wide variety of goods including cookware, furniture fabrics, carpets, food packaging, fire-fighting foams and cosmetics. They are very stable, persist for a long time in the environment and are known to be toxic to the liver, reproductive organs and immune systems of laboratory mammals.
Keller said that in a 2005 study,* PFC concentrations measured in the plasma of turtles found along the coast from Florida to North Carolina indicated that PFCs have become a major contaminant for the species. The levels of the most common PFC, perfluorooctane sulfonate (PFOS), were higher in turtles captured in the north than in the south. Data recently evaluated by NIST and College of Charleston graduate student Steven O’Connell shows that this northern trend of higher PFOS concentrations continues up into the Chesapeake Bay.
Blood chemistry analyses of PFC-contaminated loggerheads suggested damage to liver cells and the suppression of at least one immune function which could lead to a higher risk of disease. To support the “cause-effect relationship” between PFCs and illness, the researchers exposed Western fence lizards to the same PFOS levels found in loggerheads in the wild. The lizards showed significant increases in an enzyme that indicates liver toxicity. They also had signs of suppressed immune function.
These findings, Keller said, indicate that current environmental PFC exposures—at concentrations comparable to those seen in human blood samples—are putting marine species at enhanced risk of health problems from reduced immunity and may suggest a similar threat to us.
Keller reported that a recently completed study** led by colleague Margie Peden-Adams of the Medical University of South Carolina that showed PFOS is toxic to the immune systems of mice at concentrations found both in loggerhead sea turtles and humans. The ability of the mouse immune system to respond to a challenge was reduced in half by PFOS—and this occurred at the lowest level of the compound ever reported for a toxic effect.
If our immune systems have a similar sensitivity to PFOS, Keller explained, humans could be immunocompromised from current environmental exposure to PFOS.
* J. M. Keller, K. Kannan, S. Taniyasu, N. Yamashita, R.D. Day, M.D. Arendt, A.L. Segars and J. R. Kucklick. Perfluorinated compounds in the plasma of loggerhead and Kemp’s Ridley sea turtles from the southeastern coast of the U.S. Environmental Science and Technology, Vol. 39, pp. 9101-9108 (2005).
** M.M. Peden-Adams, J.M. Keller, J.G. EuDaly, J. Berger, G.S. Gilkeson and D.E. Keil. Suppression of humoral immunity in mice following exposure to perfluorooctane sulfonate (PFOS). Submitted for publication.
Media Contact: Michael E. Newman, michael.newman@nist.gov, (301) 975-3025 

Chemists Measure Copper Levels in Zinc Oxide Nanowires
Chemists at the National Institute of Standards and Technology (NIST) have been the first to measure significant amounts of copper incorporated into zinc oxide (ZnO) nanowires during fabrication. The issue is important because copper plays a significant—but not well-understood—role in important optical and electrical properties of the nanowires. Previous experiments found only trace amounts of copper.
Although zinc oxide is best known as a strong sunblock, cold remedy, itch reliever and paint pigment, nanotech engineers like it for its photoluminescence (the ability to emit light after absorbing electromagnetic radiation), field emission (the basis for advanced, high-definition flat-panel displays) and piezoelectric properties (stressing or changing shape when electricity is applied and producing electricity when stressed). ZnO nanomaterials may one day be used to improve solar cells, lasers, sensors, ultraviolet light sources, field emission sources and piezoelectric devices.
Copper enters the ZnO nanowires during fabrication. The nanowires—about 50 to 150 nanometers wide and up to 40 micrometers long—are grown on a copper substrate using a chemical vapor deposition process. The copper substrate forms droplets that absorb the zinc and oxygen vapors and deposits the ZnO on the substrate. As the nanowire grows, the zinc pushes the droplets up from the surface, but some copper remains inside the nanowire’s crystal lattice.
In a new paper,* NIST chemists report using a variety of measurement techniques to learn that the ZnO wires contain a surprising amount of copper—between 5 and 15 percent. High-resolution imaging studies of ZnO nanowires reveal that the copper manages to fit into zinc oxide’s regular crystalline structure without disrupting it. “It is in there somewhere,” explains chemist Susie Eustis. Because the copper can be easily detected when you know what to look for, she says, researchers plan to use it to better understand the crystal structure of ZnO nanowires with an eye toward manipulating the nanowires to improve performance. “The copper acts like a smart tag that you put on an animal in the wild to trace where it travels,” says Eustis.
The role copper plays in ZnO nanowires is ambiguous. Published studies differ on whether the copper increases or decreases the nanowires’ photoluminescence. Eustis and colleagues found that the copper in the nanowire increases the output of visible light but at the expense of ultraviolet emission.
In addition to determining the role copper plays in ZnO nanowires, the researchers plan to learn how to grow uniform nanowires that may one day be used in commercial products. This research is part of ongoing studies to find the best methods to determine the concentration and distribution of atoms inside nanostructures.
* S. Eustis, D.C. Meier, M.R. Beversluis and B. Nikoobakht. Analysis of copper incorporation into zinc oxide nanowires. ACS Nano Articles ASAP, published online Jan. 12, 2008.
Media Contact: Evelyn Brown, evelyn.brown@nist.gov, (301) 975-5661 

NIST Building Safety Efforts Mark Fifth Anniversary of R.I. Nightclub Fire
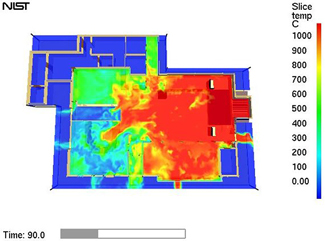 |
Computer model of fire at The Station nightclub showing temperature variation after 90 seconds at 1.5 meters (5 feet) above the floor.
Credit: NIST
View hi-resolution image |
Five years ago on Feb. 20, 2003, pyrotechnics lit during a concert at The Station nightclub in West Warwick, R.I., started a rapidly spreading fire that caused the deaths of 100 people. Within a week, the National Institute of Standards and Technology (NIST) launched a two-year investigation of the tragic event to understand exactly what happened and to make recommendations for change based on those findings.
NIST’s final report on the fire, released June 29, 2005, made 10 recommendations for increased occupant safety in nightclubs (For the complete list, see “Recommendations–NIST Investigation of The Station Nightclub Fire”.) By the time the final report was issued, seven of the 10 proposed safety improvements already had been acted upon by the state of Rhode Island and national model code development organizations. NIST supported the changes to the national model codes and urged all state and local jurisdictions to adopt them. The NIST recommendations included requirements that all nightclubs with occupancies of more than 100 people have sprinkler systems, tighter restrictions on the use of flammable materials in finish products and the maximum permitted evacuation times—90 seconds for nightclubs similar in size to or smaller than The Station.
The remaining three NIST recommendations called for more research on human behavior in emergencies, fire spread and suppression, and computer-aided decision tools—the data from which could yield further improvements in and maximize the effectiveness of these lifesaving regulations. Recent NIST research efforts are taking important steps toward achieving these goals.
One project under way seeks to improve the evaluation of portable fire extinguishers for use in public venues such as nightclubs. Laboratory fire tests at NIST’s Gaithersburg, Md., headquarters are being conducted to determine if the current method of rating and locating portable fire extinguishers in areas of assembly (such as nightclubs) is sufficient to enable typically trained nightclub staff to control a worst-case, rapidly spreading fire.
A second study is looking at how fires grow and spread on plastics, polyurethane foams and similar materials that melt and flow when burning. The goal is to develop a state-of-the-art computer model for this fire behavior that can be used to design less flammable furniture and interior wall, floor and ceiling finish materials.
Finally, NIST—working with code making organizations, other federal agencies and universities—has begun a major research program to improve the design and performance of building egress and emergency access systems through a greater understanding of human behavior in emergency situations. NIST and its partners expect this effort to eventually yield advanced building design software that accounts for how people react during evacuations.
For more information on the NIST investigation of The Station nightclub fire, go to www.nist.gov/ncst.
Media Contact: Michael E. Newman, michael.newman@nist.gov, (301) 975-3025 

Quick Links
New Strontium Atomic Clock at JILA is ‘Best in Class’
A next-generation atomic clock that tops previous records for accuracy in clocks based on neutral atoms has been demonstrated by physicists at JILA, a joint institute of the National Institute of Standards and Technology (NIST) and the University of Colorado at Boulder. The new clock, based on thousands of strontium atoms trapped in grids of laser light, surpasses the accuracy of the current U.S. time standard based on a “fountain” of cesium atoms. The work was reported last week in Science Express.
JILA’s experimental strontium clock is now the world’s most accurate atomic clock based on neutral atoms, more than twice as accurate as the NIST-F1 standard cesium clock located just down the road at the NIST campus in Boulder. The JILA strontium clock would neither gain nor lose a second in more than 200 million years, compared to NIST F-1’s current accuracy of over 80 million years.
The strontium clock uses light, which has higher frequencies than the microwaves used in NIST-F1. Because the frequencies are higher, the clock divides time into smaller units, offering record precision. Laboratories around the world are developing optical clocks based on a variety of different designs and atoms; it is not yet clear which design will emerge as the best and be chosen as the next international standard.
For more details and video see “Collaboration Helps Make JILA Strontium Atomic Clock ‘Best in Class’.” 
New NIST Video: Bill Phillips School Talk on the Science of Ultracold
The National Institute of Standards and Technology (NIST) has produced a DVD of a presentation that Nobel laureate William Phillips, a physicist at NIST, gave about the science of cold temperatures to approximately 800 students, parents and teachers at Parkland Magnet Middle School for Aerospace Technology in Rockville, Md., on Jan. 9, 2008. He showed how the properties of everyday materials—from inflated balloons that become flat as Frisbees to carnations that shatter like glass—change drastically at cold temperatures. The presentation was given in conjunction with the airing of the PBS NOVA program “Absolute Zero.”
Phillips shared the 1997 Nobel Prize in physics “for development of methods to cool and trap atoms with laser light.”
NIST created the DVD for teachers who wish to share the program with their students. To receive a copy, e-mail inquiries@nist.gov. For more information about the presentation and highlights from the DVD, see “NIST Nobel Laureate Explains ‘Science of Cold’ to 800 Middle School Students.” The PBS NOVA program “Absolute Zero” can be viewed online at www.pbs.org/wgbh/nova/zero/program.html.
New Chair, Vice Chair Elected to NIST Policy Advisory Group
A new chair and vice chair have been elected to the National Institute of Standards and Technology (NIST) Visiting Committee on Advanced Technology (VCAT), the agency’s primary private-sector policy advisory group.
The new chair is James W. Serum, president of SciTek Ventures, a consulting company that works with early stage technology companies. The new vice chair is Vinton G. Cerf, vice president and chief Internet evangelist for Google, where he identifies new enabling technologies and applications on the Internet and other platforms. Their terms start on April 1 and last for two years. The two were elected by the members of VCAT. The VCAT was established by Congress in 1988 to review and make recommendations on NIST’s policies, organization, budget and programs.
The next NIST VCAT meeting will be held on June 10-11 in Boulder, Colo. VCAT meetings are open to the public. For more information, see www.nist.gov/director/vcat/. 
NIST Releases Three-Year Plan
The National Institute of Standards and Technology (NIST) has delivered a three-year plan for its programs to Congress, as required by the America COMPETES Act passed in August 2007. The plan provides a broad vision for NIST programs during Fiscal Years 2009-2011.
In the document, NIST directs future planning towards three overarching strategic goals: help the United States to drive and take advantage of the increased pace of technological change; foster more efficient transactions in the domestic and global marketplace; and address critical national needs.
To reach these goals, the plan rests on four strategic “pillars,” including optimizing NIST’s outreach to academia and industry, for example by developing and implementing new models of public/private partnerships to promote directed basic research in key technological sectors. The document provides a framework that allows NIST to continually reevaluate itself, and retain the flexibility necessary to develop programs that address immediate national needs, whether they relate to voting standards and technology, IT security, public safety or other currently unforeseen issues.
The plan (pdf file) is available online at www.nist.gov/director/reports/Final_NIST_3y.pdf. 
Two NIST Researchers named to ‘Federal 100’
Douglas Montgomery and Stephen Quinn, two computer scientists at the National Institute of Standards and Technology (NIST), have been named to this year’s “Federal 100” by the trade publication Federal Computer Week (FCW). According to the publication, the list consists of “100 people recognized for their contributions to the federal information technology market in the past year.”
Quinn is the co-originator of the Security Content Automation Protocol, a technical framework that supports the automation of security operations in information systems (see “New Web Site Lists Validated Software Security Tools for Federal IT”). Montgomery is leading the development of a standard profile to support the federal government’s long-term implementation of Internet Protocol Version 6, the next-generation communication protocol that defines how text, voice and video will move across the future Internet (see “Revised NIST IPv6 Profile Released for Comment”).
Montgomery and Quinn will be honored at a March 24 gala in McLean, Va. FCW’s complete Federal 100 list can be viewed at www.fcw.com/events/fed100/2007/winners_list.html 
|

